Abstract
A reporter construct was created on the basis of the transcription attenuator region of the Escherichia coli tryptophan operon. Dual-fluorescent-protein genes for red fluorescent protein and cerulean fluorescent protein were used as a sensor and internal control of gene expression. The sequence of the attenuator was modified to avoid tryptophan sensitivity while preserving sensitivity to ribosome stalling. Antimicrobial compounds which cause translation arrest at the stage of elongation induce the reporter both in liquid culture and on an agar plate. This reporter could be used for high-throughput screening of translation inhibitors.
INTRODUCTION
Antibiotic resistance genes spreading among pathogenic bacteria pose the main problem for antibacterial therapy. Currently, the search for new antibiotic compounds has become one of the principal goals in molecular biotechnology. Such a search is based on high-throughput screening of synthetic chemicals or products of microbial metabolism. These methodologies can be used in combination. Identification of new natural antibiotics and testing of chemical derivatives of existing antimicrobials are both important for medicine. One form of antibacterial activity screening includes bacterial growth inhibition. Only after detection of inhibitory activity can an antimicrobial compound be purified and its mode of action and cellular target be identified. This approach suffers from several major drawbacks. First, growth inhibition requires a relatively high concentration of the compound studied. Second, at the same time, the concentration of an active antimicrobial agent in complex chemical mixtures produced by microorganisms can be too low to suppress bacterial growth. Thus, a potentially effective antimicrobial could be overlooked. Testing of the activity of synthetic compounds is also more cost-effective, if it requires smaller amounts of substances from chemical libraries, especially in high-throughput format. It is also important to classify an antimicrobial compound by its mechanism of action during the screening stage, avoiding an additional purification step. Thus, it is necessary to develop a system suitable for high-throughput screening and target classification of subinhibitory concentrations of antibacterials.
Such systems were created on the basis of beta-galactosidase (lacZ) (5, 36), green fluorescent protein (GFP) (18, 26, 29), and luciferase operon (lux) (1, 13, 19, 26, 27, 35, 41) reporter genes. For the comparative studies using reporter constructs, especially under conditions of global repression of gene expression, it is highly beneficial to use an additional reporter gene as a reference. A well-known example of a system that uses an internal reference gene is the dual-luciferase reporter system. Despite being a helpful tool to study various gene expression mechanisms, it requires the additional procedure of cell permeabilization and the consumption of costly substrates, increasing the cost of large-scale screens.
Several mechanisms of selective expression of reporter genes upon exposure to a particular class of antibacterial agents were utilized in previous reporter systems. The first approach makes use of highly specific repressor/inducer mechanisms. Detection of tetracyclines is based on the specific tetracycline binding repressor protein of the Tn10 mobile element (19). A similar system was developed for detection of beta-lactams using the ampR-ampC element from Citrobacter freundii (41). Available systems for detection of macrolide antibiotics were designed on the basis of the MphR repressor from Escherichia coli strain Tf481A (27) and induction of ErmC methyltransferase (3). The second approach for detection of antibacterials relies on stress-activated promoters. These promoters are suitable for detection of DNA-damaging agents, such as nalidixic acid and mitomycin C. recA, sulA, umuDC, and cda promoters were used to control expression of the GFP (18, 26, 29), lacZ (36), and red-shifted variant of Photinus pyralis luciferase (35) reporter genes in a number of studies in various combinations in response to genotoxic agents. For detection of oxidation damage, the katG promoter was used (26). Cell envelope stress could similarly be detected using the P3rpoH promoter (5, 36). Translation inhibitors may cause various types of responses to stress, such as cold or heat shock (42). Correspondingly, cspA and ibp promoters were applied for the detection of a number of ribosome-binding antibiotics. The cspA gene, coding for a general cold shock protein, was induced upon chloramphenicol and tetracycline treatment (5). The small heat shock protein IbpA and IbpB promoters showed maximal activity during exposure of cells to ribosome-binding aminoglycosides streptomycin, neomycin, and surprisingly, polymyxin B, affecting the outer membrane (5). Cell envelope stress induced by polymyxin B and carbenicillin increased the activity of the P3rpoH promoter (5). Replication inhibitors could be sensed by the system with an inducible plasmid copy number increase (1).
The general ability to stall the ribosome has never been used to screen for translation inhibitors. All reported systems were specifically designed for particular types of ribosome-targeting antibacterials (3, 5, 7, 19, 27). Through this study, we report a new system which selectively senses subinhibitory concentrations of ribosome-stalling inhibitors. The system uses the gene coding for red fluorescent protein (RFP) (25) as a reference gene and the gene coding for cerulean fluorescent protein (CER) (32) to monitor translation-dependent attenuation of transcription.
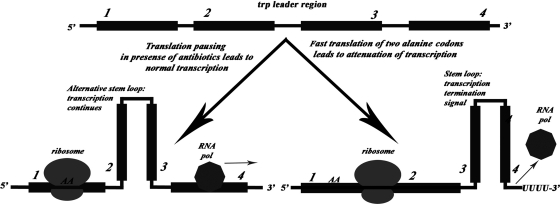
Translation attenuation mechanisms are required for the adjustment of expression of genes involved in various metabolic processes in Escherichia coli (24). The best-known example is tryptophan operon attenuation (23). The structural genes trpEDCBA, coding for enzymes involved in tryptophan biosynthesis, are preceded by the trpL gene, coding for the leader peptide. Within the leader peptide, a tandem of tryptophan codons creates a tryptophanyl-tRNA-dependent ribosome pause site (45). When paused due to tryptophan limitation, the translating ribosome affects the transcript secondary structure, preventing premature transcription termination via the Rho-independent terminator. This allows trpEDCBA genes to be transcribed and expressed. The system depends on coupling between transcription and translation and effectively measures the difference in transcription and translation rates.
We inserted the tryptophan operon leader fragment preceding the first biosynthetic gene trpE between the T5 phage promoter and CER gene. In the same plasmid, a gene coding for the RFP gene was placed under the control of a similar promoter with a deleted trp attenuation region. The reporter system was shown to depend on tryptophan concentration, as expected. We substituted tandem tryptophan codons for alanine codons, making the system independent of tryptophanyl-tRNA concentration. In this study, we demonstrate that the created translation attenuation system is sensitive to agents which specifically slow down or stop translation.
MATERIALS AND METHODS
Strains and media.
E. coli strain BW25113 and isogenic strain JW5503, devoid of the tolC gene, were kindly provided by Hironori Niki, National Institute of Genetics, Japan (2). Streptomyces venezuelae Ehrlich, Streptomyces fradiae, and Streptomyces griseus strains were obtained from the Russian Collection of Microorganisms and grown in LB medium supplied with 0.1% glucose at 30°C. E. coli strains were grown at 37°C in LB or M9 minimal medium, supplied with 100 μg/ml ampicillin if required. For growth of Actinomyces sp. strains N2 Gauze broth (glucose, 1%; peptone, 0.5%; NaCl, 0.5%; tryptone, 0.3%) was used. The culture of Micromonospora sp. strain 428/07 was grown in 100 ml N2 Gauze broth for 7 days at 25°C. After identification of the translation inhibition activity of Micromonospora sp. strain 428/07, it was renamed Micromonospora sp. strain INA01084, and under this name the organism was deposited in the strain collection of the G. F. Gauze Institute for Search for New Antibiotics.
Reporter vector construction.
To create the pRFPCER construct, two variants of native T5 promoter (33), T5-1 and T5-2 with XbaI and HindIII sites and NcoI and NdeI sites at the 5′ and 3′ ends, respectively, were cloned into pCDF Duet-1 vector (Novagen) XbaI/NcoI and HindIII/NdeI sites. Next, the RFP gene from TurboRFP (Evrogen) and the CER gene from LeGo-iCer (44) were cloned into vector NcoI/SacI and NdeI/EcorV sites. Also, the gene for antibiotic resistance was replaced by the β-lactamase gene from pKD3 (8). The DNA fragment of 0.19 kb containing the tryptophan operon regulatory region from the transcription start site to the first 10 codons of the trpE coding region was amplified by PCR from genomic DNA with 5′-CCGCGGAAGTTCACGTAAAAAGGGTATCG-3′ forward and 5′-CATATGTTGTGTTTGCATTGTTATTCTC-3′ reverse primers harboring SacII and NdeI sites (underlined), respectively; these sites were used to obtain the pRFPCER-TrpL construction. To create pRFPCER-TrpL2A, plasmid pRFPCER2-TrpL was mutated by a QuikChange PCR mutagenesis kit (Stratagene) with two pairs of primers, 5′-CGTACTGAAAGGTGCGGCGCGCACTTCCTG-3′ and 5′-CAGGAAGTGCGCGCCGCACCTTTCAGTACG-3′ for changing two tryptophan codons to alanine codons and 5′-GAAACGGGCAGTGTATTGCCGCTGCGTAAAGCAATCAG-3′ and 5′-CTGATTGCTTTACGCAGCGGCAATACACTGCCCGTTTC-3′ for compensatory changes. The JM109 E. coli strain was used for DNA cloning. DNA sequences of intermediate and final constructs were confirmed by sequencing with appropriate primers.
Dual-fluorescent-protein reporter assay in liquid medium.
Chemically competent cells made from the BW25113 strain were transformed with pRFPCER, pRFPCER-TrpL, and pRFPCER-TrpL2A plasmids. Individual clones were grown overnight in LB or M9 medium supplemented with ampicillin (100 μg/ml) diluted to an optical density (OD) of 0.005 to 0.01 (590 nm) with fresh LB or M9 broth. Following dilution, one of the following antibiotics or tryptophan was added at the indicated concentrations by a Janus (Perkin Elmer) automated workstation: erythromycin at 10, 20, or 40 μg/ml, chloramphenicol at 0.25, 0.5, or 1 μg/ml, kanamycin at 1, 2, or 4 μg/ml, tetracycline at 0.125, 0.5, or 0.625 μg/ml; streptomycin at 1.3, 2.7, or 5.3 μg/ml; spectinomycin at 2, 4, or 8 μg/ml; tobramycin at 2.5, 5, or 10 μg/ml; rifampin at 7.5, 15, or 30 μg/ml; levofloxacin at 0.0025, 0.005, or 0.01 μg/ml; ciprofloxacin at 0.00125, 0.0025, or 0.005 μg/ml; ofloxacin at 0.005, 0.01, or 0.02 μg/ml; norfloxacin at 0.005, 0.01, or 0.02 μg/ml; or tryptophan at 100 μg/ml, 1 mg/ml, or 4 mg/ml. After the addition of an antibiotic or tryptophan, cells were grown overnight at 37°C in square-well 2-ml 96-well plates with shaking. Next, the OD at 590 nm was measured to estimate the influence of an antibiotic on the growth of bacteria. Cells were washed twice with 0.9% NaCl, and the fluorescence of reporter proteins was measured by a Victor X5 2030 (Perkin Elmer) multifunctional reader, using appropriate emission/excitation filters (430/486 nm for CER and 531/595 nm for RFP). The standard deviation was derived from at least three parallel independent measurements. To measure induction of the CER gene by antibiotics, the CER gene-to-RFP gene fluorescence ratio was normalized to that in untreated cells.
Dual-fluorescent-protein reporter assay on agar.
The overnight culture of cells (BW25113 or JW5503 ΔtolC), transformed as indicated above with either pRFPCER-TrpL, pRFPCER-TrpL2A, or pRFPCER (control), was diluted to 0.05 to 0.1 OD (590 nm) units with fresh LB medium supplied with ampicillin 100 μg/ml and plated on LB agar medium (ampicillin 100 μg/ml). After drying of the plate, 1 μl of antibiotic or a disk soaked in antibiotic solution was added at the following concentrations: for antibiotics in solution, erythromycin at 5 mg/ml, chloramphenicol at 1 mg/ml, kanamycin at 1.25 mg/ml, and tetracycline at 0.25 mg/ml; for disks soaked in antibiotic solution, erythromycin at 15 μg, clarithromycin at 15 μg, roxithromycin at 30 μg, oleandomycin at 15 μg, azithromycin at 15 μg, sulfanilamide at 10 μg, polymyxin at 300 μg, rifampin at 5 μg, chloramphenicol at 30 μg, kanamycin at 30 μg, tetracycline at 30 μg, streptomycin at 30 μg, vancomycin at 30 μg, linezolid at 30 μg, lincomycin at 15 μg, clindamycin at 2 μg, nalidixic acid at 30 μg, phosphomycin at 50 μg, levofloxacin at 5 μg, norfloxacin at 10 μg, tobramycin at 10 μg, and neomycin at 30 μg. Half a disk of phosphomycin, levofloxacin, norfloxacin, or tobramycin was also used.
Fermentation broth from antibiotic-producing strains Streptomyces venezuelae Ehrlich, Streptomyces fradiae, and Streptomyces griseus was prepared according to the following procedure: a single colony of a streptomycete was inoculated into fresh LB medium supplied with 0.1% glucose and cultivated for 3 to 5 days at 30°C. Cells were harvested by centrifugation, and the supernatant was collected. The antibiotic-containing supernatant was concentrated approximately 50 times under vacuum. The obtained solution (5 to 10 μl) was applied on the surface of a confluent sheet of the reporter strain on agar. For the screening of metabolites produced by a set of soil actinomycetes, a growth medium of 45 Actinomyces sp. strains was used to soak Whatman 3MM discs (sterile; diameter, 5 mm). These discs were used along with discs soaked in solutions of pure antibiotics.
Following overnight incubation at 37°C, the petri dishes were photographed by a digital camera under UV (254-nm) illumination.
Extraction of antimicrobial compound produced by Micromonospora sp. strain 428/07 from growth medium and testing of its activity on a petri dish.
Micromonospora sp. strain 428/07 was grown in N2 Gauze broth for 7 days at 25°C with agitation. Following centrifugation to remove the cells, 30 ml of growth medium was mixed with 3 ml of chloroform and the mixture was agitated for 2 h at 4°C. Then, chloroform was separated and evaporated and the precipitate was dissolved in 200 μl of sterile Milli-Q water. The obtained solution was tested on a petri dish against a lawn of the ΔtolC strain transformed with pRFPCER-TrpL2A. A solution of strain 428/07-produced antimicrobial (3 μl) was spotted onto the plate. A solution of 1 mg/ml erythromycin (3 μl) was also spotted onto the same plate for comparison.
Testing translation inhibition in vivo and in vitro.
An individual bacterial colony was inoculated in M9 minimal medium supplied with all natural amino acids (40 mg/liter each) and grown overnight at 37°C. The obtained culture was diluted 50-fold into 10 ml of M9 medium with amino acids and grown until middle log phase (A590, ∼0.5). Cells were collected, washed twice with 0.9% NaCl, and then dissolved in M9 medium without methionine and split into several samples. Solutions of erythromycin (1 mg/ml) and, separately, a solution of strain 428/07-produced antimicrobial in appropriate concentrations (shown in Results) were added to all samples except the control sample. After 15 min of incubation, homopropargyl glycine (HPG; 25 μM; Invitrogen) was added to all samples. HPG inclusion was stopped by harvesting the cells after 15 min. Harvested cells were washed twice with 0.9% NaCl, resuspended in 150 μl of a lysis buffer (HEPES, pH 7.0, 10 mM; NaCl, 100 mM; β-mercaptoethanol, 2 mM; Brij 35, 0.15%; phenylmethylsulfonyl fluoride, 2 mM; lysozyme, 1 mg/ml), and incubated on ice for 20 min. Cell suspensions were frozen in liquid nitrogen, thawed, and then sonicated and centrifuged (18,000 rpm, 20 min). Supernatant was collected and stored at −20°C. For labeling, a Click-iT protein reaction buffer kit (Invitrogen) was used. Samples were labeled with Cy5 azide, and the quantity of labeled proteins was estimated by one-dimensional SDS-PAGE. Detection of fluorescence was made by a fluorescent scanner (Fuji). In vitro translation of luciferase mRNA was carried out in S100 extract from E. coli cells supplemented with ribosomes, according to Svetlov et al. (37). Activity of the synthesized luciferase was detected at time points of 10, 20, 30, 40, 50, and 60 min. Rifampin at a concentration of 4 μg/ml served as a control of an antibiotic that does not affect translation, while erythromycin at a concentration of 0.8 μg/ml served as a control of the translation inhibitor. A 1:50 dilution of chloroform extracted the compound produced by the 428/07 strain and was used to verify inhibition of translation.
RESULTS
Dual-fluorescent-protein reporter for monitoring translation inhibitors based on tryptophan operon attenuation region.
Attenuation of transcription of the trpEDCBA operon depends on the relative velocities of RNA polymerase and the translating ribosome. We aimed to utilize this system to create a reporter sensitive and specific to translation inhibitors. It was first necessary to create a vector carrying the genes for both RFP and CER under the control of similar but separate promoters. This would guarantee that the relative amount of fluorescent proteins would not depend on transcription initiation and plasmid replication. We used the pCDF Duet-1 vector (Novagen) for cloning of the RFP and CER genes to obtain the pRFPCER reporter (Fig. 1). A natural T5 promoter was selected, because it does not depend on most processes in a bacterial cell and can provide a constant level of transcription at different stages of growth. Between the transcription start site of the T5-2 promoter and the coding region of the CER gene, a DNA fragment containing a tryptophan operon region precisely from the transcription start site to the trpE coding region was cloned. As a result, the CER gene exactly replaced the trpE gene, following the attenuation region, creating a reporter, pRFPCER-TrpL (Fig. 1). Usage of the T5 promoter made transcription independent from the TrpR transcription repressor but not from the attenuation mechanism. Our goal was to create a reporter insensitive to a tryptophan concentration but instead suitable for monitoring the presence of translation inhibitors. To pursue this goal, we replaced tandem UGG tryptophan codons with GCG codons coding for alanine. At the same time, we introduced mutations (UCACCA to UGCCGC) to restore the secondary structure of the attenuator region (Fig. 1, right). This reporter plasmid was named pRFPCER-TrpL2A (Fig. 1).
Fig 1.
(A) Maps of pRFPCER, pRFPCER-TrpL, and pRFPCER-TrpL2A reporter plasmids. UTR, untranslated region; CDF ori, CloDF13-derived CDF replicon; bla, ampicillin resistance gene, T5-1 and T5-2, bacteriophage T5 promoters. Also shown are locations of the trpL leader coding sequence carrying W10A, W11A substitutions (trpL AA), the start of transcription (▶), and the terminator (■). (B) Secondary structure of trpL. Mutated nucleotides are indicated.
To test whether the created reporters respond to a tryptophan concentration, we grew Escherichia coli transformed with the pRFPCER-TrpL reporter in M9 minimal medium without tryptophan, as well as in the presence of 100-μg/ml, 1-mg/ml, and 4-mg/ml concentrations of tryptophan. Cells were harvested and their fluorescence of CER and RFP was measured (Fig. 2). As expected, the CER/RFP signal ratio was sensitive to the presence of tryptophan in the medium for the pRFPCER-TrpL reporter (Fig. 2, black bars). Relative CER/RFP fluorescence was several times higher upon tryptophan starvation. Thus, the reporter system that we created demonstrated its suitability for monitoring transcription attenuation. After the replacement of tryptophan codons with alanine codons, the dependence of the CER/RFP fluorescence ratio on tryptophan concentration was lost (Fig. 2, gray bars).
Fig 2.
Response of Escherichia coli transformed with reporters pRFPCER-TrpL and pRFPCER-TrpL2A to tryptophan in M9 minimal medium. Ratios of CER/RFP fluorescence with standard deviation corridors are shown.
We expected that the reporter construct pRFPCER-TrpL2A would be sensitive to translation inhibitors due to translation pausing (Fig. 3), mimicking one caused by a low abundance of Trp-tRNA (Fig. 3). To test this, we treated E. coli cells transformed with the pRFPCER-TrpL2A reporter with subinhibitory concentrations of various antibiotics (Fig. 4A). The following translation inhibitors were used: erythromycin, chloramphenicol, kanamycin, tetracycline, streptomycin, spectinomycin, and tobramycin. As a negative control, we used rifampin as an RNA polymerase inhibitor and levofloxacin, ciprofloxacin, ofloxacin, and norfloxacin as DNA gyrase inhibitors. The translation elongation inhibitors erythromycin and chloramphenicol caused an increase in the CER/RFP fluorescence ratio up to 6-fold for erythromycin and 9-fold for chloramphenicol (Fig. 4A, black bars). Other ribosome-targeting antibiotics, namely, kanamycin, tetracycline, streptomycin, spectinomycin, and tobramycin, increased the CER/RFP fluorescence ratio moderately, up to 2- to 4-fold. Control experiments with rifampin, levofloxacin, ciprofloxacin, ofloxacin, and norfloxacin displayed almost no induction within the error range (Fig. 4A, black bars).
Fig 3.
Suggested secondary structures of leader regions preceding CER gene under various conditions. (Left) Secondary structure of the leader region of mRNA transcribed from the pRFPCER-TrpL2A reporter in the presence of translation inhibitors, mimicking the secondary structure of trpL mRNA at low tryptophan concentrations; (right) secondary structure of the leader region of mRNA transcribed from the pRFPCER-TrpL2A reporter without translation inhibitors, mimicking the secondary structure of trpL mRNA at high tryptophan concentrations.
Fig 4.
Increase of CER/RFP fluorescence ratio caused by translation inhibitors. (A) Data were normalized to the CER/RFP fluorescence ratio in the absence of any antibiotics. Antibiotics used for induction are shown above the graphs, while their concentrations and MICs are indicated below the graphs. Black bars, pRFPCER-TrpL2A construct; gray bars, control construct pRFPCER devoid of attenuator. (B) The lawn of E. coli transformed with pRFPCER-TrpL2A (left), pRFPCER (center), and pRFPCER-TrpL (right) grown after local spotting of particular antibacterials (indicated): Kan, kanamycin; Cm, chloramphenicol; Ery, erythromycin; Tet, tetracycline.
The control plasmid, where CER and RFP genes have similar promoters and similar translation initiation regions without any attenuators (Fig. 4A, gray bars), displayed equal inhibition of expression of both fluorescent proteins by antibiotics, thus preserving the CER/RFP ratio. Thus, it is the attenuator region which is responsible for CER gene induction upon exposure of cells to subinhibitory concentrations of translation-stalling compounds.
Screening for translation inhibitors directly on the agar plates might be useful for primary screening of actinomycetes that produce antimicrobials. We used agar plates covered by the lawn of bacteria transformed with the pRFPCER-TrpL2A reporter plasmid. Several antibiotics were spotted onto the lawn of the reporter strain. For this test, we selected a number of antibiotics that cause translation arrest and those that cause an increase in misreading or affect translation by another mechanism. After overnight incubation at 37°C, clear inhibition zones became visible (Fig. 4B). Far from inhibition zones, the bacterial lawn showed red fluorescence due to higher expression of RFP over CER. On the border of the inhibition zones, where the antibiotic concentration was subinhibitory, circles of CER induction were clearly visible. These circles were formed under the influence of antibiotics causing ribosome stalling, such as erythromycin and chloramphenicol. Antimicrobials which do not cause ribosome stalling (kanamycin and tetracycline) do not induce CER expression. Induction of CER expression was dependent on the attenuation region (Fig. 4B). Strains transformed with the plasmids pRFPCER-TrpL and pRFPCER-TrpL2A displayed circles of CER induction around erythromycin and chloramphenicol spots, while those transformed with a parental pRFPCER construct devoid of attenuators did not induce CER, in agreement with the previous experiments for reporter induction in a liquid culture.
Testing dual-fluorescent-protein reporter with a set of pure antimicrobials.
We tested the induction of the pRFPCER-TrpL2A reporter construct against a large set of antimicrobials with various mechanisms of action. We used a set of macrolides and related compounds consisting of erythromycin, clarithromycin, roxithromycin, oleandomycin, and azithromycin to test pRFPCER-TrpL2A reporter induction. Other compounds targeting large ribosomal subunits tested were chloramphenicol, lincomycin, clindamycin, and linezolid. Several antibiotics binding the small ribosomal subunit were tested: tetracycline, streptomycin, kanamycin, neomycin, and tobramycin. The antibiotics rifampin, sulfanilamide, polymyxin, phosphomycin, vancomycin, nalidixic acid, levofloxacin, and norfloxacin, unrelated to translation, were used as controls. Several antibiotics in this list were not active against the wild-type strain of E. coli. To mimic a sensitivity pattern close to that of Gram-positive bacteria, an E. coli strain with an inactivated tolC gene, coding for an indispensable component of several drug efflux pumps, was used (12). All antimicrobials listed above were tested for pRFPCER-TrpL2A reporter induction in a filter inhibition/induction assay on a plate with a bacterial lawn (Fig. 5). All antibiotics that have no influence on translation, whatever their targets are, were found not to induce the pRFPCER-TrpL2A reporter. Among antimicrobials affecting translation, all macrolides efficiently induced the reporter gene. Apart from macrolides, chloramphenicol was one of the best inducers. A set of aminoglycosides and streptomycin either moderately induced the reporter or did not induce it at all. This reflects the well-known understanding that these antibiotics do not cause the ribosomes to stall, yet increase the rates of translation errors. Surprising results were obtained for linezolid, which occupies almost the same binding pocket on the ribosome (17) as chloramphenicol (11), yet chloramphenicol was one of the best inducers for the attenuation-based reporter and linezolid induced the reporter only slightly, so that a yellow rather than a green fluorescence was visible. Also unexpected was the difference between the native antibiotic lincomycin and its chemical derivative clindamycin. Clindamycin inhibited the wild-type E. coli strain but did not induce the reporter, while lincomycin could inhibit only a strain where the tolC gene is inactivated but still efficiently induce the reporter.
Fig 5.
Induction of CER fluorescence by antibiotics on petri plates. The lawn of E. coli BW25113 or ΔtolC (indicated) transformed with pRFPCER-TrpL2A grown after adding antibiotic disks (indicated). Antibiotics used are erythromycin (Ery), clarithromycin (Cla), roxithromycin (Rox), oleandomycin (Ole), azithromycin (Azi), sulfanilamide (Sul), polymyxin (Pol), rifampin (Rif), chloramphenicol (Cm), kanamycin (Kan), tetracycline (Tet), streptomycin (Sm), vancomycin (Van), linezolid (Linez), lincomycin (Linc), clindomycin (Cli), nalidixic acid (Nal), phosphomycin (Pho), levofloxacin (Lev), norfloxacin (Nor), tobramycin (Tob), and neomycin (Neo).
Testing dual-fluorescent-protein reporter suitability to screen antibiotic producers.
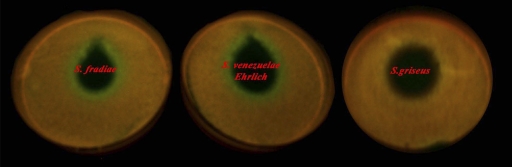
Our main goal for creation of the attenuation-based reporter system was to screen antibiotic producers in a search for translation-stalling compounds. To check the applicability of the method, we used a fermentation broth of the strains producing antibiotics which induce or do not induce the pRFPCER-TrpL2A reporter (Fig. 6). Fermentation broths of Streptomyces venezuelae Ehrlich (the chloramphenicol producer), Streptomyces fradiae (the tylosin and neomycin producer), and Streptomyces griseus (a streptomycin producer) were spotted on agar medium covered with E. coli transformed with the pRFPCER-TrpL2A reporter (Fig. 6). Medium clarified by centrifugation after growth of streptomycetes producing ribosome-stalling compounds induced clearly visible zones of CER fluorescence (Fig. 6, left and central spots), while the fermentation broth of a noninducing antibiotic producer led to a growth inhibition zone but did not increase cerulean fluorescence (Fig. 6, right spot).
Fig 6.
Induction of CER fluorescence by fermentation broths of antibiotic producers. The lawn of E. coli transformed with pRFPCER-TrpL2A grown after local spotting of fermentation broth of antibiotic producer strains S. venezuelae Ehrlich (chloramphenicol producer), S. fradiae (tylosin and neomycin producer), and S. griseus (streptomycin producer).
To test the efficiency of the new method of ribosome-stalling compound identification in a library of metabolites of natural actinomycetes, we used a set of 45 Actinomyces sp. strains found in an initial screening of soil microorganisms inhibiting growth of a methicillin-resistant Staphylococcus aureus strain. Fermentation broths of this set of soil microorganisms were used to soak filters and perform a test for induction of the pRFPCER-TrpL2A reporter in the E. coli strain with an inactivated tolC gene (Fig. 7A). One clear hit inducing the pRFPCER-TrpL2A reporter was identified.
Fig 7.
Screening of natural metabolites produced by a set of soil Actinomyces sp. strains (only a plate with the found inducer is shown). (A) The lawn of E. coli ΔtolC transformed with pRFPCER-TrpL2A grown after adding filters and soaked in fermentation broths of Actinomyces sp. strains. (B) The lawn of E. coli ΔtolC transformed with pRFPCER-TrpL2A grown after local spotting of chloroform-extracted compound produced by strain 428/07 and erythromycin (Ery) at 1 mg/ml. (C) Influence of strain 428/07 and erythromycin on nascent proteome of E. coli ΔtolC. The indicated amounts of antibiotics were added to 1 ml of cell culture, prior to addition of HPG. Cy5 fluorescence shows the amount of newly synthesized proteins after addition of the antibiotic, while the Coomassie blue-stained gel shows the amount of all proteins. (D) Inhibition of in vitro translation of luciferase mRNA by an antibiotic produced by strain 428/07. Shown are dependencies of luciferase activity in million counts per second (cps) over time of protein synthesis. Circles, activity of the luciferase synthesized in the absence of inhibitors; diamonds, activity of the luciferase synthesized in the presence of chloroform-extracted compound produced by strain 428/07 at a 1:50 dilution. Rifampin at a concentration of 4 μg/ml served as a negative control, while erythromycin at a concentration of 0.8 μg/ml served as a positive control of inhibition.
To prove that Micromonospora sp. strain 428/07, which we found to induce the pRFPCER-TrpL2A reporter, indeed produced a ribosome-stalling compound, we checked protein synthesis efficiency. A fermentation broth of Micromonospora sp. strain 428/07 was subjected to chloroform extraction to concentrate the antimicrobial compound produced. The antimicrobial compound extracted with chloroform retained the ability to induce the pRFPCER-TrpL2A reporter (Fig. 7B). A liquid culture of the E. coli strain with an inactivated tolC gene was incubated with increasing concentrations of a chloroform-extracted compound produced by Micromonospora sp. strain 428/07. In parallel, erythromycin was added to a similar E. coli culture at concentrations which cause similar growth inhibition. Protein synthesis efficiency was monitored by inclusion of a methionine analogue, homopropargyl glycine (HPG), into nascent proteins (4). Following inclusion of HPG, nascent proteins were modified with Cy5 azide in a Cu(I)-catalyzed [3 + 2] cycloaddition (22). Fluorescently labeled proteins were resolved on a denaturing gel (Fig. 7C) and scanned by a fluorescence scanner. Clearly, the antimicrobial compound produced by Micromonospora sp. strain 428/07 inhibited protein biosynthesis to almost the same extent as erythromycin.
Protein synthesis in a living cell depends on prior transcription, so it could be hypothesized that a compound produced by the 428/07 strain could inhibit transcription. To resolve this uncertainty, we performed in vitro translation of luciferase mRNA presynthesized in vitro (Fig. 7D). The transcription inhibitor rifampin served as a negative control for translation inhibition, while erythromycin served as a positive control for the ribosome-targeting antimicrobial. A clear inhibition of in vitro translation was observed for a compound produced by the 428/07 strain. Thus, the reporter described here could be used directly in screening for soil microorganisms producing compounds interfering with translation elongation.
DISCUSSION
We created a reporter system for detection of subinhibitory concentrations of antibiotics affecting translation elongation. It is based on the naturally evolved mechanism of transcription attenuation. In the cell, this mechanism is used in particular to measure the translation rate in comparison to the rate of transcription. For amino acid biosynthetic operons, such as the trpEDCBA tryptophan biosynthesis operon, the translation rate of the leader peptide, trpL, preceding an operon depends on tryptophanyl-tRNA availability (45). We used the leader region of the trpLEDCBA operon as a sensor for the efficiency of translation, but we uncoupled it from tryptophan concentration dependence by usage of the phage T5 promoter, which does not have the TrpR repressor binding site, and by replacement of UGG tryptophan codons by GCG alanine codons. Corresponding compensatory changes were introduced into the sequence of trpL RNA to preserve the attenuator secondary structure. Since alanine codons are the ones translated very efficiently, a terminator secondary structure is favored in the absence of translation inhibitors. Subinhibitory concentrations of antimicrobials causing translation stalling prevent formation of a terminator secondary structure independently of aminoacyl-tRNA availability. Accordingly, a terminator stem-loop could not be formed and the transcription proceeds to the cerulean fluorescent protein coding part. Concentrations of antimicrobial compounds in liquid culture as low as 17% (erythromycin) or 23% (chloramphenicol) of the MIC displayed detectable induction of the pRFPCER-TrpL2A reporter (Fig. 4B).
The system that we created depends on unique features of tryptophan biosynthesis operon regulation. The secondary structure of the leader region could exist either as hairpins 1-2 and 3-4 (Fig. 3) or as hairpin 2-3 (45). Hairpin 3-4 is followed by an oligouridine tract and is necessary for transcription termination. If tryptophan is in excess, translation quickly proceeds to the stop codon located between regions 1 and 2. As a result, only hairpin 3-4 could be formed, which leads to the termination of transcription (Fig. 3). A similar situation occurs for reporter construct pRFPCER-TrpL2A, if no translation inhibitor is present. If for any reason translation could not be started, both 1-2 and 3-4 hairpins are formed and transcription termination takes place as well (Fig. 3). If translation of the tryptophan leader region is limited by tryptophanyl-tRNA concentration, translation is paused at tandem UGG codons, which leads to hairpin 2-3 formation, and transcription can proceed (Fig. 3). In the reporter construct pRFPCER-TrpL2A, translation is independent from tryptophanyl-tRNA. In the presence of antibiotics targeting translation elongation, a ribosome binds mRNA and stops shortly after starting translation (Fig. 3). Antibiotics affecting translation fidelity but unable to stall a translating ribosome, like kanamycin, tobramycin, neomycin (10), and streptomycin (9), could not increase the CER/RFP activity ratio. Somewhat surprising was the inability of tetracycline to induce the pRFPCER-TrpL2A reporter. Tetracycline is known to bind the A site of the small ribosomal subunits (6, 30) and block aminoacyl-tRNA binding (34). Logically, it could have caused ribosome stalling at the elongation stage, but this apparently does not happen.
Erythromycin and other macrolides and the azalide tested here allow 6 to 8 amino acids to be polymerized (39); thus, the P site of a stalled ribosome should be located at position −12 or closer to hairpin 1-2. On the basis of the results of experiments using toe printing, helicase assay, and optical tweezers, one can state that the leading edge of the ribosome, serving as an RNA helicase, starts to melt the RNA secondary structure at 11 nucleotides from the P site (15, 31, 38). It means that any antibiotic freezing the ribosome at the step of elongation could be able to induce transcription read through the terminator and expression of cerulean fluorescent protein.
An interesting case is the group of antibiotics blocking the site occupied by the side chain of the amino acid in the A site. Chloramphenicol (11), linezolid (17), lincomycin, and its synthetic derivative clindamycin (40) bind overlapping sites in a cavity which is occupied by the methyltyrosine moiety of puromycin in the crystal structures, mimicking the peptidyl transfer intermediate states (28). Unexpected was the finding that while chloramphenicol efficiently induced the pRFPCER-TrpL2A reporter, linezolid induced the reporter only slightly, so that the zone of induction displayed yellow fluorescence, which is a mixture of red and green fluorescence. Two antibiotics occupy a site overlapping with those for chloramphenicol and linezolid. Lincomycin is a natural antibiotic which was active only against an E. coli strain with an inactivated tolC gene, while clindamycin is a lincomycin derivative which has one hydroxyl group replaced with chlorine. Clindamycin is a more potent antibiotic which was active against wild-type E. coli but did not induce the pRFPCER-TrpL2A reporter. This unexpected observation could be explained in several ways. The first hypothesis would be that more effective antimicrobials, linezolid and clindamycin, kill bacteria at low concentrations that cause ribosome stalling on a limited subset of specific mRNAs which does not include trpL. A second hypothesis could be that linezolid and clindamycin bind ribosomes predominantly before the beginning of translation and thus stall ribosomes at the very beginning of the mutated trpL coding part, preventing induction of CER. A ribosome stalled at the initiation region of mutated trpL should be located 30 nucleotides upstream from the stem 1-2 formation and should not inhibit its formation, thus being unable to induce CER gene expression. In contrast, either chloramphenicol and possibly lincomycin might bind the ribosome after the translation has started and stall the translating ribosome after several amino acids have been polymerized, or they bind to the ribosome before the beginning of translation but allow some peptide bonds to be formed before blocking the translation.
Curiously, attenuation is used in natural systems used by bacteria for detection of chloramphenicol (20, 21) and macrolides (3, 43). Both types of antibacterials were the most potent inducers of the pRFPCER-TrpL2A reporter described here. The genes cat and cmlA code for chloramphenicol acetyltransferase, inactivating the antibiotic and membrane protein involved in the transport of the drug, respectively. Both genes are preceded by short open reading frames. The ribosome-binding site of the resistance genes is blocked by a secondary structure. Stalling of translating ribosomes within the upstream open reading frame melts the secondary structure and releases a Shine-Dalgarno sequence and an initiation codon for interaction with ribosomes. Curiously, ribosome stalling in the leader region is specific for chloramphenicol, and the peptide sequence MVKTD should be encoded before the 6th codon at which stalling takes place (21). The inducible ermC gene, coding for A2058 23S rRNA methyltransferase, is necessary for making a cell resistant to macrolides, exemplified by erythromycin (14, 16). Subinhibitory concentrations of erythromycin induce ermC expression by stalling the ribosome translating the leader ermCL open reading frame. Such stalling relieves the secondary structure block for translation of the following ermC gene. In this case, the peptide sequence I6F7V8 preceding the stall site and the overall distance to the N terminus are important for induction (43). Interestingly, various macrolides and ketolides which also induce ermC expression have various requirements for the stall sequence (3). The mutated tryptophan operon leader peptide used in the reporter pRFPCER-TrpL2A with the sequence MKAIFVLKGAARTS contains an IFV subsequence which also occurs in the ermCL peptide, albeit closer to the N terminus. Whether this is a coincidence or whether it is common for stalling of peptides is still unknown. At least the cat leader peptide does not have much similarity to the leader peptide of pRFPCER-TrpL2A, despite the fact that chloramphenicol readily induces the pRFPCER-TrpL2A reporter.
It is obvious that all induction systems, whether they are artificial, such as the pRFPCER-TrpL2A reporter, or natural, as in cat, cmlA, and ermC, should have a complex dependence on the inducer antibiotic concentration for induction. If the antibiotic concentration is very low, the system would not respond to it, while when an antibiotic is present at an overwhelming concentration, it would inhibit induced gene expression as well. Thus, for every system acting similarly, one can expect a concentration of an antibiotic at which the system has maximal induction. Logically, this concentration should be subinhibitory for the cell. The advantage of the artificial reporter pRFPCER-TrpL2A described here is that we can measure CER and RFP fluorescence. A ratio between those would be a convenient measure of attenuation-based induction corrected for inhibition of reporter gene expression. Successful demonstration of the pRFPCER-TrpL2A reporter's utility in a search for translation-stalling compounds in soil microorganism products proved that it could be applied in wider screening procedures. The reporter system that we created in some cases could discard real ribosome-targeted antimicrobials (false negatives), but all compounds tested so far which cause pRFPCER-TrpL2A reporter induction caused translation stalling (no false positives).
We successfully applied our screening system to search for translation inhibitors in the fermentation broth of soil microorganisms. A positive signal was detected for the strain 428/07. Subsequent verification in vivo and in vitro allowed us to prove that the compound produced by strain 428/07 is indeed an inhibitor of translation. We hope that the screening system described here will find its application in high-throughput screening of environmental samples as well as screening for potential antimicrobials.
ACKNOWLEDGMENTS
We are grateful to Yury Rubtsov and Alex Lebedeff for their help in making the English of the manuscript readable.
This work was supported by Russian Foundation for Basic Research grants 10-04-01345-a, 11-04-12060, 11-04-01314-a, and 11-04-01018-a, Russian Ministry of Science grant 16.512.11.2108, Federal Agency for Science and Innovations grant 02.740.11.0706, and Moscow University Development Program PNR 5.13.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Anko ML, Kurittu J, Karp M. 2002. An Escherichia coli biosensor strain for amplified and high throughput detection of antimicrobial agents. J. Biomol. Screen. 7:119–125 [DOI] [PubMed] [Google Scholar]
- 2. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey M, Chettiath T, Mankin AS. 2008. Induction of erm(C) expression by noninducing antibiotics. Antimicrob. Agents Chemother. 52:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beatty KE, Xie F, Wang Q, Tirrell DA. 2005. Selective dye-labeling of newly synthesized proteins in bacterial cells. J. Am. Chem. Soc. 127:14150–14151 [DOI] [PubMed] [Google Scholar]
- 5. Bianchi AA, Baneyx F. 1999. Stress responses as a tool to detect and characterize the mode of action of antibacterial agents. Appl. Environ. Microbiol. 65:5023–5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brodersen DE, et al. 2000. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103:1143–1154 [DOI] [PubMed] [Google Scholar]
- 7. Clarebout G, Leclercq R. 2002. Fluorescence assay for studying the ability of macrolides to induce production of ribosomal methylase. Antimicrob. Agents Chemother. 46:2269–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies J, Gilbert W, Gorini L. 1964. Streptomycin, suppression, and the code. Proc. Natl. Acad. Sci. U. S. A. 51:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies J, Jones DS, Khorana HG. 1966. A further study of misreading of codons induced by streptomycin and neomycin using ribopolynucleotides containing two nucleotides in alternating sequence as templates. J. Mol. Biol. 18:48–57 [DOI] [PubMed] [Google Scholar]
- 11. Dunkle JA, Xiong L, Mankin AS, Cate JH. 2010. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. U. S. A. 107:17152–17157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fralick JA. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galluzzi L, Karp M. 2003. Amplified detection of transcriptional and translational inhibitors in bioluminescent Escherichia coli K-12. J. Biomol. Screen. 8:340–346 [DOI] [PubMed] [Google Scholar]
- 14. Gryczan TJ, Grandi G, Hahn J, Grandi R, Dubnau D. 1980. Conformational alteration of mRNA structure and the posttranscriptional regulation of erythromycin-induced drug resistance. Nucleic Acids Res. 8:6081–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartz D, McPheeters DS, Traut R, Gold L. 1988. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 164:419–425 [DOI] [PubMed] [Google Scholar]
- 16. Horinouchi S, Weisblum B. 1980. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc. Natl. Acad. Sci. U. S. A. 77:7079–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ippolito JA, et al. 2008. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 51:3353–3356 [DOI] [PubMed] [Google Scholar]
- 18. Kostrzynska M, Leung KT, Lee H, Trevors JT. 2002. Green fluorescent protein-based biosensor for detecting SOS-inducing activity of genotoxic compounds. J. Microbiol. Methods 48:43–51 [DOI] [PubMed] [Google Scholar]
- 19. Kurittu J, Karp M, Korpela M. 2000. Detection of tetracyclines with luminescent bacterial strains. Luminescence 15:291–297 [DOI] [PubMed] [Google Scholar]
- 20. Lovett PS. 1996. Translation attenuation regulation of chloramphenicol resistance in bacteria—a review. Gene 179:157–162 [DOI] [PubMed] [Google Scholar]
- 21. Lovett PS, Rogers EJ. 1996. Ribosome regulation by the nascent peptide. Microbiol. Rev. 60:366–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meldal M, Tornoe CW. 2008. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 108:2952–3015 [DOI] [PubMed] [Google Scholar]
- 23. Merino E, Jensen RA, Yanofsky C. 2008. Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol. 11:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merino E, Yanofsky C. 2005. Transcription attenuation: a highly conserved regulatory strategy used by bacteria. Trends Genet. 21:260–264 [DOI] [PubMed] [Google Scholar]
- 25. Merzlyak EM, et al. 2007. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods 4:555–557 [DOI] [PubMed] [Google Scholar]
- 26. Mitchell RJ, Gu MB. 2004. An Escherichia coli biosensor capable of detecting both genotoxic and oxidative damage. Appl. Microbiol. Biotechnol. 64:46–52 [DOI] [PubMed] [Google Scholar]
- 27. Mohrle V, Stadler M, Eberz G. 2007. Biosensor-guided screening for macrolides. Anal. Bioanal. Chem. 388:1117–1125 [DOI] [PubMed] [Google Scholar]
- 28. Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. 2000. The structural basis of ribosome activity in peptide bond synthesis. Science 289:920–930 [DOI] [PubMed] [Google Scholar]
- 29. Norman A, Hestbjerg Hansen L, Sorensen SJ. 2005. Construction of a ColD cda promoter-based SOS-green fluorescent protein whole-cell biosensor with higher sensitivity toward genotoxic compounds than constructs based on recA, umuDC, or sulA promoters. Appl. Environ. Microbiol. 71:2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pioletti M, et al. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 20:1829–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu X, et al. 2011. The ribosome uses two active mechanisms to unwind messenger RNA during translation. Nature 475:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizzo MA, Springer GH, Granada B, Piston DW. 2004. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22:445–449 [DOI] [PubMed] [Google Scholar]
- 33. Rommens J, MacKnight D, Pomeroy-Cloney L, Jay E. 1983. Gene expression: chemical synthesis and molecular cloning of a bacteriophage T5 (T5P25) early promoter. Nucleic Acids Res. 11:5921–5940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semenkov Yu P, Makarov EM, Makhno VI, Kirillov SV. 1982. Kinetic aspects of tetracycline action on the acceptor (A) site of Escherichia coli ribosomes. FEBS Lett. 144:125–129 [DOI] [PubMed] [Google Scholar]
- 35. Shapiro E, Baneyx F. 2007. Stress-activated bioluminescent Escherichia coli sensors for antimicrobial agents detection. J. Biotechnol. 132:487–493 [DOI] [PubMed] [Google Scholar]
- 36. Shapiro E, Baneyx F. 2002. Stress-based identification and classification of antibacterial agents: second-generation Escherichia coli reporter strains and optimization of detection. Antimicrob. Agents Chemother. 46:2490–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Svetlov MS, Kommer A, Kolb VA, Spirin AS. 2006. Effective cotranslational folding of firefly luciferase without chaperones of the Hsp70 family. Protein Sci. 15:242–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takyar S, Hickerson RP, Noller HF. 2005. mRNA helicase activity of the ribosome. Cell 120:49–58 [DOI] [PubMed] [Google Scholar]
- 39. Tenson T, Lovmar M, Ehrenberg M. 2003. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 330:1005–1014 [DOI] [PubMed] [Google Scholar]
- 40. Tu D, Blaha G, Moore PB, Steitz TA. 2005. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121:257–270 [DOI] [PubMed] [Google Scholar]
- 41. Valtonen SJ, Kurittu JS, Karp MT. 2002. A luminescent Escherichia coli biosensor for the high throughput detection of beta-lactams. J. Biomol. Screen. 7:127–134 [DOI] [PubMed] [Google Scholar]
- 42. VanBogelen RA, Neidhardt FC. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 87:5589–5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vazquez-Laslop N, Thum C, Mankin AS. 2008. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell 30:190–202 [DOI] [PubMed] [Google Scholar]
- 44. Weber K, Bartsch U, Stocking C, Fehse B. 2008. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol. Ther. 16:698–706 [DOI] [PubMed] [Google Scholar]
- 45. Zurawski G, Elseviers D, Stauffer GV, Yanofsky C. 1978. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc. Natl. Acad. Sci. U. S. A. 75:5988–5992 [DOI] [PMC free article] [PubMed] [Google Scholar]