Abstract
Dosing of cefepime during high blood flow (Qb; 300 ml/min), high dialysate flow (Qd; 3 liter/h) continuous venovenous hemodialysis (CVVHD) is undefined. Six patients on CVVHD had serum and effluent cefepime concentrations measured at 0.5, 1, 2, 6, and 12 h after dosing. Three patients had cefepime concentrations less than the MIC for Pseudomonas aeruginosa. A dose of 2,000 mg every 12 h or 1,000 mg every 8 h may increase time at a therapeutic concentration.
TEXT
Continuous venovenous hemodialysis (CVVHD) is a frequently used modality of continuous renal replacement therapy (CRRT) for patients with severe acute kidney injury (AKI). While CRRT may be associated with improved hemodynamics compared to intermittent hemodialysis, morbidity and mortality do not appear to be significantly improved based on current evidence (6). This may potentially be due to increased clearance of beneficial compounds, such as antibiotics.
Dosing recommendations for many drugs used in patients receiving CVVHD and other forms of CRRT have largely been determined indirectly by extrapolation from chronic dialysis studies, from in vitro assessments, or from small pharmacokinetic studies typically using low-flow CVVHD (blood flow [Qb] of 150 ml/min and dialysate flow [Qd] of 1 to 2 liters/h) (4). While there may be no mortality advantage to higher-dialysate-flow CVVHD (9), in practice clinicians often must prescribe higher dialysate flow rates to achieve lower overall targets because of unavoidable machine downtime (due to filter clotting or diagnostic testing, for example). Newer systems have been created that allow for high blood flow and high dialysate flow (2), but this can lead to increased challenges when dosing medications, as dosage recommendations using higher flows are generally not available or are not based on specific studies using these flow rates.
Antibiotics are critically important medications that require precise dosing. Cefepime is a cephalosporin with broad Gram-negative and Gram-positive activity that is commonly used in the critical care setting. Cefepime is 480 Da in size, has a volume of distribution that approaches total body water, and is approximately 20% protein bound. As cefepime levels are not routinely available in clinical laboratories, appropriate dosing in patients receiving CVVHD is a concern. For example, previous research has shown that time spent below the MIC is associated with worse bacteriologic outcomes among patients infected with Pseudomonas aeruginosa (MIC of 8 mcg/ml), a common pathogen in critically ill patients (5). Of concern, a recent pharmacokinetic study demonstrated that even on continuous venovenous hemofiltration and/or continuous venovenous hemodiafiltration at lower flow rates (Qb of 150 ml/min and Qd of 1.5 liters/h), no patient achieved a target cefepime concentration (defined by the investigators as four times the MIC for P. aeruginosa) for 60 to 70% or greater of the 48-h period studied (7).
Given the potential for inadequate dosing, we performed a pharmacokinetic analysis of cefepime in patients undergoing CVVHD at higher flow rates (Qb of 300 ml/min and Qd of 2 to 3 liters/h) in order to better elucidate dosing requirements. Over a 6-month period, all patients at our institution on CVVHD were screened for study entry. Inclusion criteria were age greater than 18 years, anuria defined as urine output less than 50 ml per day, stable dialysate flow rate, stable cefepime dose with at least 3 doses administered prior to study entry, life expectancy greater than 24 h, and attending physician approval. Cefepime dose and dialysate flow rate were determined by each patient's physician, independent of the study investigators. CVVHD was performed using an NxStage machine with a proprietary high-flux polyethersulfone membrane (2).
As current CVVHD dosing recommendations for cefepime suggest administration of 1,000 to 2,000 mg every 12 h, we measured serum and effluent (mixed dialysate and ultrafiltrate) cefepime concentrations at 0.5, 1, 2, 6, and 12 h after a given dose using high-pressure liquid chromatography (HPLC). Pre- and postfilter concentrations were measured for calculation of dialysate saturation. We assumed that cefepime clearance follows first-order kinetics in a one-compartment model (1). The primary measures of interest were trough concentration of cefepime and time with cefepime concentration <8 mcg/ml, which is consistent with the Clinical and Laboratory Standards Institute MIC breakpoints for susceptible Enterobacteriaceae and Pseudomonas aeruginosa (3) and recommendations on antibiotic dosing in CVVHD put forward by Trotman et al. (8). The study protocol was reviewed and approved by the Institutional Review Board of the University of Pennsylvania School of Medicine. Written informed consent was obtained from all participants.
Of 78 total patients screened, 18 met inclusion criteria and 6 consented to study participation (Table 1). Three patients completed the entire 12-h study, and three completed 6 h, due primarily to CVVHD treatment being paused for diagnostic testing. For the latter three patients, trough cefepime concentration was extrapolated from the decay coefficient calculated during the first 6 h. The decay constant was assumed to be equivalent to the beta-coefficient of the line of best fit on a scale of log cefepime over time. Drug concentrations in serum and effluent were determined using reversed-phase HPLC with UV detection according to previously published methods (4). The HPLC system utilized a Novapak C18 4.6-mm by 150-mm column with a guard column containing Novapak C18 inserts (Waters, Milford, MA), and the detector was set at a wavelength of 305 nm. The mobile phase consisted of acetonitrile-water (4.5:95.5 [vol/vol]) containing 50 mM trisodium citrate, adjusted to a pH of 6.0. Ceftriaxone (Sigma, St. Louis, MO) was used as the internal standard. The assay was validated in both serum and effluent, with standard curves achieving coefficients of determination (r2) of >0.998 and coefficients of variation being <4.2% for concentrations across the range of the standard curves for both fluids.
Table 1.
Characteristics of study participants (n = 6)a
| Patient | Age (yr) | Gender | Etiology of renal failure | Wt (kg) | Cefepime dose (mg) | Cefepime dose (mg/kg) | Qd (ml/h) | Qd (ml/kg/h) |
|---|---|---|---|---|---|---|---|---|
| 1 | 46 | M | Septic ATN | 95 | 2,000 | 21.1 | 2,000 | 21.1 |
| 2 | 47 | F | Septic ATN | 108 | 2,000 | 18.5 | 2,000 | 18.5 |
| 3 | 24 | M | Cardiogenic shock | 104 | 2,000 | 19.2 | 3,000 | 28.8 |
| 4 | 23 | F | Septic ATN | 49 | 2,000 | 40.8 | 3,000 | 61.2 |
| 5 | 29 | M | Cardiogenic shock | 86 | 1,000 | 11.6 | 3,000 | 34.9 |
| 6 | 56 | M | Septic ATN | 74 | 1,000 | 13.5 | 3,000 | 40.5 |
M, male; F, female; Qd, dialysate flow rate; ATN, acute tubular necrosis.
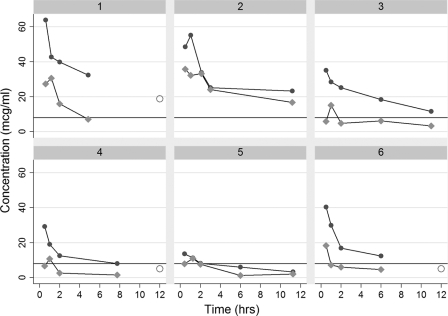
Three out of six patients had actual or extrapolated cefepime blood concentrations during the 12-h postdose period that were <8 mcg/ml, with those patients having a concentration less than 8 mcg/ml for 33%, 75%, and 83% of the treatment period (Table 2, Fig. 1). Time below the cutoff ranged from 3 to 10 h. All patients with actual or extrapolated cefepime levels of <8mcg/ml received higher dialysis doses on a ml/kg/h basis. Dialysate saturation (the ratio of dialysate concentration to plasma concentration) varied from 28 to 48% (n = 4). This is notably less than reported in prior studies (4), perhaps due to different membrane characteristics or decreased exposure time of blood to dialysate with higher flows.
Table 2.
Pharmacokinetics of cefepime in patients studieda
| Patient | Cefepime dose (mg) | Qd (ml/kg/h) | Max cefepime concn (μg/ml) | 12-h cefepime concn (μg/ml) | Half-life (h) | Time (h) < 8 mcg/mlb | % time < 8 mcg/ml | Dialysate saturation (%)c | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2,000 | 21.1 | 66.3 | 18.8* | 7.8 | 0 | 0* | 28 | Died |
| 2 | 2,000 | 18.5 | 49.3 | 22.8 | 15.0 | 0 | 0 | NA | Survived |
| 3 | 2,000 | 28.8 | 36.7 | 10.7 | 10.4 | 0 | 0 | 40 | Survived |
| 4 | 2,000 | 61.2 | 30.9 | 5.0* | 6.9 | 4* | 33* | NA | Died |
| 5 | 1,000 | 34.9 | 14.2 | 3.3 | 8.2 | 10 | 83 | 27 | Survived |
| 6 | 1,000 | 40.5 | 43.5 | 5.0* | 5.2 | 3* | 25* | 46 | Survived |
Qd, dialysate flow rate; *, extrapolated value; NA, not available.
Therapeutic concentration is defined as 8 mcg/ml.
Dialysate saturation is the effluent cefepime concentration divided by average of pre- and postfilter cefepime concentration at 6 h.
Fig 1.
Individual serum (dark gray) and effluent (light gray) concentrations in the six subjects over time. Open circles represent extrapolated 12-h serum concentrations. Solid line represents MIC of 8 μg/ml.
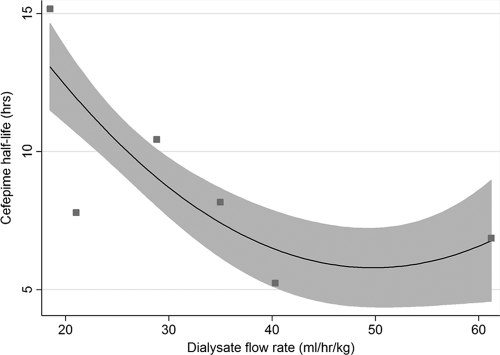
Using the pharmacokinetic data collected, we assessed the relationship between dialysate flow rate and half-life (t1/2) of cefepime at the patient level. Half-life was determined assuming an exponential decay in cefepime over time, as has been reported elsewhere (1). Half-life ranged from 5 to 15 h. There was a strong correlation between dialysate flow rate and cefepime half-life (Spearman rho = −0.78, P < 0.0001) (Fig. 2). The regression equation derived was as follows: t1/2 = −0.7205462 × Qd + 0.0072873 × Qd2 + 23.57884, where Qd is in ml/kg/h and t1/2 is in hours (r2 = 0.31). Assuming a 75-kg man (with 60% body water) receiving a Qd of 3,000 ml/h, a cefepime dose of 1,350 mg twice daily would be barely adequate to keep serum levels of >8 mcg/ml at redosing.
Fig 2.
Half-life of cefepime in six patients versus dialysis flow rate. Solid line represents line of best quadratic fit. Shaded area represents 95% confidence interval.
In summary, current dosing recommendations for cefepime may result in prolonged low blood levels in patients undergoing high Qb, high Qd CVVHD, which may lead to unfavorable bacteriologic and clinical outcomes, though organisms with a lower MIC to cefepime may still be adequately treated under current guidelines. This study enrolled a small number of patients but demonstrated significant CVVHD dose-related clearance of cefepime. During high Qb, high Qd CVVHD, based on our findings, cefepime dosing as a 2,000-mg bolus every 12 h or as a 1,000-mg bolus every 8 h should provide increased time at a therapeutic concentration. Continuous or 4-h infusions may also be considered, though the pharmacokinetic profile of this dosing methodology has yet to be evaluated in CVVHD. Further study is required to determine the optimal regimen. The current study should serve as an alert to nephrologists and intensivists to the potential for lower cefepime concentrations in patients receiving CRRT, especially at higher Qb and Qd, than those used in pharmacokinetic studies from which current dosing recommendations were derived.
ACKNOWLEDGMENTS
We have no financial disclosures to report.
This study received funding support from NxStage, Inc.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Allaouchiche B, et al. 1997. Pharmacokinetics of cefepime during continuous venovenous hemodiafiltration. Antimicrob. Agents Chemother. 41:2424–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark WR, Turk JE. 2004. The NxStage System One. Semin. Dial. 17:167–170 [DOI] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. Document M100-S20. CLSI, Wayne, PA [Google Scholar]
- 4. Malone RS, Fish DN, Abraham E, Teitelbaum I. 2001. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob. Agents Chemother. 45:3148–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (t>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents. 31:345–351 [DOI] [PubMed] [Google Scholar]
- 6. Rabindranath K, Adams J, Macleod AM, Muirhead N. 2007. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst. Rev. CD003773. [DOI] [PubMed] [Google Scholar]
- 7. Seyler L, et al. 2011. Recommended beta-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care. 15:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trotman RL, Williamson JC, Shoemaker MD, Salzer WL. 2005. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin. Infect. Dis. 41:1159–1166 [DOI] [PubMed] [Google Scholar]
- 9. VA/NIH Acute Renal Failure Trial Network 2008. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]