Abstract
The spread of the blaNDM-1 gene is gaining worldwide attentions. This gene is usually carried by large plasmids and has been discovered in diverse bacteria since it was originally found in Klebsiella pneumoniae. Here we report the complete sequences of a blaNDM-1-bearing plasmid, pNDM-BJ01, and its variant, pNDM-BJ02, isolated from clinical Acinetobacter lwoffii strains. The plasmid pNDM-BJ01 is 47.3 kb in size and cannot be classified into any known plasmid incompatibility group, thus representing a novel plasmid with an unknown maintenance mechanism. This plasmid contains both a blaNDM-1 gene and a type IV secretion system (T4SS) gene cluster. The T4SS is assigned to the P-type T4SS group, which usually encode a short, rigid pilus, and the blaNDM-1 gene is located within a composite transposon flanked by two insertion elements of ISAba125. Plasmid pNDM-BJ02 is nearly identical to pNDM-BJ01 except that one copy of the ISAba125 element is missing, and it is therefore regarded as a variant of pNDM-BJ01. Sequence alignment indicated that this blaNDM-1-containing composite transposon, which can also be captured by other mobile elements, was probably a product of multiple recombination events and can move as a whole by transposition.
INTRODUCTION
The rapid growth of antibiotic resistance has been recognized as a clinical and epidemiological problem for human health (14). The new emergence of bacteria harboring the blaNDM-1 gene, encoding the metallo-β-lactamase (MBL) NDM-1, has once again aroused public concern worldwide (1, 18, 24). NDM-1 displays the ability to hydrolyze a wide range of β-lactam antibiotics, including carbapenems, which are a mainstay for the treatment of antibiotic-resistant bacterial infections (11).
The blaNDM-1 gene was first discovered in a Klebsiella pneumoniae isolate from a Swedish patient of Indian origin (30). Bacteria carrying the blaNDM-1 gene have now been reported in many countries worldwide, such as the United States, China, Australia, France, and the Nordic countries (3, 4, 20, 22, 23). The blaNDM-1 gene was originally found in a 180-kb plasmid in K. pneumoniae (30), and later it was reported to be carried by other plasmids of from 50 to 500 kb size in various Gram-negative species (11). In China, the blaNDM-1 gene has been reported in plasmids with sizes of from ∼30 to 50 kb in Acinetobacter baumannii (4).
Complete sequencing of plasmids harboring the blaNDM-1 gene provided important information for the analysis of the genetic environment of the blaNDM-1 gene and for a better understanding of the spread of this resistance determinant. So far, three complete sequences of blaNDM-1-bearing plasmids have been reported, i.e., those of pNDM-HK (7), p271A (21), and pNDM_Dok01 (25), isolated from Escherichia coli. Sequence analysis indicated that the blaNDM-1 gene was adjacent to various insertion elements in these plasmids (for example, two IS26 elements in pNDM-HK, the ISEC33 and ISSen4 elements in p271A, and two IS903 elements in pNDM_Dok01). Here we report the complete sequences of blaNDM-1-bearing plasmids isolated from two clinical Acinetobacter lwoffii strains and a comparative analysis of available blaNDM-1-related sequences.
Case Reports
On 23 November 2010, a 62-year-old female patient was admitted to our hospital suffering from a urinary tract infection after chemotherapy treatment for pancreatic cancer. A multidrug-resistant Gram-negative bacillus, A. lwoffii (strain 10621), was found dominating her urine cultures (8). The production of a metallo-β-lactamase (MBL) by A. lwoffii 10621 was confirmed by combined disk synergy testing. According to the drug resistance pattern, the patient was treated with amikacin via intravenous drip (0.2 g, 2 times/day). After 1 week of treatment, her temperature returned to normal and the urine was negative for A. lwoffii.
On 25 December 2010, a 37-year-old female patient (Baoding, Hebei Province, China) with urinary tract infection was admitted. According to her description, before admission to our hospital she had suffered from frequent and urgent micturition and urodynia for 3 days, and the symptoms were not alleviated after treatments in the local hospital. Results of urine culture in this case showed that A. lwoffii was also the dominant bacterium. The repetitive extragenic palindromic PCR (REP-PCR) binding patterns of this isolate (no. 10659) were not the same as those of A. lwoffii 10621 in the first case (see Fig. S1 in the supplemental material), suggesting that they were not the same strain and that the two infections were not caused by clonal spread. Antibiotic susceptibility testing results showed that this isolate was resistant to ampicillin, cefazolin, cefotaxime, ceftazidime, cefpirome, ceftriaxone, imipenem, and meropenem but susceptible to amikacin, tigecycline, and colistin. PCR testing indicated that the isolate was blaNDM-1 gene positive. The patient was then treated with amikacin by intravenous drip as described above, and she recovered thereafter.
MATERIALS AND METHODS
Microbiology methods.
Bacteria were identified with the Vitek 2 system (bioMérieux Vitek Systems Inc., Hazelwood, MO). Antimicrobial susceptibility testing and interpretation were conducted by the standard disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (5). For metallo-β-lactamase (MBL) detection, combined Disk synergy testing was performed as described by Yong et al. (29). The method involves the use of an imipenem (10 μg) disk with and without EDTA (1.86 mg). REP-PCR was used for strain typing as described by Vila et al. (28).
Filter mating experiments.
Filter mating conjugation using E. coli J53 Azir as the recipient strain was performed as described previously with minor modifications (15, 16). After 24 h of incubation of donor-recipient mixtures at 37°C, cells were recovered by washing the filters in 1 ml of LB medium. Transconjugants were selected on LB agar plates supplemented with sodium azide (100 μg/ml) and ampicillin (100 μg/ml). To confirm the occurrence of transfer, 5 transconjugants from each mating were randomly selected for testing the presence of the plasmid by PCR analysis using 12 pairs of primers with products covering the whole plasmid. Conjugative transfer frequencies were calculated as the number of transconjugant cells per donor.
Sequencing and annotation.
The whole genome of A. lwoffii 10621 was sequenced using a shotgun strategy with an Illumina genome analyzer (8). In this study, a total of 12 pairs of PCR primers with overlap ends of product were designed for the confirmation of the pNDM-BJ01 structure (see Table S1 in the supplemental material) and then used for plasmid pNDM-BJ02 sequencing. PCR products generated using these 12 pairs of primers were sequenced with an ABI3730 sequencer and assembled by using the SeqMan program within the Lasergene suite version 7 (DNAStar Inc., Madison, WI). The procedures for annotation were performed as described previously (8).
Bioinformatics analysis.
A phylogenetic tree of the type IV secretion system (T4SS) family was constructed using the neighbor-joining method with 1,000 bootstrap replicates in Molecular Evolutionary Genetics Analysis software (MEGA version 4) (26). Amino acid sequences for traB/virB10 and traC/virB4 were retrieved from GenBank, and their concatenated sequences were used to generate the alignment and then for constructing the phylogenetic tree. A multiple-sequence alignment was constructed using ClustalX version 1.8 (27). Promoter searches were performed by using Softberry's BPROM (Softberry Inc., Mt. Kisco, NY), PPP-Prokaryotic Promoter Prediction (Groningen Biomolecular Sciences and Biotechnology Institute, Haren, The Netherlands [http://bioinformatics.biol.rug.nl/websoftware/ppp/ppp_start.php]), and the Neural Network Promoter Prediction program (http://www.fruitfly.org/seq_tools/promoter.html). The NCBI Basic Local Alignment Search Tool (BLAST) (17) was used repeatedly for sequence comparison and analysis.
Nucleotide sequence accession numbers.
The pNDM-BJ01 and pNDM-BJ02 plasmid sequences have been submitted to the GenBank database with accession numbers JQ001791 and JQ060896, respectively.
RESULTS
Whole-genome sequencing of A. lwoffii 10621.
The genomic draft sequence of A. lwoffii 10621 includes a ∼3.42-Mb chromosome and a ∼45-kb plasmid. Two gaps in the plasmid were closed by PCR and sequencing, and a final complete plasmid (named pNDM-BJ01) with length of 47,274 bp was obtained. The structure of pNDM-BJ01 was further confirmed by PCR using 12 pairs of primers with overlap ends of the product (see Table S1 in the supplemental material).
General features of plasmid pNDM-BJ01.
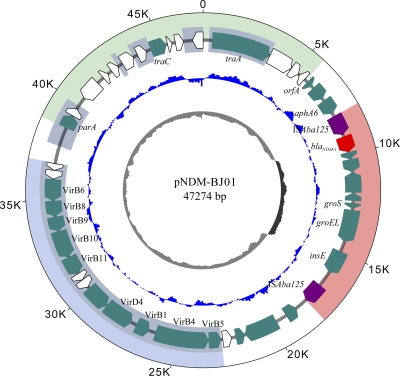
The plasmid pNDM-BJ01 contains 46 open reading frames (ORFs) with an average GC content of 40.8% (Fig. 1; see Table S2 in the supplemental material). Three different functional regions were predicted (Fig. 1): a putative transfer and replication region containing plasmid transfer genes traA and traC and plasmid-partitioning gene parA, a type IV secretion system (T4SS) gene cluster region containing the quintessential T4SS conjugative transfer genes traB and traC, and a blaNDM-1-carrying a composite transposon region flanked by two copies of ISAba125. A BLAST search showed that approximately 20 kb of pNDM-BJ01 displayed more than 70% identity with the draft genomic sequence of Acinetobacter radioresistens SK82 contig 00019 (GenBank Genome Project ID 55855). However, pNDM-BJ01 cannot be classified by the PCR-based replicon typing method (2), and no plasmid backbone sequence homologous with it can be found, indicating that it may be a novel plasmid with an unknown maintenance mechanism. The plasmid showed a relatively high transfer frequency, ranging from 9.1 × 10−3 to 1.3 × 10−2 per donor cell, to E. coli J53 Azir, suggesting a high horizontal transfer ability.
Fig 1.
Circular map of plasmid pNDM-BJ01. The circles display (from the outside) (i) coordinates in kilobase pairs, (ii) predicted coding sequences, (iii) GC skew ([G+C]/[G-C]) in a 500-bp window, and (iv) GC content plotted against 50% GC content, with light gray indicating <50% and dark gray indicating >50%. The blaNDM-1 gene and insertion element ISAba125 are colored with red and purple, respectively. ORFs in white indicate hypothetical proteins. Three major parts corresponding to the blaNDM-1-carrying composite transposon, the type IV secretion system gene cluster, and the putative transfer and replication region are shaded with light red, light blue, and light green, respectively. Homologous regions with Acinetobacter radioresistens SK82 contig 00019 are shaded with gray.
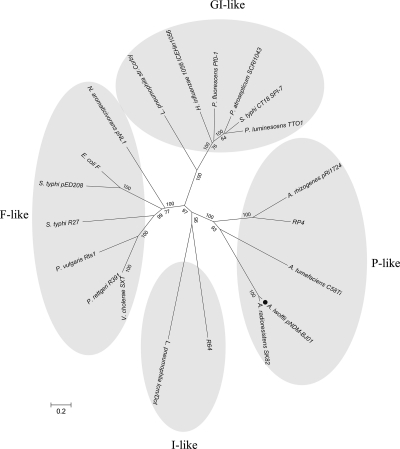
Ten genes in the predicted T4SS region show high homology with genes for putative type IV secretion system proteins in A. radioresistens SK82. Previous studies showed that genes traB/virB10 and traC/virB4, encoding quintessential T4SS proteins, are ideal for comparative amino acid alignment and appear to be sufficient to define membership in a T4SS type (9, 10). A phylogenetic tree was therefore constructed by using concatenated amino acid sequences for virB10 and virB4. The results indicated that the T4SS in pNDM-BJ01 was closely related to that in A. radioresistens SK82, and it was classified as a known P-type T4SS, members of which include those in RP4 (Pseudomonas aeruginosa), Ti (Agrobacterium tumefaciens C58), and pRi1724 (Agrobacterium rhizogenes) (Fig. 2).
Fig 2.
Phylogenetic tree of representative members of the T4SS family. The tree was constructed by the neighbor-joining method using concatenated amino acid sequences of virB10 and virB4 of the pNDM-Bj01 T4SS (●) and the previously described T4SSs. Bootstrap values of more than 50% are shown at the respective nodes (1,000 replications). The lengths of the branches indicate the divergence among the amino acid sequences. The scale bar corresponds to 20% estimated sequence divergence.
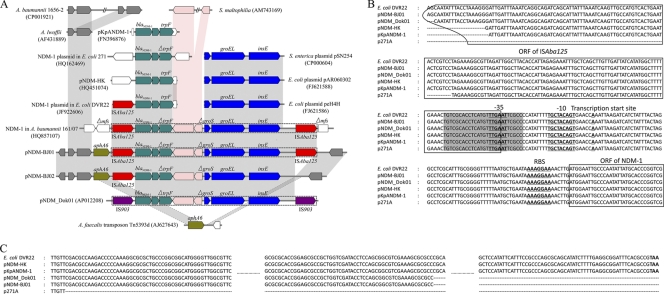
The blaNDM-1 gene was organized in a composite transposon structure within a ∼7-kb abnormal GC region bracketed between two copies of insert sequence ISAba125 which differed by 6 nucleotides, one of which resulted in the amino acid substitution Arg-60 → Gln. With both ISAba125 insertion elements, this composite transposon was 10,099 bp in length, and the transposition event of this composite transposon was confirmed by (i) a different G+C content compared with the rest of the plasmid and (ii) a 3-bp (GTT) target site duplication at the point of insertion between the aphA6 gene and its 3′ flanking region, which were continuous in Alcaligenes faecalis transposon Tn5393d (Fig. 3A). It was interesting that a nearly identical composite transposon structure (with a difference of 2 bp in a 10,099-bp length) was found to be inserted in the gene encoding a major facilitator superfamily (MFS) metabolite/H+ symporter in the chromosome of A. baumannii 161/07, which was isolated from a patient who had been repatriated to Germany from Serbia (19). Moreover, the whole component of this composite transposon (excluding ISAba125) was also captured by the IS903 mobile element in pNDM_Dok01 (Fig. 3A).
Fig 3.
Comparative analysis of blaNDM-1-carrying composite transposon sequences (A), promoter sequences of blaNDM-1 (B), and 3′-end sequences of trpF (C) in different genetic environments. (A) Different colors of ORFs denote different sequence origins. Light gray and pink shadings indicate more than 97% and less than 75% nucleotide identity, respectively. ISAba125 is colored with red. The dashed line box indicates the complete composite transposon. (B) Gray shading indicates the right inverted repeat (IR-R) of ISAba125.
Comparative analysis of the genetic environment of blaNDM-1.
The blaNDM-1 gene-containing region between two copies of ISAba125 in pNDM-BJ01 comprised three major parts (Fig. 3A). In the 5′ region, the blaNDM-1 gene was adjacent to a bleomycin resistance protein followed by a truncated trpF gene. This three-gene combination was frequently found in other genetic backgrounds: E. coli plasmids pNDM-HK, p271A, and pNDM_Dok01; the NDM-1 plasmid in E. coli DVR22; and K. pneumoniae plasmid pKpANDM-1. In the 3′ region, genes encoding the chaperonin subunits GroS (truncated) and GroEL and the transposase InsE constitute a 4-kb element showing high homology to that described in E. coli plasmids peH4H and pAR060302 and Salmonella enterica plasmid pSN254. The third part consisted of two ORFs between the truncated trpF and groS genes displaying ∼70% nucleotide homology with the genome of Stenotrophomonas maltophilia K279a. It seemed that this part was related to insertion events. However, no feature sequences, such as direct repeats or inverted repeats, can be found flanking this region therefore, exactly how the insertions happened needs to be further investigated. These results indicated that the blaNDM-1-containing region in pNDM-BJ01 was probably formed via multiple recombination events by genetic elements of different origins.
Detailed analysis of the sequence spanning ISAba125 and blaNDM-1 revealed the existence of a promoter of blaNDM-1 gene in which the −35 region was located inside the right inverted repeat (IR-R). Sequence alignment showed that all the reported NDM-1-containing sequences have the same promoter region and harbor residual nucleotides of the ISAba125 3′ sequence (including the right inverted repeat of ISAba125) in different lengths (pNDM-HK, 256 bp; pKpANDM-1, 255 bp, p271A, 194 bp; pNDM_Dok01, 273 bp; E. coli DVR22, the same copy of ISAba125 as in pNDM-BJ01) (Fig. 3B). These truncated ISAba125 ORFs had been disrupted by other insertion sequences, such as ISEC33 in p271A, IS26 in pNDM-HK and pKpANDM-1, and IS903 in pNDM_Dok01. Interestingly, available sequence information showed that the 3′ end of the blaNDM-1 gene was always adjacent to a bleomycin resistance protein followed by a trpF gene. Sequence alignment indicated that in E. coli DVR22, pNDM-HK, and pKpANDM-1 there is a complete ORF of trpF (660 bp) but that in pNDM-BJ01 and pNDM_Dok01 79 bp of its 3′ end is missing and that in p271A 271 bp of its 3′ end is missing (Fig. 3C). These results suggested that the blaNDM-1 gene was probably originally linked to ISAba125 and then disseminated in the form of truncated ISAba125 and that the sequence in E. coli DVR22 may be closely related to the ancestral form, as both ISAba125 and trpF flanking the blaNDM-1 gene were intact.
Upstream of the ISAba125 gene was a complete ORF carrying the aminoglycoside resistance gene aphA6 (12); however, the results of antibiotic susceptibility testing showed that A. lwoffii 10621 harboring pNDM-BJ01 was susceptible to amikacin, an aminoglycoside antibiotic. Analysis of the 5′ flanking region of the aphA6 gene indicated that no obvious promoter sequence could be detected by using different promoter searching programs, and upstream of the aphA6 gene were two transpose genes which were very similar to those in the genomes of A. lwoffii and A. baumannii (two copies) (Fig. 3A). Therefore, we suggest that the promoter sequence of the aphA6 gene was probably disrupted by the transposition event, thus resulting in the failure to resistant amikacin.
Characterization of pNDM-BJ02.
In the second case described above, we had the opportunity to isolate another blaNDM-1 gene-positive A. lwoffii strain harboring a plasmid (designated pNDM-BJ02) very similar in size to pNDM-BJ01. To investigate whether the two plasmids were identical, the whole pNDM-BJ02 was sequenced by primer walking of 12 PCR products; a final assembly with a length of 46,165 bp was obtained. Sequence comparison of pNDM-BJ02 with pNDM-BJ01 revealed that an 1,109-bp fragment adjacent to transposase gene insE was missing in pNDM-BJ02 (Fig. 3A). This deletion includes 94 bp of the 5′ flanking region of the ISAba125 ORF and 1,015 bp of the ORF itself (total, 1,026 bp), thus leaving 11 nucleotides of the 3′ end of ISAba125 gene and its downstream 3-bp transposition target site duplication on pNDM-BJ02. The rest of the sequences of the two plasmids are identical to each other. We therefore suggest that plasmid pNDM-BJ02 is a recent variant of pNDM-BJ01.
DISCUSSION
The New Delhi metallo-β-lactamase-1 (NDM-1) gene, blaNDM-1, has been found in diverse strains since it was first discovered in K. pneumoniae. To our knowledge, no NDM-1 gene has been reported in A. lwoffii so far, and none of the recently sequenced plasmids (pNDM-HK, p271A, and pNDM_Dok01) share a genetic link with the plasmids pNDM-BJ01 and pNDM-BJ02 described here.
Both plasmids pNDM-BJ01 and pNDM-BJ02 harbored a T4SS. It has been recognized that T4SSs not only mediate horizontal gene transfer, thus contributing to genome plasticity and the evolution of pathogens through dissemination of antibiotic resistance and virulence genes, but also are used for the delivery of bacterial effector proteins to eukaryotic host cells, thus contributing directly to bacterial pathogenicity (6, 15). The T4SS in pNDM-BJ01 was classified as a P-type T4SS, which have been reported to encode short, rigid pili (13). All conjugative plasmids bearing a P-type T4SS have a broad host range (IncP, -W, and -N) (13), which prompted us to consider pNDM-BJ01 to be a broad-host-range plasmid as well; further investigations are necessary to confirm this. Furthermore, the blaNDM-1 gene in pNDM-BJ01 is located within a composite transposon, and it might be captured by other mobile vehicles or even integrated into the chromosome, as evidenced in A. baumannii 161/07 (19). Therefore, the plasmid pNDM-BJ01, harboring both a T4SS and the blaNDM-1 gene, represents a significant threat, especially when it spreads into human pathogens.
It is very interesting that the whole sequence of the composite transposon in pNDM-BJ01 was nearly identical to that of its counterpart in the chromosome of A. baumannii 161/07 isolated from Germany, which seems to have been caused by horizontal gene transfer events. Why this happened is puzzling. First, contacts with persons from abroad who were infected with blaNDM-1-bearing bacteria cannot be excluded, as the patient was hospitalized several times before for chemotherapeutic treatment of cancer. Second, there may be a common ancestor which has not been recognized. Third, these two composite transposons may have been formed independently by chance via recombination events with similar components, which is less likely. In any case, we believe that this whole composite transposon or its variants will be found in other genetic environments in the future, captured either by ISAba125 as in pNDM-BJ01 or by other mobile elements, for example, by IS903 as in pNDM_Dok01 (25).
Concluding remarks.
We report here the complete sequences of plasmid pNDM-BJ01 and its variant pNDM-BJ02 in clinical isolates of A. lwoffii. Plasmid pNDM-BJ01, with an unknown maintenance mechanism, harbors both a blaNDM-1 gene and a P-type T4SS. The blaNDM-1 gene in pNDM-BJ01 is located inside a composite transposon structure composed of two copies of insertion sequence ISAba125. This novel plasmid represents a potential threat in the future because of its high horizontal transfer ability, and the force exerted by antibiotic abuse in China might increase this risk greatly.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National High Technology Development Program of China under grant no. 2009CB522605 and 2007CB513002, the National Key Program for Infectious Diseases of China (2012ZX10004-216 and 2008ZX10004-001-C), and the State Key Development Program for Basic Research of China (2009CB522600). G.F.G. is a leading principal investigator of the Innovative Research Group of the National Natural Science Foundation of China (NSFC) (grant no. 81021003).
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Bonomo RA. 2011. New Delhi metallo-β-lactamase and multidrug resistance: a global SOS? Clin. Infect. Dis. 52:485–487 [DOI] [PubMed] [Google Scholar]
- 2. Carattoli A, et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 2010. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States. MMWR Morb. Mortal. Wkly. Rep. 59:750. [PubMed] [Google Scholar]
- 4. Chen Y, Zhou Z, Jiang Y, Yu Y. 2011. Emergence of NDM-1-producing Acinetobacter baumannii in China. J. Antimicrob. Chemother. 66:1255–1259 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: twentieth informational supplement M100–S20 CLSI, Wayne, PA [Google Scholar]
- 6. Fronzes R, Christie PJ, Waksman G. 2009. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 7:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho PL, et al. 2011. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One 6:e17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu Y, et al. 2011. Whole-genome sequence of a multidrug-resistant clinical isolate of Acinetobacter lwoffii. J. Bacteriol. 193:5549–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juhas M, et al. 2007. Novel type IV secretion system involved in propagation of genomic islands. J. Bacteriol. 189:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juhas M, Crook DW, Hood DW. 2008. Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell. Microbiol. 10:2377–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambert T, Gerbaud G, Bouvet P, Vieu JF, Courvalin P. 1990. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob. Agents Chemother. 34:1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawley T, Klimke W, Gubbins M, Frost L. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1–15 [DOI] [PubMed] [Google Scholar]
- 14. Levy SB, Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:122–129 [DOI] [PubMed] [Google Scholar]
- 15. Li M, et al. 2011. GI-type T4SS-mediated horizontal transfer of the 89K pathogenicity island in epidemic Streptococcus suis serotype 2. Mol. Microbiol. 79:1670–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lorenzo-Diaz F, Espinosa M. 2009. Lagging-strand DNA replication origins are required for conjugal transfer of the promiscuous plasmid pMV158. J. Bacteriol. 191:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGinnis S, Madden TL. 2004. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 32:W20–W25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moellering RC., Jr 2010. NDM-1—a cause for worldwide concern. N. Engl. J. Med. 363:2377–2379 [DOI] [PubMed] [Google Scholar]
- 19. Pfeifer Y, et al. 2011. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J. Antimicrob. Chemother. 66:1998–2001 [DOI] [PubMed] [Google Scholar]
- 20. Poirel L, Al Maskari Z, Al Rashdi F, Bernabeu S, Nordmann P. 2011. NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J. Antimicrob. Chemother. 66:304–306 [DOI] [PubMed] [Google Scholar]
- 21. Poirel L, Bonnin RA, Nordmann P. 2011. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli by high-throughput genome sequencing. Antimicrob. Agents Chemother. 55:4224–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poirel L, Fortineau N, Nordmann P. 2011. International transfer of NDM-1-producing Klebsiella pneumoniae from Iraq to France. Antimicrob. Agents Chemother. 55:1821–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P. 2010. Emergence of metallo-β-lactamase NDM-1-producing multidrug resistant Escherichia coli in Australia. Antimicrob. Agents Chemother. 54:4914–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rolain JM, Parola P, Cornaglia G. 2010. New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin. Microbiol. Infect. 16:1699–1701 [DOI] [PubMed] [Google Scholar]
- 25. Sekizuka T, et al. 2011. Complete sequencing of the blaNDM-1-positive IncA/C plasmid from Escherichia coli ST38 isolate suggests a possible origin from plant pathogens. PLoS One 6:e25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 27. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vila J, Marcos MA, Jimenez de Anta MT. 1996. A comparative study of different PCR-based DNA fingerprinting techniques for typing of the Acinetobacter calcoaceticus-A. baumannii complex. J. Med. Microbiol. 44:482–489 [DOI] [PubMed] [Google Scholar]
- 29. Yong D, et al. 2002. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 40:3798–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yong D, et al. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.