Abstract
This study characterized the pharmacokinetic/pharmacodynamic profiles of the Food and Drug Administration (FDA)-approved telavancin renal dose adjustment schemes. A previously published two-compartment open model with first-order elimination and a combined additive and proportional residual error model derived from 749 adult subjects in 11 clinical trials was used to simulate the individual concentration-time profiles for 10,260 subjects (NONMEM). The dosing regimens simulated were 10 mg/kg of body weight once daily for individuals with creatinine clearances (CLCRs) of >50 ml/min, 7.5 mg/kg once daily for individuals with CLCRs of 30 to 50 ml/min, and 10 mg/kg every 2 days for those with CLCRs of <30 ml/min. The area under the concentration-time curve (AUC) under one dosing interval (AUCτ) was computed as dose/CL. The probability of achieving an AUCτ/MIC ratio of ≥219 was evaluated separately for each renal dosing scheme. Evaluation of the dosing regimens demonstrated similar AUC values across the different renal function groups. For all renal dosing strata, >90% of the simulated subjects achieved an AUCτ/MIC ratio of ≥219 for MIC values as high as 2 mg/liter. For patients with CLCRs of <30 ml/min, the probability of target attainment (PTA) exceeded 90% for both the AUC0–24 (AUC from 0 to 24 h) and AUC24–48 intervals for MICs of ≤1 mg/liter. At a MIC of 2 mg/liter, the PTAs were 89.3% and 23.6% for the AUC0–24 and AUC24–48 intervals, respectively. The comparable PTA profiles for the three dosing regimens across their respective dosing intervals indicate that the dose adjustments employed in phase III trials for complicated skin and skin structure infections were appropriate.
INTRODUCTION
Telavancin is a lipoglycopeptide antibiotic recently approved in the United States and Canada for complicated skin and skin structure infections (cSSSI) due to Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) (3). Prior to the drug entering phase II/III cSSSI trials, pharmacokinetic/pharmacodynamic (PK/PD) system analyses were performed to generate estimates of the doses to be evaluated in these trials. Studies of multiple doses and schedules demonstrated that the ratio of area under the concentration-time curve for 24 h at steady state to MIC (AUC24/MIC ratio) was the pharmacodynamic variable associated with effect in neutropenic murine-thigh infection model studies, and an AUC24/MIC ratio of 219 was identified as the exposure target associated with a 1-log reduction in colony counts from baseline for MRSA (8, 11). Since dosing regimens with a high likelihood of achieving this exposure target have been associated with successful outcomes in cSSSI phase III trials (2), a Monte Carlo simulation (MCS) employing healthy volunteer PK data as a measure of interpatient exposure variability was performed to inform dose selection. Several regimens were evaluated in the MCS, and 10 mg of telavancin/kg of total body weight administered every 24 h (q24h) was identified as the optimal dosing scheme; this regimen demonstrated a >95% probability of achieving an AUC/MIC ratio of 219 for MIC values of ≤2 mg/liter (11).
While the pre-phase II and III PK/PD system analyses suggested that telavancin at 10 mg/kg intravenously (i.v.) every 24 h was the appropriate dosing scheme, several clinically important issues merited further investigation. As with most drugs, dose selection for the phase III cSSSI studies was based on the MCS using healthy volunteer PK data. Such simulations are often considered the most conservative probability-of-target-attainment (PTA) evaluation of a new drug because volunteers are young and healthy and thus likely to have the highest drug clearances and shortest half-life values for drugs. However, because the MCS explicitly creates a distribution, it is important to understand the measure of dispersion surrounding PK estimates. Due to the limited variation surrounding PK parameters from healthy volunteer studies, it is probable that they do not fully reflect the PTA among patients in clinical practice (9).
An understanding of how the PK disposition changes as a function of creatinine clearance (CLCR) is also essential when evaluating the PK/PD profile of a drug that is renally cleared (9, 12, 13). Telavancin is eliminated primarily by the kidneys, and dose adjustments are recommended for patients with CLCRs of 10 to 50 ml/min (3). Current Food and Drug Administration (FDA)-approved dosing for cSSSI is 10 mg/kg i.v. every 24 h for patients with normal renal function, 7.5 mg/kg i.v. every 24 h for patients with CLCRs of 30 to 50 ml/min, and 10 mg/kg i.v. every 48 h for patients with CLCRs of 10 to 30 ml/min. There are no specific recommendations for dosing patients with CLCRs of <10 ml/min. The effects of renal impairment and corresponding dosing adjustment schemes on PTAs of telavancin have not been described. Such knowledge is essential because renal impairment is likely to be prevalent among patients receiving telavancin, and their dosing regimens should be adjusted accordingly.
The 2-fold objectives of this analysis were (i) to characterize the PK/PD of telavancin among patients with cSSSI and various degrees of renal function and (ii) to assess the PK/PD profiles of the renal dose adjustment schemes used in clinical practice. To accomplish the study objectives, a previously described population PK model (10) was used to simulate telavancin plasma concentration-time profiles in cSSSI patients with various degrees of renal function and evaluate the ability of dosing regimens recommended for each CLCR stratum to obtain an AUC/MIC ratio greater than the pharmacodynamic target of 219 for MIC values of 0.5, 1, and 2 mg/liter.
(This study was presented in part as a platform presentation [10] at the 20th European Congress of Clinical Microbiology and Infectious Diseases in Vienna, Austria, April 2010.)
MATERIALS AND METHODS
Telavancin population pharmacokinetic model.
Telavancin exposure profiles were estimated from a previously published open 2-compartment population PK model with a combined additive and proportional residual error model (13a) derived from 749 adult subjects in seven phase I, two phase II, and two phase III clinical trials (5–7, 14–20). The structural model was parameterized on clearance (CL), volume of the central compartment (V1), intercompartment clearance (Q), and volume of the peripheral compartment (V2). The final clearance model included effects of CLCR, weight, and gender and a flag for bacterial eradication. Body weight, CLCR, and a flag for surgery were determined to be significant sources of interindividual variability in V1; V2 was influenced by body weight, and Q was influenced only by CLCR.
Monte Carlo simulation.
Individual concentration-time profiles were simulated for 10,260 subjects (NONMEM VI; Icon, Ellicott City, MD), using the aforementioned two-compartment full-population PK model with covariates (10). Data from the cohort of 513 patients enrolled in phase II and III clinical trials were used as the distribution of covariates in the MCS (Table 1). Based on this information, body weight and CLCR values for 10,260 subjects were simulated in Matlab R2006a (Mathworks, Natick, MA). A normal distribution with a mean of 78 kg and a variance of 225 kg2 was assumed for the body weight. Creatinine clearance was simulated according to a location-and-scale factor of the Weibull distribution. These simulations were based on the assumption of independence in the distributions of body weight and CLCR, given that a scatter plot of the two parameters in the original data did not show any discernible trend (data not shown).
Table 1.
Demographic parameters in phase II and III cSSSI clinical trials of telavancin
| Parameter | Phase II (references 15 and 17) | Phase III (reference 16) | Combined |
|---|---|---|---|
| n (males, females) | 130 (72, 58) | 383 (218, 165) | |
| Body wt (kg)a | 78.34 (44.00–167.70) | 78.00 (38.60–314.00) | |
| CLCR (ml/min)a | 100.26 (28.61–150.00) | 105.61 (17.63–150.00) | |
| Age (yrs)a | 44.15 (19.62–89.45) | 44.00 (18.00–89.00) | |
| Height (cm)a | 170.00 (142.00–193.04) | 170.20 (139.70–200.70) | |
| Surgery (0, 1)b | 322, 191 | ||
| Eradication (0, 1, 2)c | 53, 312, 122 |
Shown as mean (range).
0, variable is not present; 1, variable is present.
0, not cured/eradicated; 1, cured/eradicated; 2, unknown.
The dosing regimens used in the Monte Carlo simulation were (i) 10 mg/kg once daily for individuals with CLCRs of >50 ml/min, (ii) 7.5 mg/kg for individuals with CLCRs between 30 and 50 ml/min, and (iii) 10 mg/kg every 2 days for those with CLCRs of <30 ml/min. The maximum steady-state plasma concentrations (Cmax) for each regimen were the simulated concentrations at the end of drug infusion (1 h), while the minimum steady-state plasma concentrations were the simulated predose concentrations. The AUC under one dosing interval (AUCτ) associated with each regimen was computed as dose/CL. Note that the FDA-approved dosing interval was 24 h in subjects with CLCRs of >30 ml/min and 48 h among those with CLCRs of <30 ml/min. The numbers of subjects achieving an AUCτ/MIC ratio of 219 or greater for MIC values of 0.5, 1, and 2 mg/liter were calculated for each dosing scheme (11).
Since the AUC/MIC target of 219 was derived from q24h dosing in the neutropenic mouse-thigh MRSA infection model, additional PTA analyses were conducted for subjects with severe renal impairment using a daily partitioned AUC interval of AUC0–24 and AUC24–48. In these daily partitioned PTA analyses, AUC0–24 and AUC24–48 were assumed to be 65% and 35% of the AUCτ, respectively. Unfortunately, the AUC/MIC target based on a 48-h dosing interval is unknown at this time. In the absence of an AUC/MIC target for a 48-h dosing interval, we believed that it was prudent to assess the probability of achieving the AUC/MIC target of 219 for each 24-h interval within the 48-h dosing schedule. Since it is unclear whether cumulative or noncumulative (daily partitioned) PD exposures have a greater impact on clinical outcome in these patients, we assessed both PD exposures in patients with a CLCR of <30 ml/min.
RESULTS
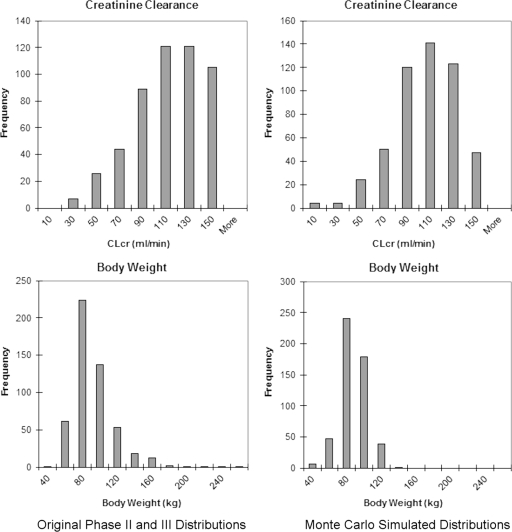
The simulations of the distributions of body weight and CLCR using normal and scale-location Weibull probability density functions were qualitatively similar to the distribution of the original data in phase II and III clinical trials of telavancin (Fig. 1). Since the distributions were comparable, the Monte Carlo simulation of 10,260 individual concentration-time profiles was based on the simulated distributions of body weight and CLCR, which were incorporated as covariates in the population model.
Fig 1.
Body weight and creatinine clearance histograms of the original and simulated populations.
Summary statistics of the simulated AUCτ values of individuals with various degrees of renal function are included in Table 2. Using the three dosing regimens, AUC values were relatively similar across the different renal function groups. Although the mean AUCτ was ∼30% higher in individuals with CLCRs of <30 ml/min, the AUC values presented in Table 2 are the AUC for one dosing interval. The dosing interval is 48 h in individuals with CLCRs of <30 ml/min. In contrast, the dosing interval is 24 h for individuals with CLCRs of ≥30 ml/min. If one considers the cumulative AUC for a 48-h interval, the total AUC is slightly higher for individuals with CLCRs of ≥30 ml/min than for those with a CLCR of <30 ml/min. However, the AUC distributions overlap considerably.
Table 2.
Simulated AUCτ and the probability of achieving an AUCτ/MIC ratio of ≥219 in subjects with various degrees of renal function
| Parameter | CLCR (ml/min) |
||
|---|---|---|---|
| <30a | 30–50b | >50c | |
| AUCτ (mean ± SD, mg · h/liter) | 1,058 ± 316 | 762 ± 238 | 776 ± 264 |
| AUCτ range (minimum, maximum; mg · h/liter) | 466, 2,071 | 318, 1,974 | 203, 2,820 |
| Probability (%) of achieving AUCτ/MIC ratio ≥ 219 at MIC | |||
| 0.5 mg/liter | 100 | 100 | 100 |
| 1 mg/liter | 100 | 100 | 100 |
| 2 mg/liter | 98.6 | 95.0 | 93.8 |
Based on 140 simulated profiles.
Based on 480 simulated profiles.
Based on 9,640 simulated profiles.
Results of the PTA analyses are provided in Tables 2 and 3. More than 99% of the simulated subjects achieved an AUCτ/MIC ratio of 219 or greater, assuming a MIC of 0.5 or 1 mg/liter, and at least 93% of the simulated population had AUCτ/MIC ratios of 219 or greater for a MIC value of 2 mg/liter (Table 2). The percentages of subjects with severe renal impairment achieving AUC0–24/MIC or AUC24–48/MIC ratios of 219 are presented in Table 3. The probability of achieving an AUC/MIC ratio of 219 exceeded 90% for both the AUC0–24 and AUC24–48 intervals at a MIC of 1 mg/liter. At a MIC of 2 mg/liter, the PTAs were 89.3% and 23.6% for the AUC0–24 and AUC24–48 intervals, respectively.
Table 3.
Simulated probability of achieving AUC0–24/MIC and AUC24-48/MIC ratios of ≥219 in subjects with severe renal impairment
| MIC (mg/liter) | Probability (%) of achieving AUCτ/MIC ratio ≥ 219 |
|
|---|---|---|
| AUC0–24/MIC ratio | AUC24–48/MIC ratio | |
| 0.5 | 100 | 100 |
| 1 | 100 | 92.4 |
| 2 | 89.3 | 23.6 |
Table 4 shows the maximum and minimum simulated steady-state concentrations of telavancin stratified by CLCR. There were no marked differences in the simulated Cmax or Cmin among patients with renal impairment from those of normal patients, based on the CLCR adjusted dose regimens. Although the Cmax and Cmin distributions were overlapping, there were differences in the mean estimates of Cmax and Cmin between CLCR strata. A higher mean Cmax was observed in individuals with a CLCR of ≥50 ml/min than in those with a CLCR of <50 ml/min. In contrast, a lower Cmin was observed for CLCRs of <30 ml/min than for the other strata. The Cmin for CLCRs of <30 ml/min reflects the predose concentration 48 h after the last dose at steady state. If one were to consider the concentration at hour 24 in the stratum of CLCRs of <30 ml/min, the point estimates of the means would be similar across all CLCR groups.
Table 4.
Summary statistics of simulated telavancin Cmax and Cmin at steady state
| Pharmacokinetic parameter | CLCR (ml/min)d |
|||
|---|---|---|---|---|
| ≥80 (n = 7,710) | 50–79 (n = 2,040) | 30–49 (n = 420) | <30 (n = 140) | |
| Cmax (μg/ml)a | ||||
| Mean ± SD (μg/ml) | 101 ± 21.8 | 101 ± 26.2 | 76.4 ± 18.6 | 82.3 ± 23.8 |
| Interquartile rangeb | 85.1, 98.9, 114 | 82.3, 97.5, 117 | 63.5, 74.7, 88.4 | 65.9, 79.6, 97.0 |
| Cmin (μg/ml)c | ||||
| Mean ± SD (μg/ml) | 10.7 ± 7.90 | 16.8 ± 10.6 | 15.9 ± 8.39 | 6.97 ± 4.99 |
| Interquartile rangeb | 4.74, 8.93, 14.6 | 9.07, 14.5, 22.4 | 9.85, 14.7, 51.8 | 3.57, 5.55, 9.95 |
Cmax = simulated value at the end of the 1-h infusion.
Interquartile range is listed as first quartile, median, and third quartile.
Cmin = predose value (telavancin was administered q24h in subjects with CLCRs of ≥30 ml/min and q48h in subjects with CLCRs of <30 ml/min).
n is number of simulated subjects.
DISCUSSION
This study distinguishes itself by using a population model-based approach to characterize the PK/PD profiles of current telavancin renal dosing schemes used in clinical practice. While valuable information for dose selection can be obtained by an MCS employing phase I data, it is imperative to validate initial dose selection as more data become available among the target population (1, 2). This is especially true for antibiotics that are renally cleared and dose adjusted for patients with renal impairment. Often, the process of selecting antimicrobial renal dosing schemes is arbitrary and based on prespecified dose adjustments (i.e., 50 to 75% dose reduction) at prespecified CLCR thresholds (i.e., <50 ml/min). In most cases, renal dose adjustment schemes are put into practice without consideration of the PK/PD profile (9, 12, 13).
The population pharmacokinetic model used in this analysis (10) was derived from 749 adult subjects in seven phase I, two phase II, and two phase III clinical trials. Overall, the model fit the data extremely well, and the PK parameters were physiologic in nature (10). While informative in understanding the behavior of telavancin in patients with various degrees of renal function, the major strength of this population PK model is its use in verifying the optimal dosing for telavancin in patients with impaired renal function. In particular, this model was embedded into a Monte Carlo simulation program and used to estimate exposure profiles for dosing regimens at fixed CLCR ranges (12). After reviewing the pharmacodynamic exposure distribution for current renal dosage regimens, our results verify that current renal dosing schemes used in clinical practice are appropriate. The exposure profiles, as measured by Cmax, Cmin, and AUCτ, were relatively comparable between regimens across the different renal strata. In addition, these regimens provided acceptable PTAs for the range of Staphylococcus aureus MIC values classified as susceptible by the FDA and Clinical and Laboratory Standards Institute (CLSI) (MIC values ≤ 1 mg/liter) (4). All proposed dosing regimens of telavancin are expected to provide an AUCτ/MIC ratio of 219 or greater in at least 99% of the population, for a MIC of 1 mg/liter or less. When the MIC was assumed to be equal to 2 mg/liter, the PTA was at least 93%. However, given that patients with severe renal impairment (CLCR of <30 ml/min) require every-other-day administration of telavancin, it was also important to consider the daily partitioned AUC values (AUC0–24, AUC24–48) given that the AUC/MIC target of 219 was derived using once-daily dosing regimens. Our analysis demonstrated that for the daily partitioned AUC values, the PTA was sufficient (>90%) for current Clinical and Laboratory Standards Institute (CLSI)- and FDA-approved breakpoints for Staphylococcus aureus (MIC ≤ 1 mg/liter) (3, 4). At a MIC of 2 mg/liter, however, the PTAs were 89.3% and 23.6% for the AUC0–24 and AUC24–48 intervals, respectively.
Several things should be noted when interpreting the results of this study. First, the PK/PD target (AUC24/MIC ratio of 219) used in this study was identified as the exposure target associated with a 1-log reduction in colony counts from baseline in the neutropenic mouse-thigh MRSA infection model. While achievement of this exposure target has been shown to have important implications for patients with cSSSI (2), data are lacking on the PK/PD target associated with optimal response using clinical data. Future studies are sorely needed to quantify the exposure targets for both efficacy and toxicity in humans. If these data become available, the utility of the FDA-approved renal dosing scheme should be revisited. Second, the AUC/MIC target of 219 was based on q24h dosing. It is currently unknown what the PK/PD target is for a 48-h dosing interval. Cognizant of this, we evaluated the AUC/MIC target for each 24-h interval within the 48-h dosing schedule for patients with a CLCR of <30 ml/min. Since patients who require q48h dosing have lower exposures during the 24- to 48-h interval than during the 0- to 24-h interval, it is imperative that further analyses assess whether cumulative or noncumulative (daily partitioned) PD exposures have a greater impact on clinical outcome. Third, given that there were subtle differences in Cmax and Cmin values across CLCR strata, future PK/PD system analyses should evaluate the clinical relevance of these findings as more data become available.
In conclusion, a robust PK/PD analysis was performed using PK data from 11 studies involving 749 patients (5–7, 14–20). The dose adjustments for renal impairment utilized in phase III protocols (7.5 mg/kg q24h for moderate renal impairment and 10 mg/kg q48h for severe renal impairment) appear appropriate based on the observed reductions in telavancin clearance among subjects with moderate and severe renal impairment (3). The concentration-time and PTA profiles were similar for the three dosing regimens across their respective dosing intervals for the different renal function groups, indicating that dose adjustments employed in phase III cSSSI trials were appropriate. All proposed telavancin dosing regimens are expected to provide an AUCτ/MIC ratio of 219 or greater in at least 90% of the population, for organisms with a MIC within current CLSI- and FDA-approved breakpoints for Staphylococcus aureus (4). Since PK/PD system analysis is an iterative process, the utility of the FDA-approved renal dosing scheme should be revisited as more clinically derived PK/PD data become available.
ACKNOWLEDGMENTS
The research and publication process were supported by Theravance, Inc., and Astellas Pharma Global Development, Inc. Editorial support (collating comments for the lead author to evaluate/incorporate) was provided by Emily Hutchinson, a medical writer at Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc. Editorial services were provided by T.P.L. of Lodise & Lodise, LLC.
J. P. Shaw, the pharmacokinetic lead at Theravance when the studies and these analyses were conducted, is acknowledged for her valuable contributions. G. L. Drusano is acknowledged for his intellectual contributions to the study and manuscript.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin. Infect. Dis. 51(Suppl. 1):S103–S110 [DOI] [PubMed] [Google Scholar]
- 2. Ambrose PG, et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 3. Astellas Pharma US, Inc, Theravance, Inc 2009. Vibativ (telavancin) for injection: prescribing information. Astellas Pharma US, Inc, Deerfield, IL, and Theravance, Inc, South San Francisco, CA [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Goldberg MR, Wong SL, Shaw JP, Kitt MM, Barriere SL. 2010. Lack of effect of moderate hepatic impairment on the pharmacokinetics of telavancin. Pharmacotherapy 30:35–42 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg MR, Wong SL, Shaw JP, Kitt MM, Barriere SL. 2010. Single-dose pharmacokinetics and tolerability of telavancin in elderly men and women. Pharmacotherapy 30:806–811 [DOI] [PubMed] [Google Scholar]
- 7. Gotfried MH, et al. 2008. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob. Agents Chemother. 52:92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hegde SS, et al. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lodise TP, Jr, et al. 2007. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob. Agents Chemother. 51:2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lodise TP, Patel N, Hedge S, Shaw J, Barriere S. April 2010. Telavancin pharmacokinetics and pharmacodynamics in patients with complicated skin and skin structure infections with varying degrees of renal function, abstr 468. 20th Eur. Congr. Clin. Microbiol. Infect. Dis., Vienna, Austria [Google Scholar]
- 11. Lodise TP, et al. April 2010. Mouse thigh MRSA infection model data and mathematical modelling to determine telavancin dosing for complicated skin and skin structure infection trials, abstr 469. 20th Eur. Congr. Clin. Microbiol. Infect. Dis., Vienna, Austria [Google Scholar]
- 12. Patel N, Scheetz MH, Drusano GL, Lodise TP. 2010. Determination of antibiotic dosage adjustments in patients with renal impairment: elements for success. J. Antimicrob. Chemother. 65:2285–2290 [DOI] [PubMed] [Google Scholar]
- 13. Patel N, Scheetz MH, Drusano GL, Lodise TP. 2010. Identification of optimal renal dosage adjustments for traditional and extended-infusion piperacillin-tazobactam dosing regimens in hospitalized patients. Antimicrob. Agents Chemother. 54:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a. Samara E, Shaw J-P, Barriere SL, Wong SL, Worboys P. 2012. Population pharmacokinetics of telavancin in healthy subjects and patients with infections. Antimicrob. Agents. Chemother. 56:2067–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw JP, et al. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stryjewski ME, et al. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stryjewski ME, et al. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683–1693 [DOI] [PubMed] [Google Scholar]
- 17. Stryjewski ME, et al. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40:1601–1607 [DOI] [PubMed] [Google Scholar]
- 18. Sun HK, et al. 2006. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob. Agents Chemother. 50:788–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong SL. 25 September 2006. Pharmacokinetics of intravenous telavancin in normal subjects and subjects [with] various degrees of renal dysfunction. Theravance study 103a-PK. Theravance, Inc, South San Francisco, CA [Google Scholar]
- 20. Wong SL, Barriere SL, Kitt MM, Goldberg MR. 2008. Multiple-dose pharmacokinetics of intravenous telavancin in healthy male and female subjects. J. Antimicrob. Chemother. 62:780–783 [DOI] [PubMed] [Google Scholar]