Abstract
Propionibacterium acnes is an important cause of orthopedic-implant-associated infections, for which the optimal treatment has not yet been determined. We investigated the activity of rifampin, alone and in combination, against planktonic and biofilm P. acnes in vitro and in a foreign-body infection model. The MIC and the minimal bactericidal concentration (MBC) were 0.007 and 4 μg/ml for rifampin, 1 and 4 μg/ml for daptomycin, 1 and 8 μg/ml for vancomycin, 1 and 2 μg/ml for levofloxacin, 0.03 and 16 μg/ml for penicillin G, 0.125 and 512 μg/ml for clindamycin, and 0.25 and 32 μg/ml for ceftriaxone. The P. acnes minimal biofilm eradication concentration (MBEC) was 16 μg/ml for rifampin; 32 μg/ml for penicillin G; 64 μg/ml for daptomycin and ceftriaxone; and ≥128 μg/ml for levofloxacin, vancomycin, and clindamycin. In the animal model, implants were infected by injection of 109 CFU P. acnes in cages. Antimicrobial activity on P. acnes was investigated in the cage fluid (planktonic form) and on explanted cages (biofilm form). The cure rates were 4% for daptomycin, 17% for vancomycin, 0% for levofloxacin, and 36% for rifampin. Rifampin cured 63% of the infected cages in combination with daptomycin, 46% with vancomycin, and 25% with levofloxacin. While all tested antimicrobials showed good activity against planktonic P. acnes, for eradication of biofilms, rifampin was needed. In combination with rifampin, daptomycin showed higher cure rates than with vancomycin in this foreign-body infection model.
INTRODUCTION
Propionibacterium acnes is a facultative anaerobic Gram-positive branching rod physiologically residing in sebaceous glands of the skin (24). It is the major agent of inflammatory acne. In addition, in 2 to 14% of cases, it is identified as the cause of various implant-associated infections, including prosthetic-joint infections, particularly shoulder prosthesis (25, 40, 43); spine implant surgery (4, 14, 19, 29); breast implant surgery (11, 27); electrophysiological cardiac devices (28); and neurosurgery involving ventricular drains and ventriculoperitoneal shunts (10). The role of P. acnes in foreign-body infections is probably underestimated due to technical reasons. Detection of anaerobes requires rapid transport to the microbiology laboratory or special transport media and needs incubation for up to 14 days due to slow growth (7, 40). Late growth and/or growth in enrichment media only is often misinterpreted as contamination. Furthermore, although P. acnes is usually introduced during surgery, clinical symptoms of low-grade infections often manifest only months to years after implantation. Therefore, the association between implant surgery and infection is not always obvious (14).
Recent studies showed that P. acnes forms biofilm on a wide range of materials (2, 26). However, little is known about the mechanisms involved in biofilm formation at the cellular and molecular levels. P. acnes is uniformly resistant to metronidazole but susceptible to several other antimicrobials, including penicillin G, ceftriaxone, vancomycin, and clindamycin (13, 16). However, the antimicrobial susceptibility is significantly reduced in biofilms, causing chronic and persistent infections that are difficult to cure without removal of the device. In addition, P. acnes can escape the immune response by resisting phagocytosis and surviving inside macrophages (37).
While the role of rifampin in eradication of staphylococcal biofilms was demonstrated in several experimental and clinical studies (1, 18, 33, 35, 39, 45), its role in P. acnes biofilm infections is less clear. The aim of this study was to investigate the activity of rifampin alone and in combination with other antimicrobials against P. acnes biofilm in vitro and in a foreign-body guinea pig infection model. In vivo studies were performed to determine the most active treatment regimen for eradication of P. acnes biofilms from implants in the clinical setting.
(Parts of the results of the present study were presented at the 21st European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Milan, Italy, 7 to 10 May 2011, and at the 2nd European Congress on Microbial Biofilms, Copenhagen, Denmark, 6 to 8 July 2011.)
MATERIALS AND METHODS
Study organism.
All experiments were performed with P. acnes strain ATCC 11827. The bacteria were stored at −70°C using the cryovial bead preservation system (Microbank; Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). For inoculum preparation for in vitro studies, one bead was spread on a blood agar plate and incubated for 72 to 96 h at 37°C anaerobically using an Anaerogen system (Oxoid, Basingstoke, Hampshire, England). One distinct colony was resuspended in 10 ml reduced (cooked) brain heart infusion (rBHI) and incubated anaerobically at 37°C. Seventy-two-hour cultures were adjusted to a turbidity of 0.5 McFarland standard (corresponding to ∼5 × 107 CFU/ml). For the inoculum for animal infection, a 72-h culture of P. acnes in rBHI was washed twice with sterile 0.9% saline before injection. The exact inoculum size was determined by CFU counting on blood agar plates incubated anaerobically. The ability of our test strain to form biofilm was confirmed by staining using Filmtracer (Invitrogen, Zug, Switzerland) and imaging with confocal microscopy.
Antimicrobial agents.
Daptomycin powder for injection was supplied by Novartis Pharma AG (Bern, Switzerland). A stock solution of 50 mg/ml was prepared in sterile 0.9% saline. Vancomycin was purchased from Teva Pharma AG (Aesch, Switzerland) as 10-mg powder ampoules. The stock solution of 50 mg/ml was prepared in sterile 0.9% saline. Levofloxacin hemihydrate injectable solution (5 mg/ml; Sanofi Aventis Pharma AG, Zurich, Switzerland) and rifampin powder (prepared in sterile water; 60 mg/ml; Sandoz AG, Steinhausen, Switzerland) were purchased from the respective manufacturers. Clindamycin (1 g) powder was purchased from Sigma and dissolved in sterile water (2 g/ml), penicillin G (25 mg/ml) was purchased from Grünenthal Pharma AG (Mitlödi, Switzerland), and ceftriaxone injectable solution (100 mg/ml) was purchased from Roche Pharma AG (Reinach, Switzerland).
Antimicrobial susceptibility of planktonic P. acnes.
The MIC and minimal bactericidal concentration (MBC) were determined by the broth macrodilution method, as described by Hall et al. (15). An inoculum of ∼1 × 106 CFU/ml was used. Serial 2-fold dilutions of the antimicrobials were prepared in rBHI. The MIC was defined as the lowest concentration of antibiotic that completely inhibited visible growth at 48 h. In addition, the MIC was determined by Etest (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions. An inoculum of 1 McFarland standard (∼1 × 108 CFU/ml) was used, and the plates were read after 48 h of anaerobic incubation at 37°C. The MBC was defined as the lowest antimicrobial concentration that killed ≥99.9% of the initial bacterial count (i.e., ≥3 log10 CFU/ml) in 48 h using rBHI (9). Growth media for daptomycin studies were supplemented with 50 mg/liter Ca2+. All experiments were performed in triplicate.
Biofilm formation on glass beads.
P. acnes biofilms were investigated using sintered glass beads (Siran carrier; SiKUG 023/02/300/A; Schott-Schleiffer AG, Muttenz, Switzerland) using a protocol adapted from previous studies (22, 42). The diameters of the beads ranged from 2 to 3 mm, and the porosity was 0.2 m2/g with a pore size of 60 to 300 μm. For biofilm formation, beads were placed in rBHI, inoculated with 2 to 3 CFU of P. acnes, and incubated anaerobically at 37°C under static conditions for 3 h, 24 h, or 72 h.
Killing of P. acnes biofilm on glass beads.
Biofilm was formed for 72 h as described above. Beads were then rinsed thrice with sterile 0.9% saline to remove planktonic bacteria, placed in rBHI containing serial 2-fold dilutions of antimicrobials, and incubated anaerobically for 24 h. After antimicrobial challenge, the beads where rinsed thrice and placed in microcalorimetric ampoules containing 4 ml rBHI. Recovering bacteria were detected by measuring heat production at 37°C for 72 h, allowing the quantification of the remaining biofilm bacteria (see below). The minimal biofilm eradication concentration (MBEC) was defined as the lowest antimicrobial concentration killing biofilm bacteria on beads, leading to absence of regrowth after 72 h of incubation in the microcalorimeter, indicated by the absence of (growth-related) heat flow. All experiments were repeated three times.
Microcalorimetric assay for quantification of biofilm on glass beads.
Replicating viable microorganisms produce heat, which can be detected with a microcalorimeter designed for precise real-time measurement (6). An isothermal microcalorimeter (TAM III; TA Instruments, New Castle, DE) was used. This instrument is equipped with 48 microcalorimetry channels, allowing independent parallel measurements. The instrumental detection limit of heat flow is 0.2 μW. The heat production is related to microbial metabolism and increases exponentially with their growth in appropriate medium (36). Microcalorimetry was recently used for investigation of staphylococcal biofilms on bone grafts and bone substitutes (8). Experiments were performed in 4-ml glass ampoules containing growth medium inoculated with sintered glass beads coated with P. acnes (with or without previous antimicrobial exposure). The ampoules were sealed and introduced first into the equilibration position for 15 min. In this time, the measuring temperature of 37.0000°C is reached, and heat disturbance by lowering the ampoule in the measuring position is minimized. The heat flow was then recorded at 10-s intervals for 72 h. The detection limit was determined at 10 μW to distinguish microbial heat production from the thermal background (e.g., nonspecific heat flow generated by degradation of the growth medium). The detection time was inversely proportional to the biofilm quantity, which allowed precise quantification of biofilm bacteria on the beads. Sterile beads (containing no bacterial biofilm) served as a negative control.
Animal model.
A foreign-body infection model in guinea pigs was used, as previously described by Zimmerli et al. (44). Male albino guinea pigs (Charles River, Sulzfeld, Germany) were housed in the Animal Care Facility at the University Hospital Lausanne, Lausanne, Switzerland. Experiments were performed according to the regulations of Swiss veterinary law. The animals were regularly weighed and observed for behavioral changes to monitor their well-being during the whole experiment. After an adaptation phase of 1 to 2 weeks, four sterile polytetrafluorethylene (Teflon) cages with 130 regularly spaced perforations 1 mm in diameter (Angst-Pfister AG, Zürich, Switzerland) were subcutaneously implanted in the flanks of the guinea pigs (weight range, 450 to 550 g). The surgery was performed under aseptic conditions, and a single dose of vancomycin (25 mg/kg of body weight) was injected intraperitoneally 30 min before skin incision. The wound clips were removed after 7 days. The sterility of the cages was confirmed by cultures of aspirated cage fluid before cage infection. The inoculation of P. acnes was performed after complete wound healing (i.e., 10 to 14 days after cage implantation) in sterile cages. The establishment of infection was confirmed by aspiration of cage fluid, followed by quantitative cultures and CFU enumeration on blood agar plates under anaerobic conditions.
Infection profile of planktonic P. acnes and persistence of biofilm P. acnes in animals.
Cages were infected by percutaneous inoculation of 200 μl of P. acnes containing 5 × 107 CFU/cage (low inoculum) or 1 × 109 CFU/cage (high inoculum). To determine the infection profile of untreated animals, cage fluid was aspirated from animals every 3 to 5 days. Two animals (one with high inoculum and one with low inoculum) were sacrificed 16 days after infection, and an additional four animals (two each with high and low inocula) were sacrificed 50 days after infection. At sacrifice, the cages were removed under aseptic conditions, placed in 5 ml rBHI, and incubated anaerobically for 10 to 14 days. After incubation, 100 μl of the medium was spread on a blood agar plate and incubated anaerobically at 37°C. A positive culture with P. acnes was defined as persistent infection.
Antimicrobial treatment of animals.
For treatment studies, infection was established with a high inoculum (1 × 109 CFU/cage). Three days after infection, quantitative cultures of aspirated cage fluid were performed, followed by starting the antimicrobial treatment. For each treatment regimen, at least three animals, each holding 4 cages, were randomized (i.e., 12 cages per treatment regimen): a control group (no antibiotic treatment), 40 mg/kg daptomycin, 10 mg/kg levofloxacin, 15 mg/kg vancomycin, 12.5 mg/kg rifampin, and the combination of rifampin with either daptomycin, levofloxacin, or vancomycin at the same doses mentioned above. All antimicrobials were injected intraperitoneally every 12 h, except daptomycin, which was given every 24 h. The duration of antimicrobial treatment was 4 days. The antimicrobial dose was determined based on pharmacokinetic studies in serum and cage fluid performed in previous studies in the same guinea pig model, mimicking antimicrobial concentrations achieved in humans (1, 5, 18, 31, 38).
Activity on planktonic and biofilm P. acnes in animals.
To determine the activity against planktonic P. acnes, cage fluid was aspirated before the start of treatment, during treatment (before administration of the last dose), and 5 days after completion of treatment. The bacterial counts were expressed as log10 CFU/ml cage fluid. To determine the activity against biofilm P. acnes, animals were sacrificed 5 days after completion of treatment, and the cages were explanted under aseptic conditions and incubated in 5 ml rBHI. After 10 days of incubation of the cages in BHI, aliquots of 100 μl were spread on a blood agar plate and incubated at 37°C for an additional 72 h. The treatment efficacy against adherent bacteria was expressed as the cure rate (as a percentage) defined as the number of cages without P. acnes growth divided by the total number of cages in the individual treatment group.
P. acnes isolates recovered from animals receiving rifampin (alone or in combination) were tested for emergence of rifampin resistance using an Etest (AB Biodisk), as described above.
Statistics.
Comparisons were performed by the Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables, as appropriate. For all tests, differences were considered significant when P values were <0.05. The graphs in the figures were plotted with Prism (version 5.04) software (GraphPad Software, La Jolla, CA).
RESULTS
Antimicrobial susceptibility of planktonic P. acnes.
Table 1 summarizes the antimicrobial susceptibility of planktonic P. acnes. The MIC values were lowest for rifampin, clindamycin, and β-lactam antibiotics (<0.2 μg/ml), whereas other antimicrobials all had an MIC of 1 μg/ml. MIC values obtained by the broth macrodilution method were congruent with results obtained with the Etest assay (differences within one dilution). The MBCs were lower for levofloxacin, rifampin, and daptomycin (≤4 μg/ml) than for vancomycin, penicillin G, and ceftriaxone (8 to 32 μg/ml). Clindamycin was only bacteriostatic. The MBC/MIC ratio was ≤4 for daptomycin and levofloxacin, indicating their bactericidal activity. Rifampin had a higher MBC/MIC ratio, due to the extremely low MIC (0.007 μg/ml) and not to a high MBC (2 μg/ml). The bactericidal concentration is achievable in vivo.
Table 1.
Antimicrobial susceptibility of planktonic and biofilm P. acnes
| Parameter | Value (μg/ml)a |
||||||
|---|---|---|---|---|---|---|---|
| Rifampin | Daptomycin | Levofloxacin | Vancomycin | Clindamycin | Penicillin G | Ceftriaxone | |
| MIC | 0.007 | 1 | 1 | 1 | 0.125 | 0.03 | 0.25 |
| MBC | 4 | 4 | 2 | 8 | 512 | 16 | 32 |
| MBC/MIC ratio | 571 | 4 | 2 | 8 | 4,096 | 5,333 | 128 |
| MBEC | 16 | 64 | 512 | 512 | 128 | 32 | 64 |
The values are medians of triplicates. The MBC was determined by broth macrodilution at 48 h. The MBEC was determined by microcalorimetry.
Antimicrobial susceptibility of biofilm P. acnes.
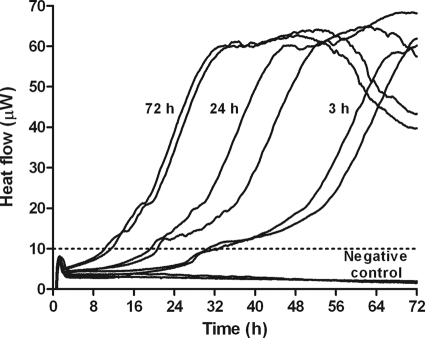
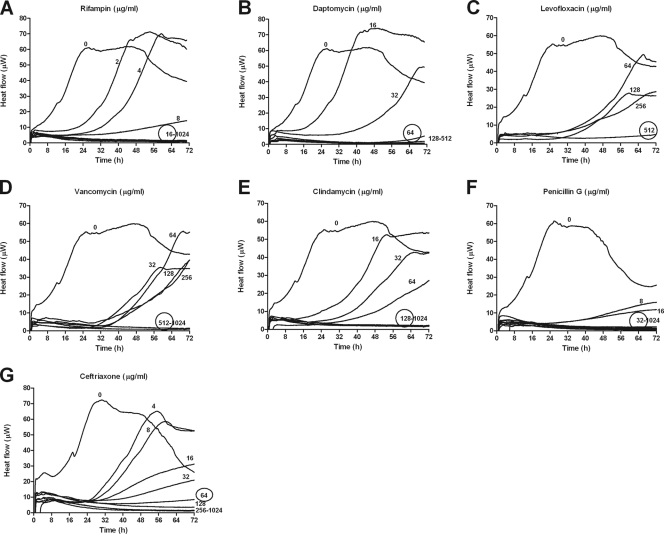
Figure 1 shows heat production by P. acnes biofilms of different ages. Young biofilms on glass beads (3 h old) were detected (e.g., heat flow exceeding 10 μW) in ∼30 h, whereas mature biofilms (72 h old) were detected in ∼8 h. Beads without biofilm did not produce any detectable heat flow. Figure 2 shows heat produced by bacteria remaining on beads after a 24-h exposure to individual antimicrobials. The MBEC was considered the concentration inhibiting regrowth after 72 h of recovery, indicated by absence of detectable heat in the microcalorimeter. Rifampin (Fig. 2A) was the most active drug for eradicating biofilm P. acnes in vitro (MBEC, 16 μg/ml), followed by daptomycin (Fig. 2B), penicillin G (Fig. 2F), and ceftriaxone (Fig. 2G), with an MBEC of ≥32 μg/ml. Clindamycin (Fig. 2E) showed activity only at 128 μg/ml. Vancomycin (Fig. 2D) and levofloxacin (Fig. 2C) were even less active, inhibiting biofilm P. acnes only at 512 μg/ml. Concentration-dependent antibiofilm activity was particularly observed with rifampin, daptomycin, and clindamycin.
Fig 1.
Heat production of P. acnes in biofilm. Heat was produced by biofilm bacteria on sintered glass beads after 3 h, 24 h, and 72 h of biofilm formation (in duplicate). The dashed line marks the detection limit of 10 μW. A sterile bead served as a negative control.
Fig 2.
MBECs determined by microcalorimetry. Shown is heat produced by recovering biofilm P. acnes after a 24-h exposure to serial dilutions of rifampin (A), daptomycin (B), levofloxacin (C), vancomycin (D), clindamycin (E), penicillin G (F), and ceftriaxone (G). The MBECs are circled. The detection limit was 10 μW.
Infection profile of planktonic P. acnes in animals.
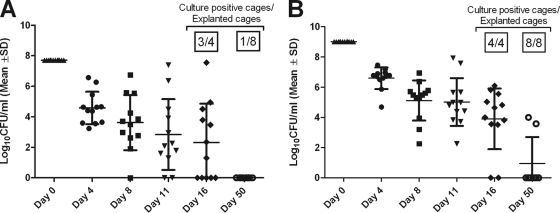
When both inocula were used, planktonic bacteria in the cage fluid decreased over time (Fig. 3). When the cages were explanted 16 days after infection, 25% of the implant-associated infections spontaneously healed after infection with the low inoculum. In contrast, after infection with the high inoculum, all infections persisted. When the infection was prolonged to 50 days, infection persisted in all 8 of the cages (100%) infected with the high inoculum, whereas 7 of the 8 cages (87.5%) infected with the low inoculum spontaneously cleared all bacteria.
Fig 3.
Infection profile of P. acnes in a foreign-body guinea pig infection model. Shown are bacterial loads in aspirated cage fluid during 50 days of infection and the percentages of culture-positive explanted cages after 16 days and 50 days of infection using a low infection inoculum of 5 × 107 CFU/cage (A) and a high infection inoculum of 1 × 109 CFU/cage (B). The values are means ± standard deviation.
In vitro activity on planktonic P. acnes.
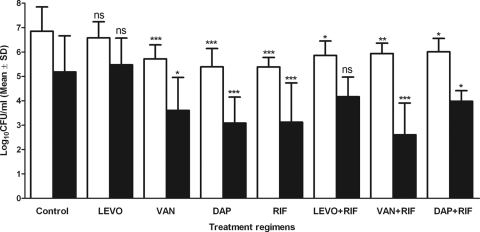
Figure 4 shows the bacterial density in aspirated cage fluid throughout the experiment. Three days after infection, before the start of treatment, the bacterial density in the cage fluid was ∼6 log10 CFU/ml. Spontaneous reduction of planktonic bacteria (−1.9 log10 CFU/ml) was observed in the cage fluid of untreated control animals during the observation period (12 days). Compared to untreated animals (during and after therapy), a significantly greater reduction in planktonic bacteria was observed with all treatment regimens, except levofloxacin alone and levofloxacin plus rifampin.
Fig 4.
Treatment efficacy against planktonic P. acnes. Shown are the bacterial loads in cage fluid aspirated during treatment (white bars) and 5 days after treatment (black bars). The values are means and standard deviations (SD). DAP, daptomycin; VAN, vancomycin; LEVO, levofloxacin; RIF, rifampin. The treatment groups are compared to the control during treatment and after treatment. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ns, not significant.
In vivo activity on biofilm P. acnes.
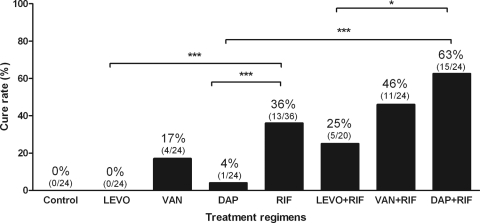
Figure 5 shows the cure rates of different antimicrobial regimens against biofilm P. acnes. No cure was observed in untreated animals (controls) and those receiving levofloxacin. Among single-drug therapies, rifampin was the most active antimicrobial, eradicating biofilms in 36% of infected cages, whereas daptomycin cured 4% and vancomycin 17% of cage infections. When combined with rifampin, the cure rate increased to 63% with daptomycin, 25% with levofloxacin, and 46% with vancomycin. No emergence of rifampin resistance was detected in animals receiving rifampin, either as a single drug or in combination treatment.
Fig 5.
Treatment activity against biofilm P. acnes. Shown are the cure rates of adherent bacteria from explanted cages. The percentages above the columns indicate the cure rates. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
DISCUSSION
P. acnes is an emerging pathogen in implant-associated infections. The growing prevalence is at least partly an artifact. It can be explained by improved diagnostic tools, such as sonication of explanted material (25, 29, 32), optimized culture conditions for anaerobes (7), and implementation of various molecular assays (34). The optimal antimicrobial treatment for P. acnes infections associated with implants has not yet been determined. It is especially unknown whether rifampin plays a favorable role similar to that in implant-associated staphylococcal infection (1, 18, 33, 35, 39, 45). Therefore, we investigated the activity of antimicrobials against biofilms in vitro and in vivo, modifying a previously established animal model (44). Conventional antimicrobials commonly used against P. acnes (β-lactams, vancomycin, and clindamycin), antibiotics with bactericidal activity on planktonic bacteria (levofloxacin), and those showing antibiofilm activity (rifampin and daptomycin) (18) were tested.
A laboratory strain of P. acnes (ATCC 11827) was chosen, exhibiting a susceptibility pattern typically observed in clinical isolates (16). The MIC values of all tested drugs for this strain were low. In contrast, the MBCs of commonly used antimicrobials, such as penicillin G (16 μg/ml), ceftriaxone (32 μg/ml), and clindamycin (512 μg/ml), were high for P. acnes infections. Interestingly, rifampin, daptomycin, and levofloxacin demonstrated low MBCs (≤4 μg/ml), suggesting superior killing of planktonic P. acnes.
In order to investigate the activities of antimicrobials against P. acnes biofilms in vitro, a microcalorimetry assay with glass beads was used. This assay allowed quantification of biofilm remaining on beads after previous exposure to the antimicrobial drug. Microcalorimetry was recently evaluated for testing antifungal activity on Candida sp. biofilms (E. Maiolo, U. Furustrand, D. Sanglard, and A. Trampuz, presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2011). In this study, the microcalorimetric assay (as shown by the MBEC values in Table 1) demonstrated the highest activity against P. acnes biofilm by rifampin, followed by penicillin G. Levofloxacin was the least active antimicrobial against P. acnes biofilms, despite bactericidal activity against planktonic P. acnes. Since guinea pigs do not tolerate β-lactams and clindamycin (showing gastrointestinal disturbance), these antimicrobials (and their combinations) could not be tested in vivo (reference 44 and unpublished observations).
When a high infection inoculum (109 CFU/cage) was injected into the tissue cage fluid of guinea pigs, P. acnes persisted on implanted cages for 50 days, despite spontaneous clearance of planktonic P. acnes from aspirated cage fluid. This finding highlights the great ability of P. acnes to adhere to the implant surface and its change from the planktonic to the biofilm phenotype. Indeed, it is a clinical observation that P. acnes is not often detected in cerebrospinal fluid or synovial fluid aspirated from prosthetic joints.
For treatment studies, a high inoculum (109 CFU/cage) was chosen, and antimicrobial treatment was started 3 days after infection. These conditions were modified from previous studies using methicillin-resistant Staphylococcus aureus (1, 18) and Enterococcus faecalis (12) in order to mimic a delayed, low-grade infection by P. acnes. In the untreated group, the number of planktonic P. acnes cells decreased over time. All treatment regimens reduced planktonic P. acnes significantly more than the spontaneous reduction in the untreated group, except levofloxacin and levofloxacin plus rifampin. The most efficient regimen against P. acnes biofilms in vivo was the combination of daptomycin and rifampin, achieving a cure rate of 63%. In our study, rifampin was the most efficient single drug, with a cure rate of 36%.
Limited data exist about treatment outcomes in a clinical setting. Rifampin in various combinations has been used in the treatment of complicated P. acnes infections, often involving implants (17, 20, 21, 30, 41). Penicillin G, linezolid, and linezolid plus rifampin were investigated against in vitro P. acnes biofilms after 14 days of exposure (3); no regrowth was detected with penicillin G and linezolid plus rifampin. A case report described a successful treatment of Propionbacterium sp. skull osteomyelitis with daptomycin (13), suggesting that the antimicrobial may be used for P. acnes bone infections.
To our knowledge, emergence of rifampin resistance has not yet been reported in Propionibacterium species. Resistance was, however, described for antimicrobials used for treatment of acne vulgaris, including MLS antibiotics (macrolides, lincosamides, and streptogramins), such as clarithromycin and clindamycin (23). No rifampin-resistant P. acnes isolate was observed in rifampin treatment failures in our animal model.
In summary, rifampin showed the highest activity against P. acnes biofilms as a single drug, both in vitro and in vivo. The combination of rifampin and daptomycin was the most active regimen against experimental P. acnes biofilms. Based on in vitro biofilm studies, the combination of rifampin and penicillin G or ceftriaxone may represent alternative options, but we were not able to investigate this in the animal model. The present study has important clinical implications, since it may initiate clinical studies with the above-mentioned antimicrobial regimens. This topic is also important because P. acnes implant-associated infections are expected to continue to increase in the future, and an optimal regimen needs to be defined.
ACKNOWLEDGMENTS
This study was supported by the Swiss National Science Foundation (K-32K1_120531).
We thank Alain Bizzini, Stefano Giulieri, and Ioana Raluca Mihailescu for critical review of the manuscript and Zarko Rajacic for practical advice.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Baldoni D, Haschke M, Rajacic Z, Zimmerli W, Trampuz A. 2009. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob. Agents Chemother. 53:1142–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bayston R, et al. 2007. Biofilm formation by Propionibacterium acnes on biomaterials in vitro and in vivo: impact on diagnosis and treatment. J. Biomed. Mater. Res. A 81:705–709 [DOI] [PubMed] [Google Scholar]
- 3. Bayston R, Nuradeen B, Ashraf W, Freeman BJ. 2007. Antibiotics for the eradication of Propionibacterium acnes biofilms in surgical infection. J. Antimicrob. Chemother. 60:1298–1301 [DOI] [PubMed] [Google Scholar]
- 4. Bemer P, et al. 2008. Significance of Propionibacterium acnes-positive samples in spinal instrumentation. Spine 33:E971–E976 [DOI] [PubMed] [Google Scholar]
- 5. Blaser J, Vergeres P, Widmer AF, Zimmerli W. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 39:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boling EA, Blanchard GC, Russell WJ. 1973. Bacterial identification by microcalorimetry. Nature 241:472–473 [DOI] [PubMed] [Google Scholar]
- 7. Butler-Wu SM, et al. 2011. Optimization of periprosthetic culture for the diagnosis of Propionibacterium acnes prosthetic joint infection. J. Clin. Microbiol. 49:2490–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clauss M, Trampuz A, Borens O, Bohner M, Illchmann T. 2010. Biofilm formation on bone grafts and bone graft substitutes: comparison of different materials by a standard in vitro test and by microcalorimetry. Acza Biomater. 6:3791–3797 [DOI] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. CLSI, Wayne, PA [Google Scholar]
- 10. Conen A, et al. 2008. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin. Infect. Dis. 47:73–82 [DOI] [PubMed] [Google Scholar]
- 11. Del Pozo JL, et al. 2009. Pilot study of association of bacteria on breast implants with capsular contracture. J. Clin. Microbiol. 47:1333–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furustrand Tafin U, et al. 2011. Gentamicin improves the activities of daptomycin and vancomycin against Enterococcus faecalis in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 55:4821–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh M, Talwani R, Gilliam BL. 2009. Propionibacterium skull osteomyelitis treated with daptomycin. Clin. Neurol. Neurosurg. 111:610–612 [DOI] [PubMed] [Google Scholar]
- 14. Haidar R, Najjar M, Der BA, Tabbarah Z. 2010. Propionibacterium acnes causing delayed postoperative spine infection: review. Scand. J. Infect. Dis. 42:405–411 [DOI] [PubMed] [Google Scholar]
- 15. Hall GS, et al. 1995. Minimum bactericidal concentrations of Propionibacterium acnes isolates from cases of chronic endophthalmitis. Diagn. Microbiol. Infect. Dis. 21:187–190 [DOI] [PubMed] [Google Scholar]
- 16. Hoeffler U, Ko HL, Pulverer G. 1976. Antimicrobiol. susceptibility of Propinibacterium acnes and related microbial species. Antimicrob. Agents Chemother. 10:387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jakab E, et al. 1996. Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. Yale J. Biol. Med. 69:477–482 [PMC free article] [PubMed] [Google Scholar]
- 18. John AK, et al. 2009. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob. Agents Chemother. 53:2719–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kowalski TJ, et al. 2007. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin. Infect. Dis. 44:913–920 [DOI] [PubMed] [Google Scholar]
- 20. Levy PY, et al. 2008. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin. Infect. Dis. 46:1884–1886 [DOI] [PubMed] [Google Scholar]
- 21. Lutz MF, et al. 2005. Arthroplastic and osteosynthetic infections due to Propionibacterium acnes: a retrospective study of 52 cases, 1995–2002. Eur. J. Clin. Microbiol. Infect. Dis. 24:739–744 [DOI] [PubMed] [Google Scholar]
- 22. Mathur T, Bhateja P, Pandya M, Fatma T, Rattan A. 2004. In vitro activity of RBx 7644 (ranbezolid) on biofilm producing bacteria. Int. J. Antimicrob. Agents 24:369–373 [DOI] [PubMed] [Google Scholar]
- 23. Nord CE, Oprica C. 2006. Antibiotic resistance in Propionibacterium acnes. Microbiological and clinical aspects. Anaerobe 12:207–210 [DOI] [PubMed] [Google Scholar]
- 24. Perry AL, Lambert PA. 2006. Propionibacterium acnes. Lett. Appl. Microbiol. 42:185–188 [DOI] [PubMed] [Google Scholar]
- 25. Piper KE, et al. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 47:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. 2003. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials 24:3221–3227 [DOI] [PubMed] [Google Scholar]
- 27. Rieger UM, Pierer G, Luscher NJ, Trampuz A. 2009. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast Surg. 33:404–408 [DOI] [PubMed] [Google Scholar]
- 28. Rohacek M, et al. 2010. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 121:1691–1697 [DOI] [PubMed] [Google Scholar]
- 29. Sampedro MF, et al. 2010. A biofilm approach to detect bacteria on removed spinal implants. Spine 35:1218–1224 [DOI] [PubMed] [Google Scholar]
- 30. Soderquist B, Holmberg A, Unemo M. 2010. Propionibacterium acnes as an etiological agent of arthroplastic and osteosynthetic infections—two cases with specific clinical presentation including formation of draining fistulae. Anaerobe 16:304–306 [DOI] [PubMed] [Google Scholar]
- 31. Trampuz A, et al. 2007. Efficacy of a novel rifamycin derivative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection. Antimicrob. Agents Chemother. 51:2540–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trampuz A, et al. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 357:654–663 [DOI] [PubMed] [Google Scholar]
- 33. Trampuz A, Widmer AF. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19:349–356 [DOI] [PubMed] [Google Scholar]
- 34. Tunney MM, et al. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 37:3281–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vergidis P, et al. 2011. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob. Agents Chemother. 55:1182–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wadsö I. 2001. Isothermal microcalorimety: current problems and prospects. J. Therm Anal. Calorim. 64:75–84 [Google Scholar]
- 37. Webster GF, Leyden JJ, Musson RA, Douglas SD. 1985. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect. Immun. 49:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Widmer AF, Frei R, Rajacic Z, Zimmerli W. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96–102 [DOI] [PubMed] [Google Scholar]
- 39. Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 14:1251–1253 [DOI] [PubMed] [Google Scholar]
- 40. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. 2008. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch. Orthop. Trauma Surg. 128:1039–1046 [DOI] [PubMed] [Google Scholar]
- 41. Zeller V, et al. 2007. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J. Infect. 55:119–124 [DOI] [PubMed] [Google Scholar]
- 42. Zimmerli W, Frei R, Widmer AF, Rajacic Z. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959–967 [DOI] [PubMed] [Google Scholar]
- 43. Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654 [DOI] [PubMed] [Google Scholar]
- 44. Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146:487–497 [DOI] [PubMed] [Google Scholar]
- 45. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279:1537–1541 [DOI] [PubMed] [Google Scholar]