Abstract
β-N-Acetylglucosaminidase (NagZ), encoded by the nagZ gene, is a critical enzyme for basal-level ampC derepression (ampC expression in the absence of β-lactam challenge) in ampD and dacB mutants of Pseudomonas aeruginosa. Three mutants with a phenotype of basal-level L1 and L2 β-lactamase derepression in Stenotrophomonas maltophilia have been reported, including KJΔDI (ampDI mutant), KJΔmrcA (mrcA mutant), and KJΔDIΔmrcA (ampDI and mrcA double mutant). In this study, nagZ of S. maltophilia was characterized, and its roles in basal-level β-lactamase derepression, induced β-lactamase activities, and β-lactam resistance of KJΔDI, KJΔmrcA, and KJΔDIΔmrcA were evaluated. Expression of the nagZ gene was constitutive and not regulated by AmpR, AmpDI, AmpN, AmpG, PBP1a, and NagZ. Introduction of ΔnagZ into KJΔDI nearly abolished basal-level derepressed β-lactamase activity; conversely, introduction of ΔnagZ into KJΔmrcA did not affect it. At least two activator ligands (ALs) are thus considered responsible for β-lactamase expression in the S. maltophilia system, specifically, the NagZ-dependent (AL1) and NagZ-independent (AL2) ligands responsible for the basal-level derepressed β-lactamase activities of KJΔDI and KJΔmrcA, respectively. The contributions of AL1 and AL2 to the induced β-lactamase activities may vary with the types of β-lactams. nagZ inactivation did not affect aztreonam-, cefoxitin-, and carbenicillin-induced β-lactamase activities, but it attenuated cefuroxime- and piperacillin-induced β-lactamase activities. Introduction of ΔnagZ into KJ, KJΔDI, KJΔmrcA, and KJΔDIΔmrcA did not significantly change the MICs of the β-lactams tested except that the MICs of cefuroxime and piperacillin moderately decreased in strains KJΔZ and KJΔDIΔZ (nagZ mutants).
INTRODUCTION
β-N-Acetylglucosaminidase (NagZ), a member of the glycosyl hydrolase 3 (GH 3) family, was first known as a cytoplasmic enzyme active toward p-nitrophenyl-β-N-acetyl-d-glucosaminide and was later found to be active toward anhydromuropeptides (11). Roles of NagZ in chromosomal ampC β-lactamase induction and β-lactam resistance were also noticed (2, 5, 25). For normal bacterial cell growth, a considerable amount of periplasmic peptidoglycan is cleaved by autolysin enzymes, with released GlcNAc-1,6-anhydromuropeptide fragments transported into the cytoplasm by AmpG permease (15, 19). The transported GlcNAc-1,6-anhydromuropeptides are competent substrates for cytosolic NagZ (encoded by nagZ) and N-acetyl-muramyl-l-alanine amidase (encoded by ampD). The ampD-processed products, GlcNAc-1,6-anhydro-MurNAc moieties or 1,6-anhydro-MurNAc moieties, are further recycled into UDP-MurNAc pentapeptides acting as a repressor ligand (RL) to repress ampC expression (12). The nagZ-processed products, 1,6-anhydromuropeptides, act as the activator ligands (ALs) for induction of chromosomal ampC β-lactamase (6). Regulatory ligands, UDP-MurNAcpentapeptide (RL) and 1,6-anhydromuropeptides (AL), competitively regulate ampC induction by directly binding to the LysR-type transcriptional regulator AmpR (8, 20). Recently, Moya et al. reported that the inactivation of dacB, encoding penicillin binding protein 4 (PBP4), triggered the CreBC two-component regulatory system and conferred high β-lactam resistance (21). Moreover, the involvement of ΔdacB in ampC overexpression and elevated β-lactam resistance has proven to be ampR, ampG, and nagZ dependent (21). Therefore, the networks of ampG-ampD-nagZ-ampR and dacB-creBC-ampG-nagZ-ampR regulons exquisitely control expression of the ampC gene and β-lactam resistance in Pseudomonas aeruginosa.
Stenotrophomonas maltophilia is a Gram-negative, nonfermentative bacillus that is an important cause of nosocomial infection. S. maltophilia is resistant to a wide range of antimicrobials because of its intrinsic resistance determinants, including β-lactamases, aminoglycoside-modified enzymes, and a qnrB-like quinolone-resistant determinant (4). Among them, two inducibly expressed L1 and L2 β-lactamases inactivate β-lactam, allowing S. maltophilia to resist virtually all β-lactams (9, 22). Like the ampR-ampC module of Enterobacteriaceae and P. aeruginosa, S. maltophilia harbors an ampR-L2 module (9, 22). To elucidate the L1 and L2 induction mechanisms of S. maltophilia, the ampN-ampG operon and the ampDI, ampR, and mrcA genes, but not nagZ, were sequentially characterized (10, 17, 18, 26). Briefly, AmpN/AmpG permease transports degraded murein sacculus from the periplasm into the cytoplasm (10). Cytoplasmic N-acetyl-muramyl-l-alanine amidase (AmpDI) encoded by ampDI, plays a negative role in the expression of β-lactamase, like AmpD in P. aeruginosa. Inactivation of ampDI causes a fully derepressed phenotype, resulting in L1 and L2 overexpression (26). The LysR-type transcriptional regulator AmpR regulates the expression of its contiguous L2 and unlinked L1 genes (18). Like dacB in P. aeruginosa, mrcA, encoding PBP1a, is also involved in basal-level β-lactamase derepression. However, ΔmrcA-derived basal-level β-lactamase derepression is creBC independent (17).
Recently, nagZ inactivation has been shown to attenuate β-lactam resistance and ampC expression in ampD mutant, dacB mutant, and ampD-dacB double-mutant strains of P. aeruginosa (27). Based on the known roles of ampDI, ampN-ampG, ampR, and mrcA in chromosomal β-lactamase gene expression (10, 17, 18, 26), the ampR-ampC system of P. aeruginosa and the ampR-L2 system of S. maltophilia share several characteristics. To date, the relationships among nagZ, the ampN-ampG-ampDI-ampR regulon, and mrcA of S. maltophilia have not been elucidated. Here, we further characterize the nagZ gene of S. maltophilia, including its significance for β-lactamase expression and β-lactam resistance in wild-type, ampDI mutant, mrcA mutant, and ampDI-mrcA double-mutant strains. Furthermore, a novel nagZ-independent but ampNG-ampDI-ampR regulon-involved mechanism for β-lactamase expression of S. maltophilia is proposed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
Table 1 lists the strains, plasmids, and primers used in this study. S. maltophilia KJ, harboring active β-lactamases L1 and L2, has been described previously (9). Tetracycline (50 μg/ml) was added to maintain selection of cells carrying pRK415 derivatives. Primers were designed based on the S. maltophilia K279a genome sequence (4).
Table 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype or propertiesa | Reference |
|---|---|---|
| S. maltophilia | ||
| KJ | Wild type; a clinical isolate from Taiwan | 9 |
| KJΔZ | S. maltophilia KJ nagZ deletion mutant; ΔnagZ | This study |
| KJΔL1 | S. maltophilia KJ L1 isogenic mutant; L1::xylE | 9 |
| KJΔL1ΔZ | S. maltophilia KJ L1 and nagZ deletion mutant; L1::xylE ΔnagZ | This study |
| KJΔL2 | S. maltophilia KJ L2 isogenic mutant; L2::xylE | 9 |
| KJΔL2ΔZ | S. maltophilia KJ L2 and nagZ deletion mutant; L2::xylE ΔnagZ | This study |
| KJΔDI | S. maltophilia KJ ampDI deletion mutant; ΔampDI | 26 |
| KJΔDIΔZ | S. maltophilia KJ ampDI and nagZ deletion mutant; ΔAmpDI ΔnagZ | This study |
| KJΔR | S. maltophilia KJ ampR deletion mutant; ΔampR | 17 |
| KJΔNG | S. maltophilia KJ ampN and ampG deletion mutant; ΔampN ΔampG | 17 |
| KJΔmrcA | S. maltophilia KJ mrcA deletion mutant; ΔmrcA | 17 |
| KJΔmrcAΔZ | S. maltophilia KJ mrcA and nagZ deletion mutant; ΔmrcA ΔnagZ | This study |
| KJΔDIΔmrcA | S. maltophilia KJ ampDI and mrcA deletion mutant; ΔampDI ΔmrcA | 17 |
| KJΔDIΔmrcAΔZ | S. maltophilia KJ ampDI, mrcA, and nagZ deletion mutant; ΔAmpDI ΔmrcA ΔnagZ | This study |
| Escherichia coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Invitrogen |
| S17-1 | λ pir+ mating strain | 26 |
| Plasmids | ||
| pEX18Tc | sacB oriT Tcr | 7 |
| pRK415 | Mobilizable broad-host-range plasmid cloning vector, RK2 origin; Tcr | 14 |
| pX1918GT | Plasmid containing the xylE-gentamicin resistance gene cassette; Ampr Gmr | 23 |
| pΔZ | pEX18Tc with an internal-deletion nagZ gene; Tcr | This study |
| pRK-Z | pRK415 with a complete nagZ gene; Tcr | This study |
| pRK188ZxylE | pRK415 with a 188-bp DNA fragment upstream from the nagZ start codon and a nagZ::xylE transcriptional fusion | This study |
| pRKDI | pRK415 with a complete ampDI gene; Tcr | 26 |
| Primers | ||
| NagZ5-F | 5′-CTGCGCAAGCTTGCCGCCGAAC-3′ | This study |
| NagZ5-R | 5′-TTGCATGCCACCACCCCGGCCAC-3′ | This study |
| NagZ3-F | 5′-CACCGCATGCTGGTCGATGAAG-3′ | This study |
| NagZ3-R | 5′-CATGATCTAGAGGAACAGCGGCAC-3′ | This study |
| NagZP-F | 5′-CTGCGCAAGCTTGCCGCCGAAC-3′ | This study |
| NagZP-R | 5′-TTGCATGCCACCACCCCGGCCAC-3′ | This study |
Added restriction enzyme recognition sites in primers are underlined.
Construction of the nagZ knockout mutant KJΔZ.
nagZ deletion mutants of strains KJ, KJΔL1, KJΔL2, KJΔDI, KJΔmrcA, and KJΔDIΔmrcA were constructed following a well-established procedure (26). Upstream 339-bp and downstream 247-bp DNA fragments of the nagZ gene were obtained by PCR using the NagZ5-F/NagZ5-R and NagZ3-F/NagZ3-R primer sets, respectively. PCR amplicons were digested with HindIII/SphI and SphI/XbaI and subsequently cloned into pEX18Tc (7). The resultant plasmid, pΔZ, has an internal 394-bp deletion in the nagZ gene. Plasmid pΔZ was mobilized into S. maltophilia KJ, KJΔL1 (9), KJΔL2 (9), KJΔDI (26), KJΔmrcA (17), and KJΔDIΔmrcA (17) via conjugation, and mutants were selected in two steps, as described previously (26). Mutant correctness was checked by colony PCR amplification (16) and sequencing.
Complementation assay.
The nagZ gene, along with the upstream 225-bp DNA fragment, was obtained by PCR using primers NagZP-F and NagZP-R. The PCR amplicon was ligated into the complementation vector pRK415 (14), resulting in the construct pRK-Z. The sequence of the cloned PCR fragment was verified by sequencing. The orientation of the complete nagZ matched that of the resident lac promoter of pRK415. The nagZ gene expressed from the recombinant plasmid pRK-Z was confirmed by quantitative reverse transcription (qRT)-PCR (data not shown).
Promoter-xylE transcriptional fusion.
We constructed pRK188ZxylE to investigate the regulation of nagZ expression. A 339-bp DNA fragment containing the partial 5′ terminus of the nagZ gene and 188 bp upstream of the nagZ gene was obtained by PCR using NagZ5-F and NagZ5-R as primers. The PCR amplicons were cloned into pRK415. A xylE gene retrieved from pX1918GT (23) was inserted following the 339-bp amplicon to create a nagZp-xylE fusion construct, pRK188ZxylE. The orientation of the xylE gene in pRK188ZxylE was opposite to that of lacZp from the pRK415 vector.
Antimicrobial susceptibility.
The susceptibilities of S. maltophilia strains to different β-lactams were determined by a 2-fold serial agar dilution method according to CLSI guidelines (3). All chemicals were purchased from Sigma. The MIC was defined as the lowest concentration of antibiotic that prevented growth of bacteria after incubation at 37°C for 18 h.
Determination of β-lactamase activity.
The procedure for β-lactamase induction is described elsewhere (18), except that different inducers were added as indicated. Cefuroxime was generally used as the inducer for determination of total β-lactamase activity; it has been proven to be a potent inducer for L1 and L2 β-lactamase (9). Cefoxitin and aztreonam are not hydrolyzed by L2 and L1 β-lactamase (9), respectively. Therefore, the inducer concentration can be kept constant during the assay process when cefoxitin and aztreonam are used as inducers in L1 and L2 deletion biological backgrounds, respectively. The β-lactamase activities were spectrophotometrically determined with 100 μM nitrocefin (Oxoid) as a substrate (18). Enzyme activity was calculated by using a molar absorption coefficient for nitrocefin of 20,500 M−1 cm−1 at 486 nm. The specific activity of β-lactamase (U/mg) was expressed as nanomoles of nitrocefin hydrolyzed per minute per milligram of protein. The protein concentration was determined using the Bio-Rad protein assay reagent, with bovine serum albumin as a standard. In all cases, mean β-lactamase activity values were obtained in three independent experiments.
Determining C23O activity.
The activity of catechol-2,3-dioxygenase (C23O), encoded by the xylE gene, was measured in intact cells, as described previously (10). The rate of hydrolysis was calculated by using 44,000 M−1 cm−1 as the extinction coefficient. One unit of enzyme activity (Uc) was defined as the amount of enzyme that converts 1 nmol substrate per minute. The specific activity of the enzyme was defined in terms of Uc per optical density at 450 nm (Uc/OD450).
Determining NAG activity.
β-1,4-N-Acetylglucosaminidase (NAG) activity was assessed by using whole-cell lysates, with p-nitrophenyl-β-N-acetyl-d-glucosaminide (Sigma) used as a substrate. An overnight culture of the assayed strain was diluted in fresh LB medium to an OD450 of 0.15. After 0.5 h of incubation, 50 μg/ml cefuroxime was added, and growth continued for 2 h. Meanwhile, a cefuroxime-free control was simultaneously prepared. Harvested cells were washed, centrifuged, and resuspended in 50 mM Tris-HCl (pH 7.4). The cells were disrupted by sonication, and a crude extract (supernatant) was obtained after centrifugation at 150,000 × g for 1 h. The 500-μl sonicated cell lysate was incubated with 400 μl 1 mM p-nitrophenyl-β-N-acetyl-d-glucosaminide at room temperature. Reactions were allowed to proceed for 1, 2, 3, or 4 h and then were quenched by adding 100 μl of 2.5 M K2CO3. The reaction mixture was centrifuged, and p-nitrophenol present in the supernatant was measured at 405 nm. Specific activity was calculated by using a molar absorption coefficient for p-nitrophenol of 20,500 M−1 cm−1 at 405 nm. One unit of enzyme activity (UnagZ) was defined as the amount of enzyme that produced 1 nmol p-nitrophenol per minute. The specific activity (UnagZ/mg) of Nag enzyme was expressed as UnagZ per milligram of protein.
Nucleotide sequence accession number.
The nucleotide sequence of the nagZ gene of S. maltophilia KJ has been deposited in GenBank under accession no. JN613812.
RESULTS
Cloning and sequence analysis of nagZ.
PCR of S. maltophilia KJ chromosomal DNA with primers NagZP-F and NagZP-R (Table 1) yielded a 1,396-bp amplicon. Sequence analysis of the PCR amplicon revealed a complete nagZ gene and its upstream 247-bp DNA fragment. A BLAST search of protein databases revealed 97 to 100%, 81 to 84%, 50 to 53%, and 47% identity to the nagZ homolog-encoded (NagZ) proteins of different S. maltophilia strains, Xanthomonas spp., Pseudomonas spp., and Escherichia coli, respectively.
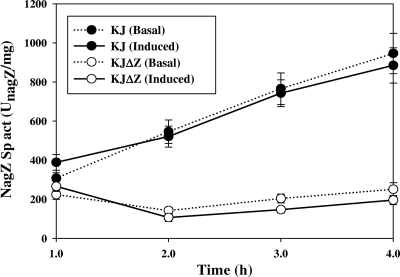
A homology study showed NagZ to be a putative β-N-acetylglucosaminidase in S. maltophilia. To assess the possibility, the β-N-acetylglucosaminidase activities of strains KJ and KJΔZ were determined under basal and cefuroxime-induced conditions, respectively. After 4 h of incubation, the β-N-acetylglucosaminidase activity of strain KJ increased from 308 to 946 UnagZ/mg and from 389 to 685 UnagZ/mg under basal and induced conditions, respectively. Still, inactivation of the nagZ gene nearly abolished the increase in β-N-acetylglucosaminidase activity regardless of the presence of the inducer (Fig. 1), which agrees with studies of E. coli nagZ (2) and P. aeruginosa nagZ (1). Hence, NagZ (a homolog of S. maltophilia K279a Smlt3538) is an active enzyme with N-acetyl-β-glucosaminidase activity in S. maltophilia KJ.
Fig 1.
NagZ activity assay of wild-type S. maltophilia KJ and its derived nagZ deletion mutant, KJΔZ, under basal and induced conditions. Cefuroxime (50 μg/ml) was used as the inducer. Results shown are mean values of three experiments ± standard deviations (SD).
Role of nagZ in induction of L1 and L2 β-lactamase genes.
NagZ is essential to produce the actual inducer molecule of 1,6-anhydroMurNAc peptides in the ampR-ampC system (2, 25). Inactivation of nagZ in some Enterobacteriaceae (24) or P. aeruginosa (1) can thus affect ampC expression. To pinpoint the extent to which nagZ is involved in the expression of L1 and L2 β-lactamase, the ΔnagZ allele was introduced into wild-type KJ, mutant KJΔL1 (L1 isogenic), and mutant KJΔL2 (L2 isogenic) (9). The resultant mutants, KJΔZ, KJΔL1ΔZ, and KJΔL2ΔZ, were analyzed in comparison with their parental strains with regard to β-lactamase activity. Inactivation of nagZ did not completely abolish β-lactamase induction (Table 2). Some 22 to 25% of induced L1 and L2 β-lactamase activities were still detectable, indicating that cefuroxime-induced β-lactamase activity is partially nagZ dependent.
Table 2.
β-Lactamase activities of S. maltophilia KJ and its derived strains
| S. maltophilia strain | β-Lactamase sp act (Una/mg) |
|
|---|---|---|
| Basal | Inducedb | |
| (A) KJ | 10 ± 1.8 | 1,628 ± 197 |
| KJΔZ | 7 ± 1.0 | 390 ± 51 |
| KJΔL1 | 5 ± 0.9 | 585 ± 65c |
| KJΔL1ΔZ | 15 ± 2.1 | 152 ± 20c |
| KJΔL2 | 9 ± 1.3 | 114 ± 18d |
| KJΔL2ΔZ | 5 ± 1.0 | 26 ± 3.2d |
| KJΔDI | 4,448 ± 752 | 4,592 ± 560 |
| KJΔDIΔZ | 88 ± 10 | 551 ± 62 |
| KJΔmrcA | 1,012 ± 190 | 2,496 ± 275 |
| KJΔmrcAΔZ | 981 ± 102 | 1,509 ± 182 |
| KJΔDIΔmrcA | 4,751 ± 876 | 5,940 ± 823 |
| KJΔDIΔmrcAΔZ | 955 ± 101 | 1,824 ± 204 |
| KJ(pRK415) | 9 ± 2.1 | 1,329 ± 150 |
| KJ(pRK-Z) | 8 ± 1.1 | 1,365 ± 143 |
| KJΔZ(pRK415) | 12 ± 1.3 | 402 ± 52 |
| KJΔZ(pRK-Z) | 10 ± 1.5 | 1,254 ± 142 |
| KJΔmrcA(pRK415) | 924 ± 112 | 2,135 ± 272 |
| KJΔmrcA(pRKDI) | 52 ± 10 | 789 ± 98 |
One unit of β-lactamase activity is defined as 1 nanomole of nitrocefin hydrolyzed per minute. The results are expressed as the mean ± standard deviation of three independent determinations.
Twenty micrograms per milliliter cefuroxime as the inducer, except for strains KJΔL1, KJΔL1ΔZ, KJΔL2, and KJΔL2ΔZ.
Twenty micrograms per milliliter cefoxitin as the inducer.
Twenty micrograms per milliliter aztreonam as the inducer.
Complementation assay.
A complementation assay was performed by introducing plasmid pRK-Z into the wild-type strain KJ and the mutant KJΔZ. Introduction of plasmid pRK-Z into the mutant KJΔZ can restore the induced β-lactamase activity to the levels of the wild-type strain (Table 2), which further confirms that NagZ is indeed a significant enzyme responsible for β-lactamase induction. However, Table 2 shows that strains KJ(pRK415) and KJ(pRK-Z) exhibited equivalent induced β-lactamase activities. The results indicate that the activity of the chromosomally encoded NagZ enzyme of S. maltophilia is potent enough to accommodate the processing of all ligand precursors and murein recycling, even under induced conditions. Therefore, nagZ did not display a dose effect on the induced β-lactamase activity.
Regulation of nagZ expression.
The regulation of nagZ expression was tested using a nagZp-xylE fusion plasmid, pRK188ZxylE. KJ(pRK188ZxylE) displayed a C23O activity of 133 ± 18 Uc/OD450, indicating the 188 bp inserted upstream of nagZ contains a functional promoter of nagZ. The C23O activity of KJ(pRK188ZxylE) was hardly changed upon the addition of β-lactam, demonstrating that nagZ gene expression is constitutive and β-lactam independent. To further investigate the effects of ampR, ampDI, ampNG, mrcA, and nagZ on nagZ expression, plasmid pRK188ZxylE was mobilized into KJΔR (17), KJΔDI (26), KJΔNG (17), KJΔmrcA (17), and KJΔZ, and the C23O activity in each pRK188ZxylE-containing strain was determined. Plasmid pRK188ZxylE displayed equivalent C23O activities in the wild-type, ΔampR, ΔampDI, ΔampNG, ΔmrcA, and ΔnagZ backgrounds under either basal or induced conditions. Accordingly, nagZ is constitutively expressed and not regulated by AmpR, AmpDI, AmpN, AmpG, and PBP1a or by the addition of β-lactam. Likewise, there was no autoregulation phenomenon in the expression of nagZ.
Role of nagZ in basal-level derepressed β-lactamase activity of mutants KJΔDI, KJΔmrcA, and KJΔDIΔmrcA.
Inactivating nagZ of P. aeruginosa notably reduces basal-level derepressed ampC expression (ampC expression in the absence of β-lactam challenge) in an ampD mutant (PAΔD) (1), a dacB mutant (PAΔdacB), and an ampD-dacB double mutant (PAΔDΔdacB) (27). The contribution of nagZ to basal-level derepressed β-lactamase activity in S. maltophilia is thus worthy of investigation. Three mutants with basal-level derepressed β-lactamase activity in S. maltophilia have been reported: KJΔDI (ampDI mutant) (26), KJΔmrcA (mrcA mutant) (17), and KJΔDIΔmrcA (17). A ΔnagZ allele was further introduced into KJΔDI, KJΔmrcA, and KJΔDIΔmrcA, yielding mutants KJΔDIΔZ, KJΔmrcAΔZ, and KJΔDIΔmrcAΔZ. Basal and cefuroxime-induced β-lactamase activities were analyzed comparatively in the paired strains KJΔDI-KJΔDIΔZ, KJΔmrcA-KJΔmrcAΔZ, and KJΔDIΔmrcA-KJΔDIΔmrcAΔZ.
In a ΔampDI background, nagZ inactivation nearly abolished basal-level derepressed β-lactamase activity (Table 2), indicating ΔampDI-derived basal-level derepressed β-lactamase activity is nagZ dependent. Still, there remained detectable β-lactamase activity in the cefuroxime-induced KJΔDIΔZ, i.e., approximately 12% of the induced β-lactamase activity of strain KJΔDI (Table 2). Surprisingly, the basal-level derepressed β-lactamase activity of KJΔmrcA was not affected by nagZ inactivation, whereas the cefuroxime-induced β-lactamase activity of KJΔmrcA decreased by about 40% owing to the introduction of ΔnagZ (Table 2). ΔnagZ had less effect on the KJΔDIΔmrcA double mutant than on the KJΔDI mutant but still reduced the basal (80%) and induced (70%) β-lactamase activity of KJΔDIΔmrcA (Table 2).
Role of nagZ in β-lactamase activities induced by different β-lactams.
It is generally recognized that different β-lactams display different binding affinities to different PBPs, which is linked to the inducibility of chromosomal β-lactamase genes. As shown above, 24% of the cefuroxime-induced β-lactamase activity of strain KJ is nagZ independent (Table 2). Whether the same is true for different β-lactams used as inducers is of interest. Carbenicillin (CAR), piperacillin (PIP), cefoxitin (FOX), and aztreonam (ATM) were the β-lactams selected as representatives of penicillin, cephalosporin, and monobactam, respectively. Table 3 demonstrates that inactivation nagZ did not significantly affect aztreonam-, cefoxitin-, and carbenicillin-induced β-lactamase activities. However, 76% and 52% of cefuroxime- and piperacillin-induced β-lactamase activities were abolished by nagZ inactivation (Table 3).
Table 3.
Induced β-lactamase activities of strains KJ and KJΔZ treated with various β-lactams
| Strain | Induced-β-lactamase sp acta (Un/mg) |
||||
|---|---|---|---|---|---|
| Aztreonam | Cefoxitin | Carbenicillin | Cefuroxime | Piperacillin | |
| KJ | 304 ± 42 | 793 ± 95 | 388 ± 40 | 1,868 ± 215 | 702 ± 84 |
| KJΔZ | 344 ± 51 | 818 ± 101 | 428 ± 52 | 448 ± 51 | 336 ± 46 |
One unit of β-lactamase activity is defined as 1 nanomole of nitrocefin hydrolyzed per minute. The results are expressed as the mean ± standard deviation of three independent determinations.
Role of nagZ in β-lactam susceptibility of mutants KJΔDI, KJΔmrcA, and KJΔDIΔmrcA.
To assess the effect of ΔnagZ on β-lactam resistance, a β-lactam susceptibility test was performed on all nagZ mutants and their parent strains (Table 4). Inactivation of nagZ of KJ and KJΔDI lowered the MICs of PIP and CXM but marginally decreased the MICs of CAR and FOX. The MICs of β-lactams tested for KJΔmrcAΔZ and KJΔDIΔmrcAΔZ were unchanged or marginally (2-fold) decreased compared to those of their parent strains, KJΔmrcA and KJΔDIΔmrcA, respectively.
Table 4.
MICs of β-lactam antibiotics for S. maltophilia KJ and its derived mutants
| Strain | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
| PIP | CAR | FOX | CXM | ATM | |
| KJ | 1,024 | 1,024 | 1,024 | 2,048 | >2,048 |
| KJΔZ | 64 | 512 | 512 | 512 | 2,048 |
| KJΔDI | 1,024 | 1,024 | 1,024 | 2,048 | >2,048 |
| KJΔDIΔZ | 256 | 512 | 512 | 512 | 2,048 |
| KJΔmrcA | 2,048 | 2,048 | 1,024 | 2,048 | >2,048 |
| KJΔmrcAΔZ | 1,024 | 1,024 | 1,024 | 1,024 | 2,048 |
| KJΔDIΔmrcA | 2,048 | 2,048 | 1,024 | 2,048 | >2,048 |
| KJΔDIΔmrcAΔZ | 1,024 | 2,048 | 1,024 | 2,048 | 2,048 |
Role of ampDI in ΔmrcA-derived basal-level β-lactamase derepression.
With NagZ and AmpD critical to ligand processing for ampC expression in the P. aeruginosa system, their roles in ΔmrcA- and ΔampDI-derived basal-level β-lactamase derepression are worthy of further study. To gain insight into the role of ampDI in ΔmrcA-derived basal-level β-lactamase derepression, an ampDI-containing plasmid, pRKDI (26), was mobilized into S. maltophilia KJΔmrcA via conjugation. Table 2 shows that introduction of pRKDI into KJΔmrcA attenuated the basal-level derepressed β-lactamase activity of KJΔmrcA. Therefore, ΔmrcA-derived basal-level β-lactamase derepression is nagZ independent and can be attenuated by ampDI overexpression.
DISCUSSION
A significant distinction between mutants KJΔmrcA and KJΔDI was observed in this study for the role of nagZ in basal-level derepressed β-lactamase activities (Table 2). Notably, nagZ inactivation significantly reduces the basal-level derepressed β-lactamase activities of KJΔDI, which agrees well with a study of P. aeruginosa (1). However, introduction of ΔnagZ into the KJΔmrcA mutant hardly influences the basal-level derepressed β-lactamase activities of KJΔmrcA (Table 2). Distinct outcomes indicate at least two different activator ligands responsible for L1/L2 expression in the S. maltophilia system. One is generated by the process of NagZ (as with KJΔDI), but the other is produced independently of NagZ (as with KJΔmrcA). For concise descriptions, we designated the former activator ligand 1 (AL1) and the latter AL2. Involvement of different activator ligands in chromosomal β-lactamase expression is not an exception. It has been proposed in the P. aeruginosa model that GlcNAc-1,6-anhydromuropeptide, generated by processing of NagZ, acts as an activator ligand for AmpC basal-level derepression in ampD and/or dacB mutants while an unidentified nagZ-independent activator ligand may participate in AmpC overexpression triggered by a classical AmpC inducer, i.e., cefoxitin (27).
Our previous studies have demonstrated that ΔmrcA- and ΔampDI-derived basal-level derepressed β-lactamase activities are ampNG permease dependent (17). Therefore, the precursors of AL1 and AL2 should be generated in the periplasm and then transported into the cytosol via the ampNG permease system. Based on similarity between AmpDI of S. maltophilia (26) and AmpD of P. aeruginosa (12, 13), The AL1 precursor generated in KJΔDI is likely the GlcNAc-1,6-anhydromuropeptides proposed in the P. aeruginosa model. In the case of mrcA inactivation, ΔmrcA-derived basal-level depressed β-lactamase activity is not affected by nagZ inactivation (Table 2, KJΔmrcA and KJΔmrcAΔZ). It can be reasonably speculated that a type of specific degraded murein fragment other than GlcNAc-1,6-anhydromuropeptide appears in KJΔmrcA. The specific degraded murein fragments generated in the periplasm can be AL2 precursors or AL2 itself. Although its structure is not known, AL2 should contain an amide bond that links anhydro-N-acetylmuramic acid and an oligopeptide of the murein subunit, since overproduced AmpDI can attenuate basal-level derepressed β-lactamase activities of KJΔmrcA (Table 2). Also, an intact amide bond in AL2 can be a critical structure for β-lactamase induction. It is worth mentioning that the GlcNAc-1,6-anhydromuropeptide proposed in Enterobacteriaceae and P. aeruginosa models is not a single entity, since it may be GlcNAc-1,6-anhydromuro-tripepetide, GlcNAc-1,6-anhydromuro-tetrapepetide, and GlcNAc-1,6-anhydromuro-pentapepetide. Therefore, it cannot be ruled out that the AL1 and AL2 proposed in this study might have a structure of anhydro-N-acetylmuramic acid with oligopeptides of different lengths (tri-, tetra-, or pentapeptides, for example).
Comparison of the induced β-lactamase activities of the wild-type KJ and of KJΔZ can elucidate the contribution of AL1 and AL2 to the induced β-lactamase activities, since NagZ dependence is a critical determinant to distinguish AL1 and AL2. If the induced β-lactamase activities of strains KJ and KJΔZ are equivalent, AL2 nearly accounts for the activator ligand responsible for the induction of β-lactamase. Inducers of aztreonam, cefoxitin, and carbenicillin are examples of this (Table 3). It can be inferred that aztreonam, cefoxitin, and carbenicillin may have significant affinity for PBP1a. However, AL1 and AL2 appear to be involved in the induced β-lactamase activities when cefuroxime and piperacillin are used as inducers (Table 3). In conclusion, the contributions of AL1 and AL2 to the induced β-lactamase activities may vary with the types of inducers (β-lactams).
Inactivation of nagZ restores the wild-type β-lactam MICs for PAO1 dacB and ampD mutants and dramatically reduces the MICs for the ampD-dacB double mutant in the P. aeruginosa system (27). In S. maltophilia, nagZ inactivation moderately attenuates the PIP and CXM resistances of the wild-type KJ and mutant KJΔDI (the MICs are reduced 4- to 16-fold) while it marginally reduces resistance to CAR and FOX, i.e., 2-fold, as shown in Table 4. The difference can be explained based on the results shown in Table 3. The activator ligands produced in the CAR- and FOX-treated bacteria are mainly non-NagZ processed (Table 3), which may reflect the marginal MIC changes of CAR and FOX in the paired strains KJ-KJΔZ and KJΔDI-KJΔDIΔZ. Both the NagZ-processed and non-NagZ-processed ligands are generated in PIP- and CXM-treated S. maltophilia; thus, the differences in the MICs of PIP and CXM are more significant in the paired strains KJ-KJΔZ and KJΔDI-KJΔDIΔZ. Nevertheless, nagZ inactivation does not significantly affect the β-lactam resistance of ΔmrcA-associated mutants (KJΔmrcA and KJΔDIΔmrcA), since the non-NagZ-processed ligand exists because of mrcA inactivation.
Several conclusions emerge. (i) When PBPs are not saturated with any β-lactam (the basal level of the wild type), the concentration of repressor ligands dominates over that of activator ligands (presumably AL1) in the cytosol. Therefore, the β-lactamase genes are repressed. (ii) Two kinds of activator ligands, NagZ processed (AL1) and non-NagZ processed (AL2), were observed with ΔampDI and ΔmrcA, respectively. AL1, likely the known activator ligand of 1,6-anhydromuropeptides of the Enterobacteriaceae model, is massively formed under the condition of ampDI inactivation, and such formation is NagZ dependent. AL2 is generated when mrcA is inactivated; formation of AL2 does not depend on NagZ, whereas AmpDI can process AL2 into a less potent form for β-lactamase induction. (iii) After adding β-lactam, the β-lactam results in saturation of certain PBPs, which depends on its affinity for different PBPs and retardation of peptidoglycan synthesis. Surplus degraded peptidoglycan fragments are transported into the cytosol by the AmpN/AmpG permease system and further processed into activator ligands for β-lactamase induction with the assistance of AmpR. The types of β-lactam and the amounts of PBPs bound by the added β-lactam determine the contributions of AL1 and AL2 to induced β-lactamase activities. Specifically, more AL2 ligand is generated when more PBP1a is bound. (iv) nagZ inactivation moderately decreases PIP and CXM MICs for the wild-type and the ampDI mutant whereas it has an insignificant effect on the CAR and FOX MICs for the wild type and the ampDI mutant and the PIP, CAR, FOX, and CXM MICs for the mrcA-associated mutants.
ACKNOWLEDGMENT
This research was supported by grant NSC 98-2320-B-039-011-MY3 from the National Science Council.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Asgarali A, Stubbs KA, Oliver A, Vocadlo DJ, Mark BL. 2009. Inactivation of the glycoside hydrolase NagZ attenuates antipseudomonal β-lactam resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:2274–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng Q, Li H, Merdek K, Park JT. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 182:4836–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing, 20th informational supplement. M100-S20 CLSI, Wayne, PA [Google Scholar]
- 4. Crossman LC, et al. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 9:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dietz H, Pfeifle D, Wiedemann B. 1996. Location of N-acetylmuramyl-L-alanyl-D-glutamylmesodiaminopimelic acid, presumed signal molecule for beta-lactamase induction in the bacterial cell. Antimicrob. Agents Chemother. 40:2173–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietz H, Pfeifle D, Wiedemann B. 1997. The signal molecule for beta-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob. Agents Chemother. 41:2113–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 8. Honore N, Nicolas MH, Cole ST. 1986. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J. 5:3709–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu RM, Huang KJ, Wu LT, Hsiao YJ, Yang TC. 2008. Induction of L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 52:1198–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang YW, et al. 2010. AmpN-AmpG operon is essential for expression of L1 and L2 beta-lactamases in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 54:2583–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 13:4684–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs C, et al. 1995. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-L-alanine amidase. Mol. Microbiol. 15:553–559 [DOI] [PubMed] [Google Scholar]
- 13. Juan C, Moya B, Perez JL, Oliver A. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high-level β-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keen NT, Tamaki S, Kobayashi D, Trollinger D. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191–197 [DOI] [PubMed] [Google Scholar]
- 15. Korfmann G, Sanders CC. 1989. ampG is essential for high-level expression of AmpC β-lactamases in Enterobacter cloacae. Antimicrob. Agents Chemother. 33:1946–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin CW, Chiou CS, Chang YC, Yang TC. 2008. Comparison of pulsed-field gel electrophoresis and three rep-PCR methods for evaluating the genetic relatedness of Stenotrophomonas maltophilia isolates. Lett. Appl. Microbiol. 47:393–398 [DOI] [PubMed] [Google Scholar]
- 17. Lin CW, Lin HC, Huang YW, Chung TC, Yang TC. 2011. Inactivation of mrcA gene derepresses the basal-level expression of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 66:2033–2037 [DOI] [PubMed] [Google Scholar]
- 18. Lin CW, Huang YW, Hu RM, Chiang KH, Yang TC. 2009. The role of AmpR in the regulation of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Res. Microbiol. 160:152–158 [DOI] [PubMed] [Google Scholar]
- 19. Lindquist S, et al. 1993. AmpG, a signal transducer in chromosomal β-lactamase induction. Mol. Microbiol. 9:703–715 [DOI] [PubMed] [Google Scholar]
- 20. Lodge J, Busby S, Piddock L. 1993. Investigation of the Pseudomonas aeruginosa ampR gene and its role at the chromosomal ampC beta-lactamase promoter. FEMS Microbiol. Lett. 111:315–320 [DOI] [PubMed] [Google Scholar]
- 21. Moya B, et al. 2009. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okazaki A, Avison MB. 2008. Induction of L1 and L2 beta-lactamase production in Stenotrophomonas maltophilia is dependent on an AmpR-type regulator. Antimicrob. Agents Chemother. 52:1525–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schweizer HP, Hoang TT. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15–22 [DOI] [PubMed] [Google Scholar]
- 24. Stubbs KA, Balcewich M, Mark BL, Vocadlo DJ. 2007. Small molecule inhibitors of a glycoside hydrolase attenuate inducible AmpC-mediated beta-lactam resistance. J. Biol. Chem. 282:21382–21391 [DOI] [PubMed] [Google Scholar]
- 25. Votsch W, Templin MF. 2000. Characterization of a beta-N-acetylglucosaminidase of Escherichia coli and elucidation of its role in muropeptide recycling and beta-lactamase induction. J. Biol. Chem. 275:39032–39038 [DOI] [PubMed] [Google Scholar]
- 26. Yang TC, Huang YW, Hu RM, Huang SC, Lin YT. 2009. AmpDI is involved in expression of the chromosomal L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 53:2902–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zamorano L, et al. 2010. NagZ inactivation prevents and reverts β-lactam resistance, driven by AmpD and PBP4 mutations, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:3557–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]