Abstract
Rhodococcus equi, the causal agent of rhodococcosis, is a severe pathogen of foals but also of immunodeficient humans, causing bronchopneumonia. The pathogen is often found together with Klebsiella pneumoniae or Streptococcus zooepidemicus in foals. Of great concern is the fact that some R. equi strains are already resistant to commonly used antibiotics. In the present study, we evaluated the in vitro potential of two equine antimicrobial peptides (AMPs), eCATH1 and DEFA1, as new drugs against R. equi and its associated pathogens. The peptides led to growth inhibition and death of R. equi and S. zooepidemicus at low micromolar concentrations. Moreover, eCATH1 was able to inhibit growth of K. pneumoniae. Both peptides caused rapid disruption of the R. equi membrane, leading to cell lysis. Interestingly, eCATH1 had a synergic effect together with rifampin. Furthermore, eCATH1 was not cytotoxic against mammalian cells at bacteriolytic concentrations and maintained its high killing activity even at physiological salt concentrations. Our data suggest that equine AMPs, especially eCATH1, may be promising candidates for alternative drugs to control R. equi in mono- and coinfections.
INTRODUCTION
Rhodococcus equi, previously known as Corynebacterium equi, is a facultative, intracellular, Gram-positive coccobacillus that causes infection in a wide variety of animal species and humans. Rhodococcosis occurs preferentially in organisms whose immune systems are compromised, either naturally or due to illness or medical treatment (21).
The infection is typically described in 1- to 6-month-old foals (40, 45) and is one of the most common causes of mortality at this age. The high susceptibility of foals to the pathogen is due to their immature immune system combined with the decrease in maternal specific antibodies transmitted through the colostrum (4). Foals are infected by R. equi while grazing or by inhalation of contaminated soil dust. The disease then occurs mainly as bronchopneumonia and less often as intestinal manifestations or septic arthritis and osteomyelitis (21, 49). The survival rate has increased dramatically since the introduction of a combination of rifampin and a macrolide such as erythromycin, or more recently azithromycin or clarithromycin for treatment (20, 25). These combinations are therefore considered to be the first-line treatment of foals for rhodococcosis. In France, a retrospective study performed on the necropsy samples of 1,617 foals between 1986 and 2006 showed that in 60% of rhodococcosis cases, R. equi was solely responsible for lethal lesions. In the other 40%, R. equi was found in coinfection with another pathogen, mainly Klebsiella pneumoniae in lung lesions (24.3%) and Streptococcus zooepidemicus (9.3%), which can sometimes cause the treatment to fail, due to the difference in antibiotic sensitivity (29, 31). Combinations of such pathogens have been described previously (29).
In humans, the infection is rare in immunocompetent patients (15, 27), but more than 300 cases have been reported in immunocompromised subjects (41, 46). Since the first human case report was described in 1967, reports of the infection have increased substantially, with more than 200 cases in the last 3 decades (23, 41, 46). The increase in cases appears to be correlated with the HIV pandemic and with expansions in transplantation medicine and cancer (44, 46). Infections in humans, especially immunocompromised patients, appear to be strongly associated with the farming environment. Contamination is likely to occur through the same route as in foals, via contaminated soil dust. However, no suggestive epidemiological exposure has been identified for half of the immunocompetent patients reported (46). The mortality rate among immunocompromised patients is relatively high, with a range of 20 to 25% among non-HIV-infected patients and 50 to 55% among HIV-infected patients (13, 24). This high mortality rate can be attributed to an incorrect or late diagnosis of the disease, as the bacterium may be mistaken for a contaminant diphtheroid or Mycobacterium species.
It is a cause for concern that the susceptibility of bacteria to rifampin and erythromycin tends to decrease over time. Buckley et al. (10) highlighted a 2-fold increase in MICs over a 10-year period. Moreover, the emergence of rhodococci resistant to these antibiotics has already been reported in humans and animals (2, 7, 10, 28). This suggests that there may be a problem in the treatment of R. equi infection, and new effective broad-spectrum drugs will be needed in the near future. Antimicrobial peptides (AMPs) have been attracting greater interest as new therapeutic molecules besides antibiotics, because of their broad spectrum of action and a lower risk of resistance acquisition (30). In general, AMPs are small cationic peptides participating in the innate immune response in almost all living organisms (including plants and invertebrates). Defensins and cathelicidins are the principal families in vertebrates. Defensins are characterized by intramolecular disulfide bonds and a β-sheet structure, while the predominant secondary structure of cathelicidins is α-helical (14). AMPs are active against Gram-negative and Gram-positive bacteria, viruses, fungi, and parasites. The principal mechanism of action of these peptides is attributed to membrane destabilization leading to cell lysis. Several AMPs are potential alternative antimicrobial drugs, and some have already undergone clinical trials (http://clinicaltrials.gov). To our knowledge, 30 mature antimicrobial peptides have already been identified in horses, but only a few of them have been characterized (8, 9, 35). Among them are the α-defensin DEFA1 and the cathelicidin eCATH1.
The aim of the present study was to evaluate the in vitro potential of these two equine antimicrobial peptides for the treatment of rhodococcosis and the effect on its associated pathogens.
MATERIALS AND METHODS
Antibiotics and antimicrobial peptides.
The antimicrobial peptide eCATH1 was chemically synthesized by GenScript USA Inc. (Piscataway, NJ) and dissolved in 10 mM acetic acid. DEFA1 was chemically synthesized (Biosyntan GmbH, Berlin, Germany), refolded as previously described by Jung et al. (26) for another synthetic peptide, and dissolved in 0.01% trifluoroacetic acid. Nisin (reference number [ref] N5764; Sigma-Aldrich, St. Louis, MO) was suspended in 20 mM HCl to obtain a 100-μg/ml stock solution. Erythromycin (ref E5389) and rifampin (ref R7382) were purchased from Sigma-Aldrich (St. Louis, MO), and stock solutions were freshly diluted in sterile water prior to each experiment.
CD spectroscopy and liposome preparation.
Circular dichroism (CD) measurements were performed on a Jasco J-720 spectropolarimeter (Japan Spectroscopic, Tokyo, Japan) using suitable quartz cuvettes (Helma GmbH, Germany) with different cell lengths. Each CD spectrum represents the mean of three scans at a bandwidth of 2 nm and a data pitch of 1 nm. The scanning speed was adjusted to 5 nm/min with a response time of 8 s. CD experiments were performed in the absence and presence of liposomes in order to compare the secondary structure of the peptides in aqueous and hydrophobic environments. Liposomes were prepared essentially as described by Pick et al. (34) using defined phospholipids and 50 mM sodium phosphate buffer, pH 5.2. Initially crude liposome samples were refined by passing them over a NAP-5 column (Amersham Biosciences). The eluate served as a stock suspension for the subsequent experiments and was stored at 4°C. The phospholipids purchased from Avanti Polar Lipids Inc. (Alabaster, AL) were l-α-phosphatidyl-dl-glycerol (PG) and l-α-phosphatidylcholine (PC). Due to the net negative charge at the membrane surface, PG liposomes serve as highly simplified models for bacterial membranes, whereas PC liposomes serve as corresponding models for eukaryotic membranes due to their electrostatic neutral surface.
Eight hundred microliters of the liposome stock, diluted 1:100 in 50 mM sodium phosphate buffer, pH 7.0, was applied to one chamber of a tandem quartz cuvette (2 × 4.375 mm). The other chamber was filled with the peptide sample. eCATH1 was applied in a 50 mM sodium phosphate buffer, pH 7.0, at a concentration of 9 μg/ml. DEFA1 was applied in a sodium phosphate buffer, pH 5.2, at a concentration of 17 μg/ml. After initial measurements of separated peptide and liposome samples, the tandem cuvette was inverted 40 times to mix the sample. The spectra of the mixed samples were recorded after 20 min of incubation.
Antimicrobial assays.
MICs of eCATH1 for the reference strains R. equi ATCC 33701 P+, S. zooepidemicus CIP 103228T, and K. pneumoniae CIP 82.91T were determined using the standard broth microdilution method outlined by the CLSI M07-A8 document (12) with modifications proposed by the Hancock laboratory to avoid AMP adsorption to the microplate (R. E. Hancock, unpublished data). One hundred microliters of bacterial suspension at 5 × 105 CFU/ml in Mueller-Hinton broth (MHB) was incubated on polypropylene Nunc microplates with 11 μl of serially diluted peptides in 10 mM acetic acid supplemented with 0.2% bovine serum albumin (BSA). BSA was used to avoid the adsorption of cationic peptides to the test microplate. Plates were sealed and incubated at 37°C for 24 to 48 h until visible growth. Cultures without the peptides were used as positive controls. Noninoculated MHB was used as a negative control. The MIC was defined as the lowest concentration of the peptide causing an 80% decrease in turbidity compared to the growth of a control well. The experiments were carried out in triplicate.
Salt tolerance.
The salt tolerance of eCATH1 was determined using the microdilution assay as described previously with minor modifications (26). The R. equi ATCC 33701 P+ suspension was adjusted to 104 to 105 bacteria per ml in 10 mM sodium phosphate, pH 7.2, supplemented with 1% brain heart infusion (BHI) broth and sodium chloride at final concentrations of 0, 50, 100, or 150 mM. Eleven-microliter portions of the eCATH1 dilutions (range of final concentrations tested: 0.22 to 35.5 μg/ml) were added to 100 μl of the bacterial suspension and incubated at 37°C for 2.5 h at 250 rpm before CFU were determined. The eCATH1 solvent (10 mM acetic acid) served as the negative control. The antibacterial activity of the peptide was given either as a 90% lethal dose (LD90) or as minimum bactericidal concentrations (MBCs) (99.9% killing). Each experiment was performed in triplicate.
SEM.
One milliliter of a mid-exponential-phase culture of R. equi ATCC 33701 P+ (optical density at 600 nm [OD600] = 0.5 to 0.6) was exposed to 100 μg/ml of eCATH1, DEFA1, or peptide diluents, as a control, in BHI broth for 5 min at 37°C. The cell/peptide ratio used for the scanning electron microscopy (SEM) assay was at least 20 times higher than in the conditions used to determine the MIC values of these peptides in order to observe the effect of the peptides on the bacterial membrane before their lysis. Bacteria were sedimented by centrifugation at 4,000 × g for 5 min and washed twice in phosphate-buffered saline (PBS), pH 7.2. The pellets were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, at 4°C overnight. Meanwhile, cells were dispersed and sedimented on Thermanox coverslips coated with poly-l-lysine. The cells were then rinsed in 0.2 M cacodylate buffer, pH 7.4, in the presence of 0.2 M sucrose and postfixed for 1 h with 1% osmium tetroxide in 0.1 M cacodylate buffer, pH 7.4, in the presence of 0.1 M sucrose (at 4°C protected from light). Bacteria were then washed in 0.2 M cacodylate buffer, pH 7.4, in the presence of 0.2 M sucrose and dehydrated in progressive baths of ethanol (70 to 100%). Samples were critical point dried (CPD 030; Leica Microsystems), sputtered with platinum (JFC 1300; Jeol), and observed with a Jeol 6400F scanning electron microscope at the Electron Microscopy Center of the University of Caen Basse-Normandie (CMABio, France).
Synergy study.
R. equi P103 P− was used to test the antimicrobial combinations by the checkerboard titration method using 96-well polypropylene microtiter plates. The experiment was performed in triplicate. Concentrations tested ranged from 0.031 to 2 times the MIC of each respective antimicrobial (eCATH1, erythromycin, and rifampin). The final inoculum was verified by counting. Positive (containing no antimicrobials) and negative growth controls were included. Microtiter plates were sealed and incubated at 37°C for 48 h. alamarBlue (Invitrogen, Cergy Pontoise, France), a colorimetric redox indicator without known effects on organism growth (3), was used for the reading of checkerboard plates. Growth was determined visually by observing the reduction in the colorimetric redox indicator (a color change from blue to purple or pink). The lowest fractional inhibitory concentration (FIC) index was calculated according to the following equation: FIC index = FICA + FICB = A/MICA + B/MICB, where A and B are the MICs of drug A and drug B in combination, MICA and MICB are the MICs of drug A and drug B alone, and FICA and FICB are the FICs of drug A and drug B. The FIC indexes were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifferent; and >4.0, antagonism (1). In some cases, the interpretation may vary (6); therefore, a second method (“two-well method”) was used for confirmation. The two-well method defined synergy as the absence of turbidity in the two wells containing 0.25× MIC of both drugs and 2× MIC of both drugs. Antagonism was defined as the presence of turbidity in both of these wells, whereas indifference was defined as all other possibilities (16).
Cytotoxicity.
The effects of eCATH1 and DEFA1 on plasma membrane integrity of RK13 CCL-87 and Vero CCL-81 cell lines were assessed by lactate dehydrogenase (LDH) release assay. Epithelial cell lines (ATCC) were grown according to the ATCC guidelines. The viability of test cells prior to cytotoxicity assays exceeded 99%, as determined by exclusion of the vital dye trypan blue. The LDH release assay was carried out according to the manufacturer's instructions using a commercially available kit (TOX-7; Sigma-Aldrich, Saint Louis, MO). Briefly, cells were incubated with serial dilutions (ranging from 0.31 to 100 μg/ml) of either eCATH1, DEFA1, nisin, or 1% (vol/vol) Triton X-100 (positive control) for 24 h. Nisin was used here as a “safe control” because of its generally recognized as safe (GRAS) status in the food industry. The percentage of cytotoxicity was calculated as described by Vaucher et al. (43). Each experiment was performed in triplicate, and values were expressed as mean ± standard error.
Hemolytic activity.
The hemolysis of fresh defibrinated sheep (bioMérieux, Marcy l'Etoile, France) and horse (collected from a healthy 8-year-old mare) erythrocytes was evaluated in triplicate using a hemoglobin release assay (36). Briefly, red blood cells were rinsed three times with PBS (pH 7.2) by centrifugation for 15 min at 800 × g and resuspended in PBS, pH 7.2, at a final concentration of 4% (vol/vol). Samples of 100 μl of suspension were transferred to a microplate and treated with eCATH1, DEFA1, nisin (safe control), or 1% (vol/vol) Triton X-100 (positive control) at 37°C for 1 h. After centrifugation at 1,000 × g for 5 min, supernatants were transferred to a clean microtiter plate where hemoglobin release was monitored by measuring the absorbance at 414 nm. The percentage of hemolysis was calculated as follows: (AT − AC)/(AX − AC) × 100, where AT is the experimental absorbance of treated supernatants, AC is the control absorbance of untreated cell supernatant, and AX is the absorbance of 1% (vol/vol) Triton X-100-lysed cells.
Resistance studies.
Ten microliters of R. equi P103 P− suspension was seeded from the well equal to one-half of the MIC to a fresh microtiter plate containing 75 μl of antimicrobial dilution and 65 μl of fresh MHB (48). Antimicrobial dilutions (of eCATH1, rifampin, or erythromycin) were double the desired concentration (0.25× MIC to 4× MIC of the agent) as a 2-fold dilution was applied in the microplate. Plates were sealed and incubated at 37°C. Due to the slow growth of R. equi, MICs were determined every 48 h for 50 passages (580 generations) or the selection of resistance for the antibiotics tested. Clones with a lower sensitivity to eCATH1 were frozen at −80°C until further analysis. Because the resistance and susceptibility MIC breakpoints for R. equi have not been defined, the strain was considered as resistant for rifampin at a MIC of >8 μg/ml and resistant for erythromycin at a MIC of >4 μg/ml. To verify the stability of resistance to eCATH1, frozen clones with a lower sensitivity to the peptide were transferred one to four times on unsupplemented agar medium before MIC measurement, as described in “Antimicrobial assays” above.
RESULTS
CD spectroscopy.
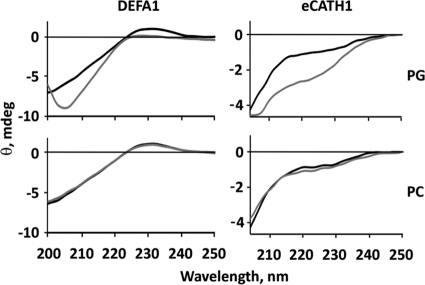
In the absence of liposomes, DEFA1 had a circular dichroism (CD) spectrum whose maximum was at 230 nm, which is typical for proteins with a β-sheet structure (Fig. 1). After mixing DEFA1 with PG liposomes, precipitates were formed leading to strong light scattering. This effect hindered the interpretation of the secondary structure content of DEFA1 by CD spectroscopy. In contrast, no precipitation was observed after mixing DEFA1 with PC liposomes. The CD spectra of DEFA1 had the same shape before and after mixing with PC liposomes, indicating a constant secondary structure content.
Fig 1.
Impact of phospholipid membranes on the secondary structure of DEFA1 and eCATH1. The secondary structures of both DEFA1 (left) and eCATH1 (right) were investigated by CD spectroscopy in the absence (black lines) and presence (gray lines) of liposomes composed of negatively charged (PG, top) or electrostatically neutral (PC, bottom) phospholipids.
Regarding eCATH1, the CD spectra exhibited the typical shape of a linear peptide in the absence of liposomes (Fig. 1). After mixing eCATH1 with PC liposomes, there was no detectable change of secondary structure composition, comparable to DEFA1, but there was a change after eCATH1 mixing with PG liposomes. Interaction of the peptide with the liposomal membranes changed the secondary structure from linear to an α-helical character with the typical minimum at 222 and 208 nm.
Antimicrobial activity.
The antibacterial activity of eCATH1 and DEFA1 on reference strains of R. equi (ATCC 33701 P+ and P103 P−), K. pneumoniae (CIP 82.91T), and S. zooepidemicus (CIP 103228T) is presented in Table 1. Equine CATH1 inhibited the growth of the Gram-positive bacteria R. equi (3.5 μg/ml, 1.1 μM) and S. zooepidemicus (7.1 μg/ml, 2.3 μM) and was also effective against the Gram-negative bacterium K. pneumoniae (7.1 μg/ml, 2.3 μM) at low micromolar concentrations. Similar results were obtained for DEFA1 with the Gram-positive strains: R. equi ATCC 33701 P+ and S. zooepidemicus were inhibited by 10 μg/ml (2.45 μM) and 5 μg/ml (1.22 μM) of peptide, respectively. However, K. pneumoniae was not sensitive to DEFA1, as no growth inhibition was observed at the maximal concentration (40 μg/ml; 9.81 μM) tested.
Table 1.
MICs of DEFA1 and eCATH1 in R. equi, K. pneumoniae, and S. zooepidemicus reference strains
| Microorganism | MIC (μg/ml) |
|
|---|---|---|
| DEFA1 | eCATH1 | |
| Rhodococcus equi ATCC 33701 P+ | 10 | 3.5 |
| Rhodococcus equi P103 P− | NDa | 3.5 |
| Klebsiella pneumoniae CIP 82.91T | >40 | 7.1 |
| Streptococcus zooepidemicus CIP 103228T | 5 | 7.1 |
ND, not determined.
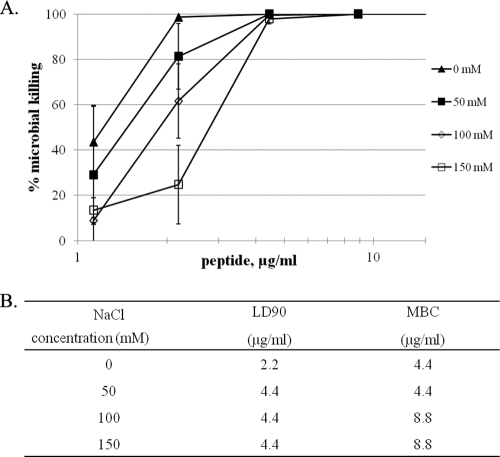
In the absence of sodium chloride, eCATH1 was able to kill 90% of R. equi ATCC 33701 P+ at 2.2 μg/ml (LD90) and 99.9% (MBC) at 4.4 μg/ml (Fig. 2). The MBC and MIC values were similar, indicating that eCATH1 has a bactericidal rather than bacteriostatic effect on R. equi. In the presence of 50 mM sodium chloride, the LD90 was twice as high but the MBC remained at 4.4 μg/ml. At a physiological salt concentration (150 mM), eCATH1 maintained its relatively high killing activity, since the MBC was increased only by a factor of two (8.8 μg/ml) compared with the no-salt conditions (Fig. 2).
Fig 2.
Salt dependence of the antimicrobial activity of eCATH1. The salt tolerance was tested against R. equi ATCC 33701 P+ by measuring the MBCs in the presence of 0, 50, 100, and 150 mM sodium chloride. (A) Mortality curves: values are expressed as the mean of three independent experiments ± standard error. (B) LD90s and MBCs in the absence or presence of salt.
The antibacterial activity of eCATH1 in combination with DEFA1 or an antibiotic (rifampin, erythromycin) against R. equi P103 P− is shown in Table 2. Interestingly, there was synergy when eCATH1 was combined with the RNA polymerase inhibitor rifampin (FIC < 0.5). However, this peptide was indifferent in combination with DEFA1 or erythromycin (0.5 < FIC ≥ 4). All these data were confirmed by the two-well method (data not shown).
Table 2.
Antibacterial interaction of eCATH1 with DEFA1, rifampin, or erythromycin in R. equi P103 P−
| Drug combination | FICa | Interaction |
|---|---|---|
| DEFA1-eCATH1 | 0.53 | Indifferent |
| Rifampin-eCATH1 | 0.49 | Synergy |
| Erytromycin-eCATH1 | 1.25 | Indifferent |
FIC indices indicate synergy when the value is <0.5 and antagonism when the value is >4.
SEM.
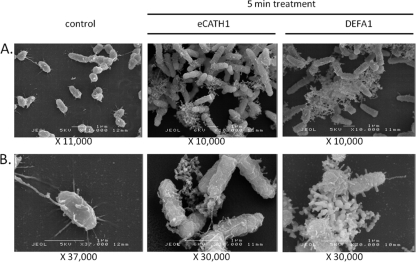
SEM was used to examine the effect of eCATH1 and DEFA1 on the morphology of R. equi ATCC 33701 P+. In the negative control, the bacterial cell membrane was intact, with a mucoid aspect, and small filopodium-like structures were observed. A similar morphology has also been described based on micrographs published for another reference strain of R. equi (39). Cellular fragments were absent in the control sample. Treatment of bacteria with 100 μg/ml of DEFA1 or eCATH1 appeared to result in membrane destabilization already after 5 min of incubation. Both peptides clearly led to altered membrane morphology. Moreover, numerous cellular fragments could be observed after treatment, indicating cellular lysis (Fig. 3).
Fig 3.
Scanning electron micrographs of R. equi ATCC 33701 P+ treated with DEFA1 or eCATH1. R. equi in mid-logarithmic-phase growth was incubated with 100 μg/ml of antimicrobial peptide eCATH1 or DEFA1 or with the peptide solvent (as a negative control) for 5 min. Observation at low (A) and high (B) magnifications.
Cytotoxicity of the antimicrobial peptides.
The cytotoxicity of eCATH1 and DEFA1 was assessed by the LDH release assay using RK13 and Vero epithelial cells and a maximum of 100 μg/ml of peptide (Fig. 4). For both cell lines, eCATH1 did not significantly affect the plasma membrane integrity of cells. Compared to the negative control, no cytotoxicity was observed up to 50 μg/ml, and only minor cytotoxicity (10%) was observed for the Vero cell line at 100 μg/ml (Fig. 4A). The RK13 cells were unaffected by 100 μg/ml of eCATH1 (Fig. 4B). In contrast, DEFA1 was found to be more cytotoxic, with a substantial effect on Vero cells at 50 μg/ml. At 100 μg/ml of DEFA1, the membrane integrity of RK13 and Vero cells was strongly affected (Fig. 4A and B). In addition, eCATH1 and DEFA1 were tested for hemolytic activity against sheep and horse erythrocytes. For both peptides, no significant hemolytic activity (<3%) was observed up to 100 μg/ml (data not shown).
Fig 4.
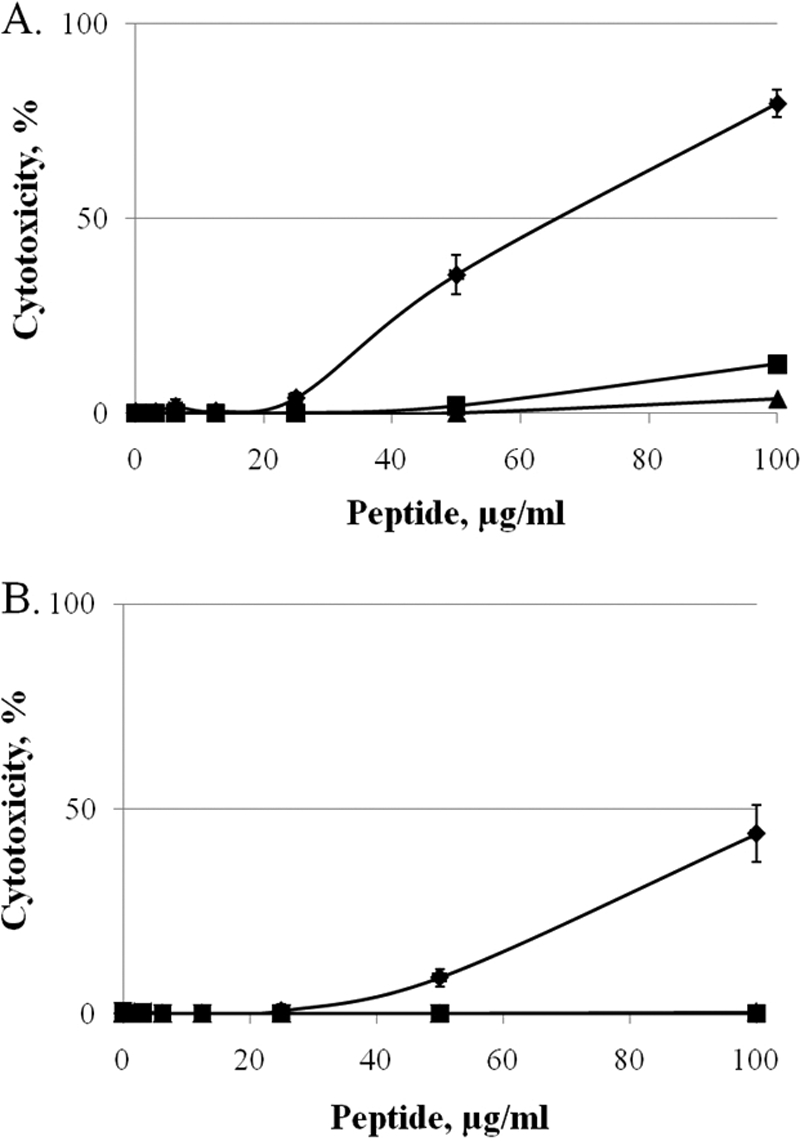
Cytotoxic activity of DEFA1, eCATH1, and nisin. (A and B) Vero CCL-81 (A) and RK13 (B) cells were incubated with increasing amounts of DEFA1 (♦), eCATH1 (■), and nisin as a safe control (▲). Cytotoxicity was measured spectrophotometrically using the LDH release assay.
Resistance study.
When cultures of R. equi P103 P− were serially transferred 50 times in the presence of subinhibitory concentrations, the final MIC of eCATH1 was slightly higher than with the value determined initially. The MIC of the peptide increased 2-fold after 34 passages (400 generations) and then by 4-fold after 43 passages (500 generations) and remained stable until the end of the experiment (50 passages). In comparison, resistance to rifampin or erythromycin appeared more quickly, after 4 passages (45 generations) or 10 passages (115 generations), respectively. The lower sensitivity of the strain to eCATH1 appeared to be only transient, since a single passage in a peptide-free medium led to the reversion to the MIC of the parent strain (data not shown).
DISCUSSION
Giacometti et al. (17–19) analyzed the activity of several AMPs as well as different conventional antibiotics in R. equi. MICs of these peptides were comparable to or higher than those of DEFA1 and eCATH1. Moreover, eCATH1 had bactericidal activity similar to that of vancomycin (median MBCs, 4 μg/ml), used to treat rhodococcosis in humans, and a higher activity than rifampin and clarithromycin (median MBCs, 32 μg/ml), commonly used to treat rhodococcosis in foals (17–19). As DEFA1 was more or less ineffective against the rhodococcosis-associated pathogen K. pneumoniae, eCATH1 appears to be more suitable in terms of therapeutic usage. Therefore, we investigated its tolerance at physiological salt concentrations. The activities of several AMPs differ greatly in the presence or absence of salt (22, 42); however, eCATH1 was still able to kill R. equi at low micromolar concentrations (also at higher salt concentrations) despite a slight decrease in bactericidal activity at physiological sodium chloride concentrations. Moreover, we observed that the MBC of eCATH1 against R. equi at salt concentrations of 150 mM was more than 10 times lower than the cytotoxic concentration for epithelial cells. In addition, eCATH1 did not harm erythrocytes, making this peptide more useful for therapy. These data are consistent with the findings of Skerlavaj et al. (37), who also observed the salt tolerance of eCATH1 and the lack of cytotoxicity against human and horse erythrocytes.
Bell and Gouyon (5) do not advocate the use of antimicrobial peptides to treat human or animal diseases because they may increase host sensitivity to infections. Although resistance mechanisms to AMPs have been described in different bacterial species (32), naturally sensitive strains are unlikely to acquire stable resistance because AMPs can interact with membranes without a specific target. Therefore, resistance involves biochemical modifications to the entire membrane, incurring metabolic costs that may be too high to maintain over several generations (47, 48). Only a few studies have been performed in vitro on the experimental evolution of resistance among a continuous exposition of antimicrobial peptide. Perron et al. (33) exposed different strains of Escherichia coli and Pseudomonas fluorescens to increasing concentrations of pexiganan, a synthetic analog of magainin. Most of the strains developed stable resistance after 80 passages. In two other studies, the decrease in sensitivity of bacteria to antimicrobial peptides, when detectable, was found to be modest, took much longer to select in comparison to conventional antibiotics, and was transient (30, 38, 48). Our data are consistent with these studies as only a modest decrease in sensitivity (4-fold) was observed after 50 passages (580 generations). In contrast, when we tested conventional antibiotics under the same selection conditions it became evident that mutants resistant to erythromycin and rifampin appeared after only 11 and 4 passages, respectively. Moreover, the lower sensitivity of the strain to eCATH1 was reversed by a single passage in a peptide-free medium. Although our in vitro study showed that the acquisition of stable resistance to eCATH1 by R. equi is unlikely to occur, in a therapeutic setting any new antieffective has to be carefully monitored again in the in vivo situation.
Interestingly, a synergistic effect was observed between eCATH1 and the RNA polymerase inhibitor rifampin. Previous studies have already reported such interactions between AMPs and hydrophobic antibiotics (reviewed in reference 11), and it was hypothesized that AMPs allow the antibiotic to access its intracellular target by permeabilization of the bacterial membrane. Therefore, AMPs represent a way of enhancing the activity of classical antibiotics, but even more they could potentially tackle the problem of the rise in multidrug-resistant pathogens.
Both DEFA1 and eCATH1 interacted with liposomal membranes that have a net negative surface charge. In contrast, there was no interaction with liposomes with a neutral surface. Furthermore, the tested liposomes were free of any membrane proteins. Therefore, the electrostatic attraction was apparently sufficient and probably solely responsible for mediating the initial interaction of DEFA1 and eCATH1 with their target membranes. Whereas DEFA1 already had a β-sheet secondary structure character in an aqueous environment, eCATH1 was linear. CD measurement results clearly showed that eCATH1 adopted an α-helical fold upon interaction with the liposomal membranes. Together with the absence of a precipitation effect as observed for DEFA1, both peptides might exert different mechanisms. However, according to SEM, the comparable killing effects of both peptides appeared to be mediated through a rapid membrane disruption of the bacteria. Further studies are necessary to clarify the interplay of structure and activity/cytotoxicity that might also explain the different MIC values and cytotoxicities of both peptides.
In conclusion, the in vitro therapeutic potential of equine DEFA1 for rhodococcosis treatment was found to be lower than envisaged mainly due to its cytotoxicity but also due to its inefficacy in killing K. pneumoniae. In contrast, eCATH1 proved to be a promising candidate for the development of a suitable drug in the fight against rhodococcosis in mono- and coinfections and warrants further research in view of its in vivo potential in human or veterinary medicine. Furthermore, among the other equine peptides that have not yet been expansively studied in vitro, there might be additional promising candidates for the treatment of rhodococcosis and other infectious diseases in humans and animals.
ACKNOWLEDGMENTS
This work was supported by grants from the European Regional Development Fund, the Région Basse-Normandie, the Institut Français du Cheval, and the French Agency for Food, Environmental and Occupational Health Safety.
We are grateful to Séverine Cauchard and Laurent Hébert.
Footnotes
Published ahead of print 9 January 2012
REFERENCES
- 1. Anonymous. 1992. Synergism testing: broth microdilution checkerboard and broth macrodilution methods. American Society for Microbiology, Washington, DC [Google Scholar]
- 2. Asoh N, et al. 2003. Emergence of rifampin-resistant Rhodococcus equi with several types of mutations in the rpoB gene among AIDS patients in northern Thailand. J. Clin. Microbiol. 41:2337–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker CN, Banerjee SN, Tenover FC. 1994. Evaluation of Alamar colorimetric MIC method for antimicrobial susceptibility testing of gram-negative bacteria. J. Clin. Microbiol. 32:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barton MD, Hughes KL. 1980. Corynebacterium equi: a review. 50:60–80 [Google Scholar]
- 5. Bell G, Gouyon PH. 2003. Arming the enemy: the evolution of resistance to self-proteins. Microbiology 149:1367–1375 [DOI] [PubMed] [Google Scholar]
- 6. Bonapace CR, Bosso JA, Friedrich LV, White RL. 2002. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 44:363–366 [DOI] [PubMed] [Google Scholar]
- 7. Boyen F, Pasmans F, Haesebrouck F. 2011. Acquired antimicrobial resistance in equine Rhodococcus equi isolates. Vet. Rec. 168:101. [DOI] [PubMed] [Google Scholar]
- 8. Bruhn O, Grötzinger J, Cascorbi I, Jung S. 2011. Antimicrobial peptides and proteins of the horse - insights into a well-armed organism. Vet. Res. 42:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruhn O, et al. 2007. A novel horse alpha-defensin: gene transcription, recombinant expression and characterization of the structure and function. Biochem. J. 407:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckley T, McManamon E, Stanbridge S. 2007. Resistance studies of erythromycin and rifampin for Rhodococcus equi over a 10-year period. Irish Vet. J. 60:728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cassone M, Otvos L., Jr 2010. Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev. Anti Infect. Ther. 8:703–716 [DOI] [PubMed] [Google Scholar]
- 12. CLSI 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—eighth edition, M07-A8 CLSI, Wayne, PA [Google Scholar]
- 13. Cornish N, Washington JA. 1999. Rhodococcus equi infections: clinical features and laboratory diagnosis. Curr. Clin. Top. Infect. Dis. 19:198–215 [PubMed] [Google Scholar]
- 14. De Smet K, Contreras R. 2005. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett. 27:1337–1347 [DOI] [PubMed] [Google Scholar]
- 15. Devi P, Malhotra S, Chadha A. 2011. Bacteremia due to Rhodococcus equi in an immunocompetent infant. Indian J. Med. Microbiol. 29:65–68 [DOI] [PubMed] [Google Scholar]
- 16. Eliopoulos GM, Moellering RC. 1996. Antimicrobial combinations, p 330–396 In Lorian V. (ed), Antibiotics in laboratory medicine, 4th ed The Williams & Wilkins Co., Baltimore, MD [Google Scholar]
- 17. Giacometti A, Cirioni O, Barchiesi F, Fortuna M, Scalise G. 1999. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 44:641–645 [DOI] [PubMed] [Google Scholar]
- 18. Giacometti A, et al. 2005. In vitro activity of citropin 1.1 alone and in combination with clinically used antimicrobial agents against Rhodococcus equi. J. Antimicrob. Chemother. 56:410–412 [DOI] [PubMed] [Google Scholar]
- 19. Giacometti A, et al. 2005. In vitro activity and killing effect of uperin 3.6 against gram-positive cocci isolated from immunocompromised patients. Antimicrob. Agents Chemother. 49:3933–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giguere S, et al. 2004. Retrospective comparison of azithromycin, clarithromycin, and erythromycin for the treatment of foals with Rhodococcus equi pneumonia. J. Vet. Intern. Med. 18:568–573 [DOI] [PubMed] [Google Scholar]
- 21. Giguere S, Prescott JF. 1997. Clinical manifestations, diagnosis, treatment, and prevention of Rhodococcus equi infections in foals. Vet. Microbiol. 56:313–334 [DOI] [PubMed] [Google Scholar]
- 22. Goldman MJ, et al. 1997. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553–560 [DOI] [PubMed] [Google Scholar]
- 23. Golub B, Spink FGWW. 1967. Lung abscess due to Corynebacterium equi. Report of first human infection. Ann. Intern. Med. 66:1174–1177 [DOI] [PubMed] [Google Scholar]
- 24. Harvey RL, Sunstrum JC. 1991. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev. Infect. Dis. 13:139–145 [DOI] [PubMed] [Google Scholar]
- 25. Hillidge CJ. 1987. Use of erythromycin-rifampin combination in treatment of Rhodococcus equi pneumonia. Vet. Microbiol. 14:337–342 [DOI] [PubMed] [Google Scholar]
- 26. Jung S, et al. 2011. Human beta-defensin 2 and beta-defensin 3 chimeric peptides reveal the structural basis of the pathogen specificity of their parent molecules. Antimicrob. Agents Chemother. 55:954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kedlaya I, Ing MB, Wong SS. 2001. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin. Infect. Dis. 32:E39–E46 [DOI] [PubMed] [Google Scholar]
- 28. Kenney DG, Robbins SC, Prescott JF, Kaushik A, Baird JD. 1994. Development of reactive arthritis and resistance to erythromycin and rifampin in a foal during treatment for Rhodococcus equi pneumonia. Equine Vet. J. 26:246–248 [DOI] [PubMed] [Google Scholar]
- 29. Lavoie JP, Fiset L, Laverty S. 1994. Review of 40 cases of lung abscesses in foals and adult horses. Equine Vet. J. 26:348–352 [DOI] [PubMed] [Google Scholar]
- 30. Marr AK, Gooderham WJ, Hancock RE. 2006. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 6:468–472 [DOI] [PubMed] [Google Scholar]
- 31. Mauger C. 2009. D.V.M. thesis Retrospective study of equine rhodococcosis observed at autopsy on 1617 foals at the “LERPE” (AFSSA, Dozulé) from 1986 to 2006. Ecole Nationale Vétérinaire de Toulouse, Toulouse, France: (In French.) [Google Scholar]
- 32. Nizet V. 2006. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 8:11–26 [PubMed] [Google Scholar]
- 33. Perron GG, Zasloff M, Bell G. 2006. Experimental evolution of resistance to an antimicrobial peptide. Proc. Biol. Sci. 273:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pick U. 1981. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch. Biochem. Biophys. 212:186–194 [DOI] [PubMed] [Google Scholar]
- 35. Scocchi M, et al. 1999. Novel cathelicidins in horse leukocytes(1). FEBS Lett. 457:459–464 [DOI] [PubMed] [Google Scholar]
- 36. Shin SY, et al. 2001. Antibacterial, antitumor and hemolytic activities of alpha-helical antibiotic peptide, P18 and its analogs. J. Pept. Res. 58:504–514 [DOI] [PubMed] [Google Scholar]
- 37. Skerlavaj B, Scocchi M, Gennaro R, Risso A, Zanetti M. 2001. Structural and functional analysis of horse cathelicidin peptides. Antimicrob. Agents Chemother. 45:715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinberg DA, et al. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sydor T, et al. 2008. A mycolyl transferase mutant of Rhodococcus equi lacking capsule integrity is fully virulent. Vet. Microbiol. 128:327–341 [DOI] [PubMed] [Google Scholar]
- 40. Takai S, Sasaki Y, Tsubaki S. 1995. Rhodococcus equi infection in foals: current concepts and implication for future research. J. Equine Sci. 6:105–119 [Google Scholar]
- 41. Topino S, Galati V, Grilli E, Petrosillo N. 2010. Rhodococcus equi infection in HIV-infected individuals: case reports and review of the literature. AIDS Patient Care STDS 24:211–222 [DOI] [PubMed] [Google Scholar]
- 42. Travis SM, et al. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vaucher RA, da Motta Ade S, Brandelli A. 2010. Evaluation of the in vitro cytotoxicity of the antimicrobial peptide P34. Cell Biol. Int. 34:317–323 [DOI] [PubMed] [Google Scholar]
- 44. Weinstock DM, Brown AE. 2002. Rhodococcus equi: an emerging pathogen. Clin. Infect. Dis. 34:1379–1385 [DOI] [PubMed] [Google Scholar]
- 45. Yager JA. 1987. The pathogenesis of Rhodococcus equi pneumonia in foals. Vet. Microbiol. 14:225–232 [DOI] [PubMed] [Google Scholar]
- 46. Yamshchikov AV, Schuetz A, Lyon GM. 2010. Rhodococcus equi infection. Lancet Infect. Dis. 10:350–359 [DOI] [PubMed] [Google Scholar]
- 47. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]
- 48. Zhang L, et al. 2005. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 49:2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zink MC, Yager JA, Smart NL. 1985. Corynebacterium equi infection in horses, 1958-1984: a review of 131 cases. Am. J. Vet. Res. 46:2171–2174 [PMC free article] [PubMed] [Google Scholar]