Abstract
In response to global concerns over the spread of the New Delhi metallo-β-lactamase gene 1, blaNDM-1, a monthly surveillance program was initiated in September 2010. All carbapenem-resistant Gram-negative strains forwarded to our facility are screened for this gene. To date, 321 carbapenem-resistant isolates, encompassing 11 bacterial species, have been tested. In February 2011, two strains of Providencia stuartii, submitted from a military hospital in Afghanistan, tested positive for blaNDM-1. Both strains were identical by pulsed-field gel electrophoresis (PFGE). blaNDM-1 was carried on a large plasmid, pMR0211, which was sequenced by emulsion PCR and pyrosequencing. pMR0211 is 178,277 bp in size and belongs to incompatibility group A/C. The plasmid consists of a backbone with considerable homology to pAR060302 from Escherichia coli, and it retains many of the antibiotic resistance genes associated with it. The plasmid also shares common elements with the pNDM-HK plasmid, including blaNDM-1, armA, and sul1. However, gene orientation is reversed, and a 3-kb fragment from this region is absent from pMR0211. pMR0211 also contains additional genes, including the aminoglycoside-modifying enzyme loci aadA and aac(6′), the quinolone resistance gene qnrA, a gene with highest homology to a U32 family peptidase from Shewanella amazonensis, and the blaOXA-10 gene. The finding of this gene in an intrinsically colistin-resistant species such as Providencia stuartii is especially worrisome, as it renders the organism resistant to nearly every available antibiotic. The presence of multiple insertion sequences and transposons flanking the region containing the blaNDM-1 gene further highlights the potential mobility associated with this gene.
INTRODUCTION
During the past 2 decades, metallo-β-lactamases have risen from relative obscurity to their current status at the forefront of antibiotic resistance research (8). Potent carbapenemase activity and resistance to clinical β-lactamase inhibitors, combined with increasing association with highly mobile genetic elements (8, 13, 22), makes them one of the most serious challenges facing infection control programs in recent years (17, 19, 23).
The gene of New Delhi metallo-β-lactamase 1 (blaNDM-1) constitutes a case study in the epidemiology of metallo-β-lactamase transmission. Since its initial identification in 2008 (25), blaNDM-1 has been identified in many countries (1, 11, 22), and the global distribution of this gene is imminent (20). blaNDM-1 is found in an ever-widening number of bacterial species and is strongly associated with large, highly mobile plasmids that carry numerous other antibiotic resistance genes (22, 25). Like the VIM-type metallo-β-lactamases, NDM-1 confers resistance to all β-lactam agents except the monobactams, but mobile DNA elements associated with blaNDM-1 transmission routinely carry numerous other antibiotic resistance genes, including those encoding resistance to monobactams (12, 13, 22, 23).
With increasing surveillance for blaNDM-1, a concomitant increase in the variety of plasmids associated with its transmission has been reported (12, 13, 22, 23). Plasmid sizes vary considerably, ranging from 50 to >400 kb (23), but some common associations have been noted. For example, Walsh and coworkers identified 12 plasmids from 20 bacterial species in an environmental survey of New Delhi, and all five of the Enterobacteriaceae in that study carried blaNDM-1 on a 140-kb plasmid of the incompatibility group A/C (IncA/C) type (23). In contrast, blaNDM-1 was found on an 89-kb plasmid of incompatibility group IncL/M from a strain of Escherichia coli recovered in Hong Kong (12).
In September 2010, a high-throughput real-time PCR assay for detecting blaNDM-1 was developed, and monthly screening of carbapenem-resistant clinical isolates from within the Military Health System was implemented at the Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research (15, 16). To date, 13 hospitals, including 5 in war zones, submit an average of 550 isolates each month, including carbapenem-resistant Gram-negative organisms. In February 2011, a strain of Providencia stuartii submitted from a military hospital in Afghanistan tested positive for blaNDM-1. Sequencing revealed 100% homology to the archetypal blaNDM-1 gene from Klebsiella pneumoniae (13), and plasmid analyses indicated that the gene was present on a large plasmid termed pMR0211.
In this report, we describe the structure of pMR0211, a 178-kb plasmid carrying the blaNDM-1 gene. The plasmid shares considerable homology to plasmid pAR060302 from E. coli (4), as well as to genes previously described in pNDM-HK, including blaNDM-1, armA, and sul1 (12). The plasmid also contains additional open reading frames, including the aminoglycoside-modifying enzyme loci aadA and aac(6′), the quinolone resistance gene qnrA, a U32 family peptidase gene, and the blaOXA-10 gene.
MATERIALS AND METHODS
Case report.
On 4 March 2011, two isolates of Providencia stuartii obtained on 26 January from the blood of a burn patient in a U.S./Coalition medical facility in Afghanistan were found to harbor the blaNDM-1 gene by real-time PCR as part of an ongoing monthly surveillance for this gene. The patient was an Afghan national who was transferred from a local national facility in Kabul 5 days after injury to the intensive care unit 25 km north of Kabul for the treatment of severe burns and inhalational injury following a gas explosion. The patient received unspecified, broad-spectrum antibiotics at the local national hospital, which were changed to levofloxacin, piperacillin-tazobactam, and vancomycin upon arrival at the U.S./Coalition medical facility. No history of prior illness or travel exposures was obtained. The patient was coinfected with a carbapenem-resistant, blaNDM-1-negative Pseudomonas aeruginosa strain as well as a less resistant P. aeruginosa strain and Proteus mirabilis. Computed tomography imaging of the brain showed global effacement and herniation. The patient died from complications due to severe injuries and central nervous system infections 12 days after injury.
Surveillance isolates.
MRSN participants submitted an average of 26 carbapenem-resistant Gram-negative clinical isolates each month since September 2010. Resistance to carbapenems in Enterobacteriaceae is defined according to the updated Clinical and Laboratory Standards Institute guidelines (7) (for imipenem, ≤1 μg ml−1 is sensitive and ≥4 μg ml−1 is resistant; for meropenem, ≤1 μg ml−1 is sensitive and ≥4 μg ml−1 is resistant; for ertapenem, ≤0.25 μg ml−1 is sensitive and ≥1 μg ml−1 is resistant; and for doripenem, ≤1 μg ml−1 is sensitive and ≥4 μg ml−1 is resistant). Identification and antibiotic susceptibilities of isolates were confirmed at the central facility using three commercially available platforms: the Vitek II (bioMérieux, Inc., NC), the BD Phoenix automated microbiology system (Becton Dickinson, NJ), and the Microscan WalkAway (Siemens, PA).
To date (May 2011), 213 carbapenem-resistant isolates have been submitted through the surveillance initiative for blaNDM-1 testing. Isolates were from blood, surveillance, respiratory, urine, and wound cultures from health care facilities in Washington, DC, Maryland, Virginia, North Carolina, Hawaii, Iraq, and Afghanistan. Acinetobacter baumannii and P. aeruginosa were the predominant species (52.1 and 38%, respectively). Other species included Aeromonas veronii (0.9%), Burkholderia cepacia (0.5%), Citrobacter freundii (0.5%), Enterobacter cloacae (0.9%), E. coli (2.8%), K. pneumoniae (2.3%), Pseudomonas stutzeri (0.5%), P. stuartii (0.9%), and Serratia marcescens (0.5%).
Processing of bacterial samples.
All bacterial isolates were cultured overnight on blood agar, and single colonies were resuspended in 200 μl of sterile phosphate-buffered saline (PBS). Ten-μl aliquots were treated with 20 μl Lyse-and-Go PCR reagent in a 96-well plate format per the manufacturer's instructions for the isolation of total DNA (Thermo Fisher Scientific, MA). Two-μl aliquots of the resulting lysate were used directly for real-time PCR and sequencing.
Real-time PCR assay for the detection of blaNDM-1.
Real-time PCR primers targeting the blaNDM-1 gene were designed and validated using MIQE (for minimum information for the publication of quantitative real-time PCR experiments) guidelines (3) with Primer Express 2.0 (Applied Biosystems, CA) from the blaNDM-1 sequence deposited at GenBank under accession number FN396876 by Yong et al. (25). All isolates were tested for the presence of blaNDM-1 using primer pair NDMRT1-F (GGC CAC ACC AGT GAC AAT ATC A) and NDMRT1-R (CAG GCA GCC ACC AAA AGC) on a Light Cycler 480 (Roche Applied Science, IN). To ensure efficient lysis, isolates were tested in parallel using a universal 16S real-time primer pair (18). Reaction conditions were performed in 20-μl volumes in 96-well plates with an annealing temperature of 56°C. Positive (K. pneumoniae CDC1000529 and K. pneumoniae NCTC 13443) and negative (K. pneumoniae ATCC 8049 and K. pneumoniae ATCC 1708) controls were included in duplicate on every plate. All samples were tested in triplicate from three biological replicates (i.e., three separate Lyse-and-Go preparations).
The blaNDM-1 genes from both P. stuartii strains (MRSN 2154 and MRSN 2155) were sequenced by Macrogen Corp. (Rockville, MD) using the primer pair NDMPCR-F (CCA TGC GGG CCG TAT GAG TGA TT) and NDMPCR-R (AAG CTG AGC ACC GCA TTA GCC G), which amplifies a 763-bp region of the blaNDM-1 gene. The amplification of the same region from K. pneumoniae CDC1000529 was sequenced in parallel as a control. Sequences were assembled using SeqMan and aligned using MegAlign (DNAStar Inc., WI).
DNA extraction and pyrosequencing.
Twenty colonies were collected and used for the extraction of total DNA (chromosomal and extrachromosomal). DNA was purified using the bacterial genomic DNA purification kit (EdgeBio, MD) with the following modifications. Following the isopropanol precipitation and pelleting of the DNA by centrifugation, the supernatant was centrifuged for an additional 15 min at 4°C to ensure the full recovery of plasmid DNA. Both pellets were processed separately and combined after dissolution in 10 mM Tris-HCl.
One microgram of the total DNA extract was subjected to DNA fragmentation using the Covaris S2 system (Covaris, Inc., MA), and a rapid ligation (RL) genomic shotgun library was prepared using the GS FLX Titanium rapid library preparation kit (Roche 454 Life Sciences, CT). After purification, the RL library was resolved on a 0.8% agarose gel. Fragments ranging in size from 600 to 800 bp were collected and subjected to DNA gel extraction. Subsequent emulsion PCR and pyrosequencing using the Genome Sequencer FLX system (Roche 454 Life Sciences) were performed as described by the manufacturer.
Pyrosequencing data analysis and assembly of pMR0211.
Sequencing reads were assembled to consensus de novo assembly contigs using the Roche Genome Sequencer FLX software GSAssembler, version 2.5.3. All de novo contigs were subjected to direct megablast (http://blast.ncbi.nlm.nih.gov/) against GenBank's nucleotide collection (nr/nt) with the Entrez query set to bacterial plasmids. Two plasmids, pAR060302 and pNDM-HK, showed the highest identities with contig sequences and were used as query sequences to align all contigs. A draft plasmid sequence for pMR0211 was created using the sequences of pAR060302 and pNDM-HK as larger scaffolds from the information generated by the contig branching structure. The scaffold assembly and draft completed sequence were verified by mapping against the sequence reads to ensure contiguous coverage crossing adjacent contigs. The authenticity of the assembly was further tested by PCR amplification using a set of primer pairs and the total DNA extract. Amplification product sizes were estimated using 1% agarose gel electrophoresis and compared to the expected sizes.
Conjugation.
Conjugation experiments were performed using E. coli J53AzR as the recipient as described previously (23), except that MacConkey agar containing 100 μg/ml sodium azide and 8 μg/ml arbekacin was used in addition to MacConkey agar containing 100 μg/μl sodium azide and 0.5 μg/ml of meropenem for selection. Plasmids were extracted using S1 nuclease digestion and pulsed-field gel electrophoresis (PFGE) as described previously (13). Plasmid bands were excised from agarose gels, and the detection of blaNDM-1, blaOXA-10, and armA was performed by PCR and real-time PCR. Plasmid extracts were also tested for uidE, ompF, and the 16S gene, which are chromosomally encoded in E. coli using species-specific primers developed previously (unpublished results).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in the course of this work have been deposited in GenBank under accession numbers JF826283 (MRSN 2154), JF826284 (MRSN 2155), and JF826285 (MRSNKpNDM). The sequence of plasmid pMR0211 has been deposited at GenBank with accession number JN687470.
RESULTS
Characterization of clinical isolates.
P. stuartii MRSN 2154 and MRSN 2155 were resistant to carbapenems (ertapenem, imipenem, and meropenem) and susceptible to the monobactam aztreonam. The isolates also were resistant to all other antibiotics tested, including colistin (MIC > 256 μg/ml via Etest) (Table 1). Pulsed-field gel electrophoresis (PFGE) revealed 99.5% identity between both strains.
Table 1.
Antibiotic susceptibilities for NDM-1-positive and -negative isolates from the same patient
| Antibioticb | MICa (μg/ml) |
|||
|---|---|---|---|---|
| P. stuartii (MRSN 2154/2155) | P. aeruginosa (MRSN 2152) | P. aeruginosa (MRSN 2153) | P. mirabilis (MRSN 2150) | |
| Tobramycin | >8 | >8 | >8 | ≤1 |
| Ampicillin-sulbactam | >16/8 | >16/8 | >16/8 | 16/8 |
| Piperacillin-tazobactam | >64/4 | >64/4 | >64/4 | ≤2/4 |
| Cefepime | >16 | >16 | >16 | ≤2 |
| Cefoxitin | >16 | >16 | >16 | ≤4 |
| Ceftazidime | >16 | >16 | >16 | ≤2 |
| Ceftriaxone | >32 | >32 | >32 | ≤2 |
| Aztreonam | ≤2 | >16 | >16 | ≤2 |
| Colistinc | >256 | - | 2 | - |
| Ertapenem | >4 | - | - | ≤0.5 |
| Imipenem | >8 | ≤4 | >8 | ≤4 |
| Meropenem | >8 | ≤4 | ≥16 | ≤4 |
| Ciprofloxacin | >2 | 2 | >2 | >2 |
| Levofloxacin | >4 | 4 | >4 | >4 |
| Tetracycline | >8 | >8 | >8 | - |
| Tigecyclinec | 4 | - | - | - |
| Trimethoprim-sulfamethoxazole | >2/38 | - | - | >2/38 |
MICs were confirmed and replicated on Vitek2, Phoenix, and Microscan automated systems.
All isolates were resistant to gentamicin. A dash indicates not tested or inherently resistant.
Etest result.
Two strains of P. aeruginosa also were isolated, one from the blood and one from a wound of the patient. One was of particular concern, because it was resistant to all 15 antibiotics tested (Table 1) but was blaNDM-1 negative. The other P. aeruginosa isolate displayed multiple resistance also, but it retained carbapenem susceptibility and intermediate susceptibility to fluoroquinolones (ciprofloxacin and levofloxacin). The P. mirabilis strain was susceptible to 10 of the 15 antibiotics tested (Table 1).
Conjugation of plasmid pMR0211 with J53AzR.
Plasmid pMR0211 was readily transferred from P. stuartii MRSN 2154 to E. coli J53AzR with a frequency of (3.4 ± 0.7) × 10−5 per donor cell at 37°C. All transconjugants displayed resistance to meropenem, imipenem, and arbekacin relative to the recipient parent strain. Plasmid analyses showed that all transconjugants contained a plasmid of ∼180 kb in size which was positive by PCR for the armA, blaNDM-1, and blaOXA-10 genes but negative for chromosomally carried uidE, ompF, and the 16S gene.
Pyrosequencing, assembly, and annotation of pMR0211.
An average coverage depth of 18.5×, with an average quality of QV64 for the host P. stuartii genome and the pMR0211 plasmid sequence, were obtained using Roche GS FLX Titanium pyrosequencing of the shotgun RL library for isolate MRSN2154. Seventeen de novo assembly contigs which were homologous to plasmid pAR060302 or pHK-NDM1 were used to assemble the complete nucleotide sequence for plasmid pMR0211. Seventeen regions which covered individual or two consecutive repeats and junctions were amplified to confirm correct assembly. Sizes of the PCR products determined on agarose gel are unambiguously consistent with expected sizes based on the assembled complete pMR0211 sequences (see Table S1 and Fig. S1 in the supplemental material).
pMR0211 was functionally annotated using the xBASE server (www.xbase.ac.uk) (6), with E. coli strain K-12, substrain DH10B, serving as the reference genome. The 148 proteins predicted computationally (see Table S2 in the supplemental material) were compared against the set of proteins encoded by pAR060302 using BLAST (26). Homologs for predicted proteins that did not match a product from pAR060302 were identified via blastp or blastx analysis using the nonredundant protein data set maintained by the National Center for Biotechnology Information (NCBI).
Structure of pMR0211.
The total length of pMR0211 is 178,277 bases, with an average GC content of 51.44% (Fig. 1 and 2). In silico PCR with the pMR0211 sequence and the replicon typing primers constructed by Carattoli and colleagues indicated that plasmid pMR0211 belongs to incompatibility group A/C (5).
Fig 1.
Structure of pMR0211. (A) Alignment of pAR060302 (4) and pNDM-HK (12) with pMR0211 showing areas of homology between all three plasmids. White shading (on pAR060302 and pNDM-HK only) indicates areas with no homology to pMR0211. Red shading indicates regions with homology to pAR060302. Green shading indicates regions with homology to pNDM-HK. Blue shading (on pMR0211 only) indicates regions with no orthologs in pAR060302 and pNDM-HK. Solid lines connecting the plasmids indicate insertion and deletion events in pMR0211 compared to the other plasmids. The sizes of the respective plasmids are indicated at the 3′ terminus. (B) Circular representation of plasmid pMR0211. Colored shading represents genes and regions with homology to pAR060302, pNDM-Hk, and pMR0211 as described above. The repA gene and putative antibiotic resistance genes with their respective orientations are indicated in the outermost region by block arrows with the same color scheme. White block arrows indicate the orientation and position of putative insertion sequence elements and transposons.
Fig 2.
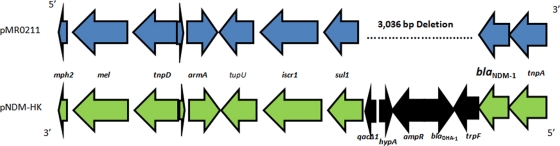
Structure of the blaNDM-1 gene and surrounding regions in pMR0211 and pNDM-HK. Gene orientation is reversed in both plasmids, as indicated. A 3,036-bp deletion in pMR0211 is indicated by the dotted line on pMR0211 and corresponds to the genes shaded in black on pNDM-HK. Putative gene annotations were assigned using xBASE (www.xbase.ac.uk) (6).
Regions of homology to pAR060302 and pNDM-HK (Fig. 1) were defined using the BLAST and ClustalW software (14). The orthologous regions between pAR060203 and pMR0211 retain the genes involved in plasmid maintenance and transfer as well as multiple antibiotic resistance genes, including the chloramphenicol resistance gene floR, the tetracycline resistance gene tetA, the streptomycin resistance genes strA and strB, the sulfonamide resistance genes sul1 and sul2, the class C β-lactamase ampC, and the quaternary ammonium resistance gene sugE1 (see Table S2 in the supplemental material) (4).
Sequence analysis reveals several alterations that distinguish pMR0211 from pAR060302. First, nucleotides 6838 to 8524 of pAR060302 are absent from pMR0211. This 1,687-bp region contains an IS91 element containing a gene encoding an IS1274 transposase protein. In addition, pMR0211 has a 53-bp deletion corresponding to nucleotides 23178 to 23229 of pAR060302. This deletion affects a pair of inverted insB2 sequences. pMR0211 also differs from pAR060302 with respect to two prominent insertion/deletion events (Table 2). The first is the deletion of 8,342 bp from pAR060302 (positions 115225 to 123566) that encodes the aadA, aacC, groS, and groEL loci. This region is replaced by a 5,983-bp insert in pMR0211-containing genes encoding a chloramphenicol acetyltransferase (cmlA7), a class D carbapenemase (blaOXA-10), and an aminoglycoside resistance protein (aadA1) (Table 2 and Fig. 1). The second event is a deletion of 14.2 kb from pAR060302 (132151 to 146351), which includes a cluster of mercury resistance genes (4). In pMR0211, this region is replaced by two distinct insertions of 12.7 and 17.3 kb. The 12.7-kb insertion shares close homology to a portion of plasmid pNDM-HK that contains the blaNDM-1 gene. The orientation of this region is reversed in pMR0211, and this region also has a 3-kb deletion that removes the blaDHA-1, ampR, and hypA loci and part of the qacΔ1 gene found in pNDM-HK (Fig. 1B). The 17.3-kb insertion encodes 13 predicted open reading frames, including genes with homology to the aminoglycoside-modifying enzyme loci aadA and aac(6′), a gene encoding a product similar to a protein of unknown function (DUF1696) from Shewanella woodyi ATCC 51908, a gene encoding a U32 family peptidase with homology to the collagenase PrtC, the quinolone resistance gene qnrA, and the sulfonamide resistance gene sul1 (Table 2 and Fig. 1). These regions are surrounded by multiple insertion elements and transposons, including elements from the ins and isc family of insertion sequences (Table 2; also see Table S2 in the supplemental material).
Table 2.
Open reading frames present in pMR0211 but absent from pAR060302 and pNDM-HK
| pMR0211 locus | Functiona | Position |
Directionb | |
|---|---|---|---|---|
| Start | End | |||
| 0100 | Transposase, insB4 | 113534 | 114250 | + |
| 0101 | Integrase, intI1 | 114284 | 114679 | − |
| 0102 | Chloramphenicol resistance gene, cmlA7 | 116271 | 117578 | + |
| 0103 | Beta-lactamase OXA-10 precursor, blaOXA-10 | 117843 | 118643 | + |
| 0104 | Aminoglycoside-modifying enzyme, aadA | 118660 | 119451 | + |
| 0124 | 3′-Aminoglycoside phosphotransferase type VI, aac(6′) | 141819 | 142598 | − |
| 0125 | Transposase, insB | 142704 | 143444 | − |
| 0126 | Transposase IS3/IS911 family | 143531 | 143848 | − |
| 0127 | Transposase, iscR1 | 145261 | 146802 | − |
| 0128 | Protein of unknown function, DUF1696 | 147246 | 147620 | − |
| 0129 | U32 family peptidase (related to collagenase prtC) | 147631 | 148410 | − |
| 0130 | No known homology | 148420 | 149265 | + |
| 0131 | No known homology | 149954 | 150109 | − |
| 0132 | Quinolone resistance gene, qnrA1 | 151427 | 152083 | − |
| 0133 | Transposase, iscR1 | 152482 | 154023 | − |
| 0134 | Sulfonamide resistance gene, sul1 | 154428 | 155354 | − |
| 0135 | Aminoglycoside-modifying enzyme, aadA | 155777 | 156571 | − |
| 0136 | Transposase, insB3 | 157351 | 158067 | − |
Functional categories were assigned as described in Results.
Gene orientation is relative to that of the repA sequence: +, 5′ to 3′; −, 3′ to 5′.
DISCUSSION
In this study, we report on the complete sequence of a plasmid carrying the blaNDM-1 gene belonging to incompatibility group A/C. The plasmid was isolated from a strain of P. stuartii that was recovered from a patient undergoing treatment for extensive burn injuries at a hospital in Afghanistan. Although P. stuartii is not considered a priority ESKAPE pathogen as defined by the Infectious Disease Society of America (2), the finding of a highly mobile blaNDM-1-carrying plasmid in Providencia that is intrinsically resistant to polymixins (21) is of great concern, because such strains thus become resistant to virtually all currently available antibacterials. This pan-resistant phenotype is evident in the strain of Providencia described herein, as evidenced by resistance to all antibiotics except aztreonam.
The sequence of pMR0211 further demonstrates the remarkable mobility of the blaNDM-1 gene and the ever-expanding number of plasmid backbones associated with its carriage. The plasmid backbone shares extensive homology (>98%) with pAR060302, a plasmid isolated from a dairy calf in Illinois (4), and is a distinct component of a larger lineage of plasmids that are distributed among E. coli, Yersinia pestis, Y. ruckeri, Photobacterium damselae, and Salmonella enterica (4). pAR060302 contains multiple insertion sequences and transposons, harbors an array of antibiotic resistance genes, and is readily transferable to E. coli recipient cells, all characteristics that are shared by pMR0211. In pMR0211, the plasmid backbone has retained two of the four antibiotic resistance regions, the floR region and the aadA region identified by Call and coworkers (4), and it carries 22 putative transposons and associated insertion sequences. pMR0211 contains three other regions that mark its divergence from pAR060302. The first is a 5.9-kb region containing genes encoding a chloramphenicol acetyltransferase (cmlA7), a class D carbapenemase (blaOXA-10), and an aminoglycoside resistance protein (aadA1) flanked by two transposons. BLAST analysis of this sequence reveals that it is composed of two overlapping sequences with 100% homology to regions in the K. pneumoniae subsp. pneumoniae plasmid pKPN5; a class 1 integron in Salmonella enterica, P. aeruginosa, and A. veronii; E. coli plasmid R751; and in the genome of A. baumannii AYE. The second region is a 12.7-kb insertion that shares almost 100% homology to a region in pNDM-HK and harbors the class B metallo-β-lactamase blaNDM-1, the 16S methylase armA, the macrolide resistance genes mel and mph, and the sulfonamide resistance gene sul1. The region has a 3-kb deletion in pMR0211 compared to pNDM-HK that removes the blaDHA-1, ampR, and hypA loci and part of the qacΔ1 gene. The final region encompasses a 17.3-kb insert with some notable features. In addition to carrying the quinolone resistance gene qnrA, the region also contains genes encoding two aminoglycoside-modifying enzymes, aadA and aac(6′), and an additional copy of sul1 (bringing the total copy number of this gene to 3 in the entire plasmid). Two additional loci, pMR0211_0130 and pMR0211_0131, have no known homology to any sequence in GenBank. Interestingly, a BLAST analysis of this region indicates that the sequence with no known homology has been inserted into mobile elements associated with other bacterial species, including plasmids pKP96 and pNICED61 from K. pneumoniae and Vibrio fluvialis, respectively, a class I integron associated with plasmids in C. freundii, K. pneumoniae, Enterobacter cloacae, E. coli, Aeromonas punctate, and P. mirabilis and with a chromosomal region in A. baumannii AYE.
To date (August 2011), the sequences of two blaNDM-1-carrying plasmids have been submitted to GenBank: an 88.8-kb plasmid, designated pNDM-HK, isolated from a strain of E. coli in Hong Kong (HQ451074) (12), and p271A, a 35.9-kb plasmid isolated from E. coli (JF785549). Both sequences show that the blaNDM-1 gene is associated with insertion sequences and transposons, although the plasmid backbone and structure of the mobile elements associated with blaNDM-1 vary. Similarly, the sequencing of the regions surrounding blaNDM-1 in E. coli DVR22 (JF922606) and Acinetobacter baumannii strain 161/07 (HQ857107) revealed the presence of multiple insertion sequences and transposons. These structural features are evident in pMR0211 also, with five different transposons and insertion sequences surrounding the region containing the blaNDM-1 gene.
In recent years, plasmids belonging to the IncA/C incompatibility group have received increased attention, primarily due to their broad host range and ability to confer resistance to a diverse group of antimicrobial agents (9). They have been identified in numerous bacterial species, and sequence analysis of these plasmids has revealed that they share considerable homology (4, 10, 24). Interestingly, the IncA/C plasmids are increasingly associated with blaNDM-1 carriage (9, 11, 23), and a recent study has shown that plasmids carrying blaNDM-1 among Enterobacteriaceae isolated from New Delhi all were from incompatibility group A/C and were approximately 140 kb in size (23). The sequencing of these plasmids would provide valuable information on the epidemiology of incompatibility group A/C plasmids in this region and their association with Enterobacteriaceae.
In conclusion, pMR0211 represents a vivid example of a highly mobile megaplasmid that encodes an extensive arsenal of antibiotic resistance genes. Like other plasmids isolated in this geographical region (13, 23), it belongs to the IncA/C family, further highlighting the role of these plasmids in the dissemination of blaNDM-1. These plasmids present a serious and growing challenge to health care workers and patients throughout the world.
Supplementary Material
ACKNOWLEDGMENTS
We greatly acknowledge Brandi Limbago and Kamile Rasheed at the Centers for Disease Control (CDC), Atlanta, GA, for their prompt response to our request for strain K. pneumoniae NDM-1 (CDC1000529). We also acknowledge Phil Bossart and his entire laboratory team and Peter Chung for their continued collaborative efforts.
The material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the true views of the Department of the Army or the Department of Defense.
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Bonomo RA. 2011. New Delhi metallo-beta-lactamase and multidrug resistance: a global SOS? Clin. Infect. Dis. 52:485–487 [DOI] [PubMed] [Google Scholar]
- 2. Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 3. Bustin SA, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 4. Call DR, et al. 2010. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob. Agents Chemother. 54:590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carattoli A, et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 6. Chaudhuri RR, et al. 2008. xBASE2: a comprehensive resource for comparative bacterial genomics. Nucleic Acids Res. 36:D543–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CLSI 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed M07-A8 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Cornaglia G, Giamarellou H, Rossolini GM. 2011. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect. Dis. 11:381–393 [DOI] [PubMed] [Google Scholar]
- 9. Fernandez-Alarcon C, Singer RS, Johnson TJ. 2011. Comparative genomics of multidrug resistance-encoding IncA/C Plasmids from commensal and pathogenic Escherichia coli from multiple animal sources. PLoS One 6:e23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fricke WF, et al. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J. Bacteriol. 191:4750–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammerum AM, et al. 2010. Global spread of New Delhi metallo-beta-lactamase 1. Lancet Infect. Dis. 10:829–830 [DOI] [PubMed] [Google Scholar]
- 12. Ho PL, et al. 2011. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One 6:e17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 15. Lesho E, et al. 2011. Surveillance, characterisation, and preservation of multidrug-resistant bacteria. Lancet Infect. Dis. 11:8–10 [DOI] [PubMed] [Google Scholar]
- 16. Lesho E, et al. 2011. Joint collaboration enhances infection control at home and abroad: the maiden voyage of the multidrug-resistant organism repository and surveillance network. Mil. Med. 176:241–243 [DOI] [PubMed] [Google Scholar]
- 17. Livermore DM, Woodford N. 2006. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413–420 [DOI] [PubMed] [Google Scholar]
- 18. Nadkarni MA, Martin FE, Jacques NA, Hunter N. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266 [DOI] [PubMed] [Google Scholar]
- 19. Nordmann P, Poirel L, Toleman MA, Walsh TR. 2011. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 66:689–692 [DOI] [PubMed] [Google Scholar]
- 20. Rolain JM, Parola P, Cornaglia G. 2010. New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin. Microbiol. Infect. 16:1699–1701 [DOI] [PubMed] [Google Scholar]
- 21. von Graevenitz A, Nourbakhsh M. 1972. Antimicrobial resistance of the genera Proteus, Providencia and Serratia with special reference to multiple resistance patterns. Med. Microbiol. Immunol. 157:142–148 [DOI] [PubMed] [Google Scholar]
- 22. Walsh TR, Toleman MA. 2011. The new medical challenge: why NDM-1? Why Indian? Expert Rev. Anti Infect. Ther. 9:137–141 [DOI] [PubMed] [Google Scholar]
- 23. Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11:355–362 [DOI] [PubMed] [Google Scholar]
- 24. Welch TJ, et al. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yong D, et al. 2009. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203–214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.