Abstract
Rifampin is a major drug used to treat leprosy and tuberculosis. The rifampin resistance of Mycobacterium leprae and Mycobacterium tuberculosis results from a mutation in the rpoB gene, encoding the β subunit of RNA polymerase. A method for the molecular determination of rifampin resistance in these two mycobacteria would be clinically valuable, but the relationship between the mutations and susceptibility to rifampin must be clarified before its use. Analyses of mutations responsible for rifampin resistance using clinical isolates present some limitations. Each clinical isolate has its own genetic variations in some loci other than rpoB, which might affect rifampin susceptibility. For this study, we constructed recombinant strains of Mycobacterium smegmatis carrying the M. leprae or M. tuberculosis rpoB gene with or without mutation and disrupted their own rpoB genes on the chromosome. The rifampin and rifabutin susceptibilities of the recombinant bacteria were measured to examine the influence of the mutations. The results confirmed that several mutations detected in clinical isolates of these two pathogenic mycobacteria can confer rifampin resistance, but they also suggested that some mutations detected in M. leprae isolates or rifampin-resistant M. tuberculosis isolates are not involved in rifampin resistance.
INTRODUCTION
Leprosy and tuberculosis persist as important global public health concerns. Rifampin, a major drug used to treat these two infectious diseases, has a molecular mechanism of activity involving the inhibition of DNA-dependent RNA polymerase (15). In Escherichia coli, this enzyme is a complex oligomer comprised of four subunits, α, β, β′, and σ, encoded by rpoA, rpoB, rpoC, and rpoD, respectively. Rifampin binds to the β subunit of RNA polymerase and results in transcription inhibition (15). Mutations in the rpoB gene, encoding the β subunit of RNA polymerase, reportedly result in resistance to rifampin in several mycobacterial species, including Mycobacterium leprae and Mycobacterium tuberculosis (9, 21). The former has not yet been cultured on artificial media; it requires 11 to 14 days to double in experimentally infected mice. Therefore, it is difficult to determine the rifampin susceptibilities of M. leprae isolates. The standardized method using a mouse footpad takes more than half a year to determine the rifampin susceptibility of M. leprae isolates and requires 5 × 103 M. leprae bacilli (3), which require almost a year to prepare. In vitro drug susceptibility testing for M. leprae using a radioactive reagent requires more (107) M. leprae cells (7). In contrast, mutations in the rpoB gene of M. leprae can be detected in a few days or less. It would be very helpful if mutations responsible for rifampin resistance could be determined without performing mouse footpad testing. The main mutations that confer rifampin resistance to M. tuberculosis are located in the 81-bp core region of the rpoB gene, encompassing codons 507 to 533, known as the rifampin resistance-determining region (RRDR) (17, 18). About 95% of rifampin-resistant M. tuberculosis strains have a mutation in this region (18, 20). Four mutations, D516V, H526Y, H526D, and S531L, are most commonly associated with the high-level rifampin resistance of M. tuberculosis strains (4, 10, 19), but some other mutations in the 81-bp region have not yet been confirmed completely as being responsible for rifampin resistance.
We have established a method to determine the mutations responsible for the dapsone resistance of M. leprae using recombinant Mycobacterium smegmatis strains (16). In the present study, we assessed the applicability of the determination of rifampin resistance for analysis. We then analyzed rpoB mutations conferring rifampin resistance to M. leprae and M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli DH5α was used for DNA cloning. M. smegmatis mc2155 was used as a mycobacterial host to produce strains for drug susceptibility testing. Plasmids pYUB854 and phAE87 were kindly provided by W. R. Jacobs, Jr. (Department of Microbiology and Immunology, Albert Einstein College of Medicine, New York, NY). M. smegmatis mc2155 and its transformants were grown in Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI) supplemented with 0.5% bovine serum albumin (fraction V), 0.2% glucose, 0.085% NaCl, 0.2% glycerol, and 0.1% Tween 80.
Site-directed mutagenesis.
The wild-type rpoB genes of M. leprae and M. tuberculosis were amplified from M. leprae Thai-53 and M. tuberculosis H37Rv by PCR and cloned into pMV261. Site-directed mutagenesis was performed by using PCR with DNA polymerase (Takara PrimeStar HS; Takara Bio Inc., Kyoto, Japan) and the primers presented in Table 1. PCR products were purified and phosphorylated with T4 kinase and ATP and were then ligated to make them circular. The ligation mixture was used to transform E. coli DH5α cells, and kanamycin-resistant colonies were isolated. Plasmids were extracted from the transformants. The mutated sequences were then confirmed by sequencing. The inserts of the plasmids were also cloned into pNN301 (16). Mutations introduced into the M. leprae rpoB or M. tuberculosis rpoB gene are listed in Table 2.
Table 1.
Primers used for this study
| Primer | Sequencea | Application |
|---|---|---|
| M. smegmatis | ||
| MSRBUF | GCCTTAAGGAGGAGAAGGACGAGGCCAC | rpoB disruption, upstream forward |
| MSRBUR | GCTCTAGACAAGATGCATCCTTCCAGCA | rpoB disruption, upstream reverse |
| MSRBDF | GCAAGCTTTCGCGCAACGAATCCGCGTC | rpoB disruption, downstream forward |
| MSRBDR | GCACTAGTAGCGCACGCAGCTTCTTCTG | rpoB disruption, downstream reverse |
| MSRBF | TGGTCAAGCAGTTCCTCAAC | Detection of rpoB disruption, forward |
| MSRBR | CGTTGTTGACGATGATCTCG | Detection of rpoB disruption, reverse |
| M. leprae | ||
| MLRBWTF | GCGGATCCGTGCTGGAAGGATGCATCTT | Cloning of M. leprae rpoB, forward |
| MLRBWTR | GCGTTAACCTAAGCCAGATCTTCTATGG | Cloning of M. leprae rpoB, reverse |
| MLRBWTF1 | CAGTTCATGGATCAGAACAACCCTC | Introduction of point mutation at codons 507 and 508 |
| MLRBWTF2 | TGTCGGCGCTGGGCCCGGGTGGTTT | Introduction of point mutation at codon 526 |
| MLRBWTF3 | TTCGCACTACGGCCGGATGTGCCCG | Introduction of point mutation at codon 547 |
| MLRBWTR1 | CGACAGCTGGCTGGTGCCGAAGAAT | Introduction of point mutation at codons 513, 516, and 517 |
| MLRBWTR2 | GCCGGCGCTTGTGGGTCAGGCCCGA | Introduction of point mutation at codons 531, 532, and 533 |
| MLRB507GGG | CGACAGCTGGCTGGTCCCGAAGAAT | Introduction of point mutation GGC507→GGG |
| MLRB507AGC | CGACAGCTGGCTGGTGCTGAAGAAT | Introduction of point mutation GGC507→AGC |
| MLRB508ACA | CGACAGCTGGCTTGTGCCGAAGAAT | Introduction of point mutation ACC508→ACA |
| MLRB513GTG | GTGTTCATGGATCAGAACAACCCTC | Introduction of point mutation CAG513→GTG |
| MLRB516AAT | CAGTTCATGAATCAGAACAACCCTC | Introduction of point mutation GAT516→AAT |
| MLRB517CAT | CAGTTCATGGATCATAACAACCCTC | Introduction of point mutation CAG517→CAT |
| MLRB526TAC | GCCGGCGCTTGTAGGTCAGGCCCGA | Introduction of point mutation CAC526→TAC |
| MLRB531TTG | TGTTGGCGCTGGGCCCGGGTGGTTT | Introduction of point mutation TCG531→TTG |
| MLRB531TGG | TGTGGGCGCTGGGCCCGGGTGGTTT | Introduction of point mutation TCG531→TGG |
| MLRB532TCG | TGTCGTCGCTGGGCCCGGGTGGTTT | Introduction of point mutation GCG532→TCG |
| MLRB533CCG | TGTCGGCGCCGGGCCCGGGTGGTTT | Introduction of point mutation CTG533→CCG |
| MLRB547ATC | GGGTGCACGTCACGGATCTCTAGCC | Introduction of point mutation GTC547→ATC |
| M. tuberculosis | ||
| MTRBWTF | GCGAATTCTTGGCAGATTCCCGCCAGAG | Cloning of M. tuberculosis rpoB, forward |
| MTRBWTR | GCAAGCTTTTACGCAAGATCCTCGACAC | Cloning of M. tuberculosis rpoB, reverse |
| MTRBWTF1 | AATTCATGGACCAGAACAACCCGCT | Introduction of point mutation at codons 507, 508, 510, 511, 512, and 513 and deletion of codons 506-508 |
| MTRBWTF2 | CTGTCGGCGCTGGGGCCCGGCGGTC | Introduction of point mutation at codons 522, 523, 526, and 531 |
| MTRBWTR1 | GGCTCAGCTGGCTGGTGCCGAAGAA | Introduction of mutation at codons 514, 516, 518, 519, and 521; deletion of codon 518; and insertion of TTC between codons 514 and 515 |
| MTRBWTR2 | TCGGCGCTTGTGGGTCAACCCCGAC | Introduction of point mutations TCG531→TTC and TCG531→TTG |
| MTRB507AGC | GGCTCAGCTGGCTGGTGCTGAAGAA | Introduction of point mutation GGC507→AGC |
| MTRB507GAT | GGCTCAGCTGGCTGGTATCGAAGAA | Introduction of point mutation GGC507→GAT |
| MTRB508CAC | GGCTCAGCTGGCTGTGGCCGAAGAA | Introduction of point mutation ACC508→CAC |
| MTRB508GCC | GGCTCAGCTGGCTGGCGCCGAAGAA | Introduction of point mutation ACC508→GCC |
| MTRB510CAT | GGCTCAGATGGCTGGTGCCGAAGAA | Introduction of point mutation CAG510→CAT |
| MTRB511CCG | GGCTCGGCTGGCTGGTGCCGAAGAA | Introduction of point mutation CTG511→CCG |
| MTRB513AAT1 | TGCTCAGCTGGCTGGTGCCGAAGAA | Introduction of point mutation CAA513→AAT |
| MTRB513AAT2 | ATTTCATGGACCAGAACAACCCGCT | Introduction of point mutation CAA513→AAT |
| MTRB513GAA | CGCTCAGCTGGCTGGTGCCGAAGAA | Introduction of point mutation CAA513→GAA |
| MTRB516GAG | AATTCATGGAGCAGAACAACCCGCT | Introduction of point mutation GAC516→GAG |
| MTRB516CAC | AATTCATGCACCAGAACAACCCGCT | Introduction of point mutation GAC516→CAC |
| MTRB516GTC | AATTCATGGTCCAGAACAACCCGCT | Introduction of point mutation GAC516→GTC |
| MTRB521ATG | AATTCATGGACCAGAACAACCCGAT | Introduction of point mutation CTG521→ATG |
| MTRB522TTG | TCGGCGCTTGTGGGTCAACCCCAAC | Introduction of point mutation TCG522→TTG |
| MTRB523GCG | TCGGCGCTTGTGGGTCAACGCCGAC | Introduction of point mutation GGG523→GCG |
| MTRB523GGC | TCGGCGCTTGTGGGTCAAGCCCGAC | Introduction of point mutation GGG523→GGC |
| MTRB526CTC | TCGGCGCTTGAGGGTCAACCCCGAC | Introduction of point mutation CAC526→CTC |
| MTRB526TAC | TCGGCGCTTGTAGGTCAACCCCGAC | Introduction of point mutation CAC526→TAC |
| MTRB526GAC | TCGGCGCTTGTCGGTCAACCCCGAC | Introduction of point mutation CAC526→GAC |
| MTRB526TTC | TCGGCGCTTGAAGGTCAACCCCGAC | Introduction of point mutation CAC526→TTC |
| MTRB526AAC | TCGGCGCTTGTTGGTCAACCCCGAC | Introduction of point mutation CAC526→AAC |
| MTRB526CGC | TCGGCGCTTGCGGGTCAACCCCGAC | Introduction of point mutation CAC526→CGC |
| MTRB526CAA | TCGGCGCTTTTGGGTCAACCCCGAC | Introduction of point mutation CAC526→CAA |
| MTRB529AAA | TTTGCGCTTGTGGGTCAACC | Introduction of point mutation CGA529→AAA |
| MTRB531TTC | CTGTTCGCGCTGGGGCCCGGCGGTC | Introduction of point mutation TCG531→TTC |
| MTRB531TTG | CTGTTGGCGCTGGGGCCCGGCGGTC | Introduction of point mutation TCG531→TTG |
| MTRB506d | GGCTCAGCTGGCTGAACTCCTTGAT | Introduction of mutation 506-508del |
| MTRBin514TTC | AATTCTTCATGGACCAGAACAACCC | Introduction of mutation 514insTTC |
| MTRBd518 | AATTCATGGACCAGAACCCGCTGTC | Introduction of mutation 518del |
Restriction sites are underlined.
Table 2.
Rifampin and rifabutin susceptibilities of the recombinant M. smegmatis strains
| Mutation | Rifampin |
Rifabutin |
Reference(s) | ||
|---|---|---|---|---|---|
| MIC (μg/ml) | Fold increasea | MIC (μg/ml) | Fold increase | ||
| M. leprae | |||||
| Wild type | 1 | 0.25 | |||
| GGC507→GGG (silent) | 1 | 1 | 0.25 | 1 | This study |
| GGC507→AGC (G507S) | 0.5 | 0.5 | 0.125 | 0.5 | 3 |
| ACC508→ACA (silent) | 1 | 1 | 0.25 | 1 | This study |
| CAG513→GTG (Q513V) | 32 | 32 | 8 | 32 | 3 |
| GAT516→AAT (D516N) | 32 | 32 | 2 | 8 | 14 |
| CAG517→CAT (Q517H) | 1 | 1 | 0.25 | 1 | 11 |
| CAC526→TAC (H526Y) | 32 | 32 | 8 | 32 | 14 |
| TCG531→TTG (S531L) | 32 | 32 | 4 | 16 | 3, 14 |
| TCG531→TGG (S531W) | 32 | 32 | 8 | 32 | 14 |
| GCG532→TCG (A532S) | 1 | 1 | 0.25 | 1 | 11 |
| CTG533→CCG (L533P) | 32 | 32 | 4 | 16 | 14 |
| GTC547→ATC (V547I) | 1 | 1 | 0.25 | 1 | This study |
| M. tuberculosis | |||||
| Wild type | 1 | 0.25 | |||
| GGC507→AGC (G507S) | 0.5 | 0.5 | 0.125 | 0.5 | 1 |
| GGC507→GAT (G507D) | 0.5 | 0.5 | 0.125 | 0.5 | 1 |
| ACC508→CAC (T508H) | 0.5 | 0.5 | 0.125 | 0.5 | 1 |
| ACC508→GCC (T508A) | 1 | 1 | 0.25 | 1 | 1 |
| CAG510→CAT (Q510H) | 1 | 1 | 0.25 | 1 | 22 |
| CTG511→CCG (L511P) | 16 | 16 | 1 | 4 | 1, 12 |
| CAA513→AAT (Q513N) | 8 | 8 | 0.5 | 2 | 1 |
| CAA513→GAA (Q513E) | 32 | 32 | 2 | 8 | 1 |
| GAC516→GAG (D516E) | 8 | 8 | 0.5 | 2 | 12 |
| GAC516→CAC (D516H) | 1 | 1 | 0.25 | 1 | 1 |
| GAC516→GTC (D516V) | 32 | 32 | 2 | 8 | 12, 21, 22 |
| CTG521→ATG (L521M) | 1 | 1 | 0.125 | 0.5 | 21 |
| TCG522→TTG (S522L) | >32 | >32 | 8 | 32 | 21 |
| GGG523→GCG (G523A) | 1 | 1 | 0.125 | 0.5 | 1 |
| GGG523→GGC (silent) | 1 | 1 | 0.25 | 1 | 1 |
| CAC526→CTC (H526L) | 32 | 32 | 4 | 16 | 12, 22 |
| CAC526→TAC (H526Y) | >32 | >32 | 8 | 32 | 12, 22 |
| CAC526→GAC (H526D) | >32 | >32 | 8 | 32 | 12, 22 |
| CAC526→TTC (H526F) | >32 | >32 | 4 | 16 | 1 |
| CAC526→AAC (H526N) | 32 | 32 | 2 | 8 | 8 |
| CAC526→CGC (H526R) | 32 | 32 | 8 | 32 | 12, 22 |
| CAC526→CAA (H526Q) | 8 | 8 | 0.5 | 2 | 1 |
| CGA529→AAA (R529K) | 32 | 32 | 4 | 16 | 22 |
| TCG531→TTC (S531F) | 32 | 32 | 4 | 16 | 1 |
| TCG531→TTG (S531L) | 32 | 32 | 8 | 32 | 21, 22 |
| 506-508delb | 16 | 16 | 0.5 | 2 | 5 |
| 514insTTCc | >32 | >32 | 8 | 32 | 12,22 |
| 518deld | 32 | 32 | 2 | 8 | 22 |
Fold increase in MIC compared to the wild-type sequence.
Deletion of codons 506 to 508.
Insertion of TTC between codons 514 and 515.
Deletion of codon 518.
Disruption of the rpoB gene on the M. smegmatis chromosome.
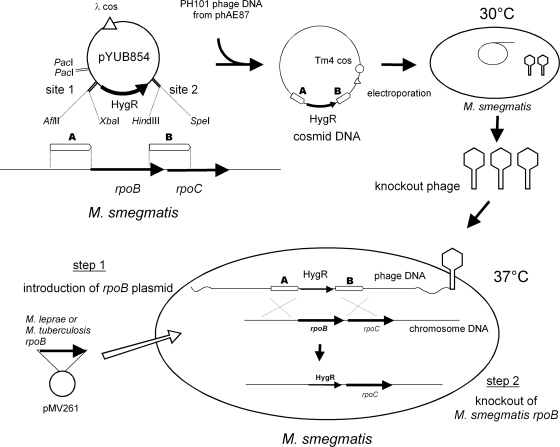
M. smegmatis mc2155 cells were transformed with plasmids carrying the M. leprae or M. tuberculosis rpoB gene with or without a point mutation. Recombinants were selected on LB medium containing kanamycin. Allelic-exchange mutants were constructed by using a temperature-sensitive mycobacteriophage method described in a previous report (2). Using the M. smegmatis mc2155 genome sequence (GenBank accession number CP000480), the upstream and downstream flanking DNA sequences were used to generate a deletion mutation in the rpoB gene (MSMEG_1367). To disrupt the rpoB gene, DNA segments from 1,119 bp upstream through 21 bp downstream of the initiation codon of M. smegmatis rpoB and from 39 bp upstream through 941 bp downstream of the termination codon were cloned directionally into the cosmid vector pYUB854, which contains a res-hyg-res cassette and a cos sequence for lambda phage assembly. The plasmids thus produced were digested with PacI and ligated into PH101 genomic DNA excised from the phage-plasmid hybrid (phasmid) phAE87 by PacI digestion. The ligated DNA was packaged (GigaPackIII Gold packaging extract; Stratagene, La Jolla, CA). The resultant mixture was used for the transduction of E. coli STBL2 cells (Life Technologies Inc., Carlsbad, CA) to yield cosmid DNA. After E. coli was transduced and the transductants were plated onto hygromycin-containing medium, phasmid DNA was prepared from the pooled antibiotic-resistant transductants and electroporated into M. smegmatis mc2155. Bacterial cells were incubated at 30°C to produce the recombinant phage. The M. smegmatis transformant carrying the M. leprae or M. tuberculosis rpoB gene was infected with the produced temperature-sensitive phage at 37°C for allelic exchange, and kanamycin-resistant and hygromycin-resistant colonies were isolated. Two colonies for each point mutation were subjected to subsequent tests.
Drug susceptibility testing.
The MIC values for M. smegmatis recombinant clones were determined by culture on Middlebrook 7H10 agar plates containing 2-fold serial dilutions of rifampin (0.25 to 32 μg/ml) or rifabutin (0.0625 to 8 μg/ml). The MIC value for each strain was defined as the lowest concentration of the drug necessary to inhibit bacterial growth.
RESULTS
Construction of recombinant M. smegmatis strains.
In our previous study, we sequenced the rpoB regions of M. leprae clinical samples isolated in Vietnam and detected several mutations (11). In addition to these mutations, we detected some mutations (GGC→GGG at codon 507, ACC→ACA at codon 508, and GTC→ATC at codon 547) in clinical specimens from Vietnam and other countries (our unpublished data). We prepared plasmids with mutations in the M. leprae and M. tuberculosis rpoB genes. Each plasmid has one of 40 mutations (12 for M. leprae rpoB and 28 for M. tuberculosis rpoB) presented in Table 2. The mutated sequences were confirmed by sequencing. Plasmids carrying the M. leprae or M. tuberculosis rpoB gene with or without a point mutation were introduced individually into M. smegmatis. The M. smegmatis transformants were subjected to allelic exchange to disrupt the rpoB gene on their own chromosome (Fig. 1). The isolation of rpoB-disrupted mutants carrying the pNN301-rpoB constructs was unsuccessful. Consequently, the recombinant strains with pMV261-rpoB constructs were used for subsequent tests. PCR analysis confirmed that the M. smegmatis rpoB sequences in the recombinant strains with pMV261-rpoB constructs were replaced by hygromycin resistance gene sequences (see Fig. S1 in the supplemental material). All strains showed growth rates comparable to that of wild-type M. smegmatis.
Fig 1.
Construction of recombinant M. smegmatis strains for rifampin susceptibility testing.
Drug susceptibility.
The rifampin susceptibilities and rifabutin susceptibilities of the recombinant M. smegmatis strains were tested (see Fig. S2 in the supplemental material). The MIC values of rifampin and rifabutin for the recombinant M. smegmatis strains and the fold increases in MIC compared to the wild-type sequences are presented in Table 2. It should be noted that the MIC values for the M. smegmatis strains might be shifted from those for M. leprae or M. tuberculosis because of their differences in cell wall permeability and other factors. The MIC value of rifampin for the recombinant M. smegmatis strain with the wild-type sequence of the M. leprae rpoB or M. tuberculosis rpoB gene was 1 μg/ml. Most strains that had a mutation at codon 511, 513, 516, 522, 526, 531, or 533 showed rifampin resistance. In contrast, strains that had a mutation at codon 507, 508, 517, 521, 523, or 532 showed MIC values of rifampin comparable to those for the wild-type sequence. The MIC values of rifabutin for the recombinant M. smegmatis strains with the wild-type sequence of the M. leprae rpoB or M. tuberculosis rpoB gene were 0.25 μg/ml. Generally, rifabutin was more efficacious than rifampin in terms of concentration.
DISCUSSION
To functionally replace the rpoB gene of M. smegmatis with the M. leprae or M tuberculosis counterpart, we used a method established in our previous study (16). Because rpoB is a necessary gene for bacterial growth, this genetic locus cannot be disrupted without compensating for its activity. Therefore, we first introduced the rpoB gene of M. leprae or M. tuberculosis into M. smegmatis using vector plasmids of two types before disrupting the rpoB gene on the M. smegmatis chromosome. One vector was pMV261, a multicopy shuttle plasmid. The other was a single-copy integrative shuttle plasmid, pNN301. However, the isolation of rpoB-disrupted mutants carrying pNN301-rpoB constructs was unsuccessful, probably because of insufficient RpoB expression.
We tested 2 silent mutations and 10 mutations that change amino acid residues for M. leprae (Fig. 2). Codons 516, 526, 531, and 533 in the M. leprae rpoB gene are known to be codons responsible for rifampin resistance. However, it remains unclear whether or not mutations that have not been reported previously can confer rifampin resistance. Our results show that not all mutations in the rpoB gene detected in M. leprae clinical samples confer rifampin resistance. M. leprae is not cultivable. Therefore, it has been very difficult to analyze the mutation-susceptibility relationship. Using recombinant M. smegmatis, however, we can analyze it in a few weeks. We also tested 1 silent mutation, 24 mutations that change amino acids, 2 deletions, and 1 insertion for M. tuberculosis. Some mutations did not confer rifampin resistance, which is inconsistent with the susceptibility of the M. tuberculosis clinical isolates reported previously. Most mutations at codon 516, 526, or 531 showed rifampin resistance. It is interesting that the strains with the mutation GAC516→CAC for D516H were not rifampin resistant. All other mutations at codon 516 showed rifampin resistance. The mutation GAC516→CAC in M. tuberculosis was reported for a strain with multiple mutations and should not be involved in rifampin resistance.
Fig 2.
Mutations introduced into the M. leprae rpoB gene or M. tuberculosis rpoB gene and rifampin susceptibility. The consensus amino acid sequence of M. leprae RpoB and M. tuberculosis RpoB between codons 506 and 565 is shown. The M. leprae rpoB sequence and codons are shown above the consensus amino acid sequence. The M. tuberculosis rpoB sequence and codons are shown below the consensus sequence. Mutated codons that gave rise to rifampin resistance are surrounded by ovals. Mutated codons that showed levels of rifampin susceptibility comparable to those of the wild-type sequences are surrounded by rectangles.
Rifabutin, a spiropiperidyl rifampin, is a rifamycin derivative that is more active than rifampin against slow-growing mycobacteria, including M. tuberculosis and M. avium-M. intracellulare complex strains, in vitro and in vivo. It is also active against some rifampin-resistant strains of M. tuberculosis (6, 13). Our results indicate that some mutations (e.g., GAT516→AAT of M. leprae and GAC516→GAG of M. tuberculosis) show weak resistance to rifabutin.
Molecular methods designed to detect drug resistance have some limitations. In some cases, the identified mutations are not related to the acquisition of resistance. Caution is necessary when considering mutations, especially if the mutation detected in clinical isolates is not reported very often. For example, Q510H and L521M mutations were detected in rifampin-resistant M. tuberculosis isolates (21, 22), but our results suggest that these mutations are not responsible for rifampin resistance (Table 2). The method used for this study can directly assess the influence of designated mutations in rpoB. If the mutations can confer rifampin resistance, we can eliminate the possibility that genetic variation in some region other than rpoB on the chromosome of the clinical isolates is responsible for the resistance. Bahrmand et al. previously reported the high-level rifampin resistance of M. tuberculosis isolates with multiple mutations within the rpoB gene (1). Our method might also be useful for analyzing multiple mutations detected in the rpoB gene of clinical isolates to determine the contribution of each single mutation to rifampin resistance.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Health, Labor, and Welfare (Emerging and Re-Emerging Infectious Diseases).
Footnotes
Published ahead of print 17 January 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Bahrmand AR, Titov LP, Tasbiti AH, Yari S, Graviss EA. 2009. High-level rifampin resistance correlates with multiple mutations in the rpoB gene of pulmonary tuberculosis isolates from the Afghanistan border of Iran. J. Clin. Microbiol. 47:2744–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bardarov S, et al. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007–3017 [DOI] [PubMed] [Google Scholar]
- 3. Cambau E, et al. 2002. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin. Infect. Dis. 34:39–45 [DOI] [PubMed] [Google Scholar]
- 4. Cavusoglu C, Turhan A, Akinci P, Soyler I. 2006. Evaluation of the Genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 44:2338–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chikamatsu K, Mizuno K, Yamada H, Mitarai S. 2009. Cross-resistance between rifampicin and rifabutin among multi-drug resistant Mycobacterium tuberculosis strains. Kekkaku 84:631–633 (In Japanese.) [PubMed] [Google Scholar]
- 6. Dickinson JM, Mitchison DA. 1987. In vitro activity of new rifamycins against rifampicin-resistant M. tuberculosis and MAIS-complex mycobacteria. Tubercle 68:177–182 [DOI] [PubMed] [Google Scholar]
- 7. Franzblau SG, Hastings RC. 1988. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32:1758–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hauck Y, Fabre M, Vergnaud G, Soler C, Pourcel C. 2009. Comparison of two commercial assays for the characterization of rpoB mutations in Mycobacterium tuberculosis and description of new mutations conferring weak resistance to rifampicin. J. Antimicrob. Chemother. 64:259–262 [DOI] [PubMed] [Google Scholar]
- 9. Honore N, Cole ST. 1993. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob. Agents Chemother. 37:414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huitric E, Werngren J, Jureen P, Hoffner S. 2006. Resistance levels and rpoB gene mutations among in vitro-selected rifampin-resistant Mycobacterium tuberculosis mutants. Antimicrob. Agents Chemother. 50:2860–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kai M, et al. 2011. Analysis of drug-resistant strains of Mycobacterium leprae in an endemic area of Vietnam. Clin. Infect. Dis. 52:e127–e132 [DOI] [PubMed] [Google Scholar]
- 12. Kapur V, et al. 1994. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase beta subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J. Clin. Microbiol. 32:1095–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luna-Herrera J, Reddy MV, Gangadharam PR. 1995. In-vitro and intracellular activity of rifabutin on drug-susceptible and multiple drug-resistant (MDR) tubercle bacilli. J. Antimicrob. Chemother. 36:355–363 [DOI] [PubMed] [Google Scholar]
- 14. Maeda S, et al. 2001. Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob. Agents Chemother. 45:3635–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClure WR, Cech CL. 1978. On the mechanism of rifampicin inhibition of RNA synthesis. J. Biol. Chem. 253:8949–8956 [PubMed] [Google Scholar]
- 16. Nakata N, Kai M, Makino M. 2011. Mutation analysis of the Mycobacterium leprae folP1 gene and dapsone resistance. Antimicrob. Agents Chemother. 55:762–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramaswamy S, Musser JM. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3–29 [DOI] [PubMed] [Google Scholar]
- 18. Rattan A, Kalia A, Ahmad N. 1998. Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives. Emerg. Infect. Dis. 4:195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rigouts L, et al. 2007. Newly developed primers for comprehensive amplification of the rpoB gene and detection of rifampin resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 45:252–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Telenti A, et al. 1993. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647–650 [DOI] [PubMed] [Google Scholar]
- 21. Williams DL, et al. 1994. Characterization of rifampin-resistance in pathogenic mycobacteria. Antimicrob. Agents Chemother. 38:2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang B, et al. 1998. Relationship between antimycobacterial activities of rifampicin, rifabutin and KRM-1648 and rpoB mutations of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 42:621–628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.