Abstract
Toxoplasma gondii is a parasite that generates latent cysts in the brain; reactivation of these cysts may lead to fatal toxoplasmic encephalitis, for which treatment remains unsuccessful. We assessed spiramycin pharmacokinetics coadministered with metronidazole, the eradication of brain cysts and the in vitro reactivation. Male BALB/c mice were fed 1,000 tachyzoites orally to develop chronic toxoplasmosis. Four weeks later, infected mice underwent different treatments: (i) infected untreated mice (n = 9), which received vehicle only; (ii) a spiramycin-only group (n = 9), 400 mg/kg daily for 7 days; (iii) a metronidazole-only group (n = 9), 500 mg/kg daily for 7 days; and (iv) a combination group (n = 9), which received both spiramycin (400 mg/kg) and metronidazole (500 mg/kg) daily for 7 days. An uninfected control group (n = 10) was administered vehicle only. After treatment, the brain cysts were counted, brain homogenates were cultured in confluent Vero cells, and cysts and tachyzoites were counted after 1 week. Separately, pharmacokinetic profiles (plasma and brain) were assessed after a single dose of spiramycin (400 mg/kg), metronidazole (500 mg/kg), or both. Metronidazole treatment increased the brain spiramycin area under the concentration-time curve from 0 h to ∞ (AUC0–∞) by 67% without affecting its plasma disposition. Metronidazole plasma and brain AUC0–∞ values were reduced 9 and 62%, respectively, after spiramycin coadministration. Enhanced spiramycin brain exposure after coadministration reduced brain cysts 15-fold (79 ± 23 for the combination treatment versus 1,198 ± 153 for the untreated control group [P < 0.05]) and 10-fold versus the spiramycin-only group (768 ± 125). Metronidazole alone showed no effect (1,028 ± 149). Tachyzoites were absent in the brain. Spiramycin reduced in vitro reactivation. Metronidazole increased spiramycin brain penetration, causing a significant reduction of T. gondii brain cysts, with potential clinical translatability for chronic toxoplasmosis treatment.
INTRODUCTION
Toxoplasmosis is an important opportunistic infection in immunocompromised patients (12, 23). Infection in humans can occur via direct inoculation of tachyzoites, by the oral-fecal transmission of oocysts from domestic cats, through tissue cysts in undercooked meat, or by transfer from mother to fetus (24). The life cycle of Toxoplasma gondii is complex. The sexual cycle takes place in animals, with cats being the final host and humans the intermediate host during its asexual life cycle, which can result in dormant infections. T. gondii is able to cross the intestinal membrane, disseminate in body tissues, cross the blood-brain barrier (BBB), and migrate to the central nervous system (23). It is an obligate intracellular replication parasite (12), releasing more tachyzoites, which may form cysts containing bradyzoites that evade the immune system and remain in tissues (33).
Chronic toxoplasmosis is associated with tissue-localized cysts, primarily in the brain (3). It is asymptomatic in healthy humans, although some may develop symptoms (22). However, in immunocompromised hosts, the cysts may rupture and the bradyzoites revert to tachyzoites, causing acute-on-chronic toxoplasmosis (33), including toxoplasmic encephalitis, which may be fatal (22), especially in HIV patients (20). In vitro studies that mimic an immunocompromised state have shown that the conversion occurs within 1 week regardless of the age of the brain cysts (5). In immunocompetent hosts, the presence of cysts caused by T. gondii (referred to here as “T. gondii cysts”) in the brain seems to be associated with various neurological disorders (27), including cryptogenic epilepsy (37), migraine (25), schizophrenia (38), Malloret meningitis (26), and affective (36) and behavioral (11) disorders.
Treatment of chronic toxoplasmosis is hampered by the poor drug brain penetration to achieve therapeutic concentrations. The combined administration of sulfadiazine and pyrimethamine has shown efficacy against acute toxoplasmosis (18) but failed against chronic cerebral toxoplasmosis (10). In addition, the prolonged use of these drugs may cause hematologic and renal toxicity (7). Other combination treatments include atovaquone and clindamycin, which are effective during the acute infection (1, 8) and reduced the severity of toxoplasmic encephalitis relapses (9). However, low bioavailability, lack of brain penetration, and incipient resistance (17) hamper the full therapeutic potential of this combination. Another treatment option is spiramycin, a macrolide antibiotic, which is effective against acute toxoplasmosis, less toxic than other drugs, and able to achieve high concentrations in the placenta (30). Spiramycin has an oral bioavailability of ca. 35%, demonstrates low plasma protein binding (20%; which may explain the extensive tissue penetration), and is associated with hepatic to active metabolites (32). The elimination half-life (t1/2) is around 6 to 8 h; spiramycin is excreted in bile and breast milk (13), and ca. 14% is recovered unchanged in urine and 80% through feces (35). However, in spite of significant tissue penetration, spiramycin demonstrates poor penetration across the BBB and does not reach effective concentrations in the brain due to the presence of the efflux transporters multidrug-resistant protein 2 (Mrp2) and P-glycoprotein, for which spiramycin is a substrate (14, 35).
Thus, we hypothesized that enhanced spiramycin brain uptake may be attained with the coadministration of a second drug to inactivate the efflux pumps present in the BBB reaching effective concentrations to eliminate T. gondii brain cysts. To validate this hypothesis, the pharmacokinetics and brain uptake of spiramycin were evaluated upon metronidazole coadministration. Subsequently, the ability to eradicate brain cysts was assessed in a validated model of chronic toxoplasmosis in mouse (5). The selection of metronidazole was based on its ability to inhibit the efflux pumps and to enhance the brain distribution of other drugs that lack brain penetration (34). In addition, metronidazole has no activity against T. gondii (21); therefore, it was not expected to affect the assessment of spiramycin's effectiveness against brain cysts.
MATERIALS AND METHODS
Ethics.
Prior to the initiation of the pharmacokinetic and efficacy studies, the Institutional Research and Ethics Committee of the International Medical University (Kuala Lumpur, Malaysia) reviewed and approved all experimental protocols.
Pharmacokinetic study.
BALB/c mice (male, 8 to 10 weeks of age) were purchased from the University Putra Malaysia (Kuala Lumpur, Malaysia) and kept under a 12-h light cycle in the animal holding facility and allowed food and water ad libitum. Spiramycin and metronidazole were purchased from Sigma-Aldrich (USA) and stored at 4°C protected from light. Dosing solutions were prepared with ultra pure water and administered using a 22G feeding needle (Braintree Scientific, Inc., USA) after overnight fast. The plasma and brain pharmacokinetic profiles were determined after administration of 400 mg/kg, a single oral dose of spiramycin (control group), or after coadministration with 500 mg of metronidazole/kg 30 min before the dose of spiramycin (study group). Metronidazole was administered 30 min prior to the dose of spiramycin to allow tissue uptake and distribution of metronidazole in order to maximize its effect at the BBB. An additional group was given 500 mg of metronidazole/kg alone. At predetermined time points after spiramycin administration (0.5, 1, 2, 2.5, 4, 6, 8, 10, and 12 h), mice (n = 4 per time point in each study arm) were euthanized by cervical dislocation, and blood samples were collected via cardiac puncture with heparinized (Sigma-Aldrich, Germany) syringes (Terumo Corp., The Philippines) and transferred to 1.5-ml Eppendorf tubes. The brains were then harvested, rinsed with saline, and stored at −35°C. Plasma was separated by centrifugation at 1,500 rpm for 10 min at 4°C and kept at −35°C until analysis.
The plasma and brain concentrations of spiramycin and metronidazole were measured simultaneously by high-pressure liquid chromatography (HPLC) (6). Briefly, 100 μl of plasma was added to 200 μl of HPLC-grade acetonitrile (Merck, Germany), vortex mixed, and centrifuged (15,000 rpm, 15 min, 4°C), and the supernatant was transferred to microvials. The brain tissue was homogenized at 35,000 rpm in a mixture of acetonitrile (70%) and ultrapure water (30%) at a 1:2 brain/solvent ratio in an ice bath. An aliquot of homogenate was centrifuged (15,000 rpm, 15 min, 4°C), and the supernatant was transferred to HPLC vials. Extracted plasma and brain samples were injected (20 μl) into a C18 Phenomenex reversed-phase column (150 by 3.5 mm, 5-μm particle size) attached to an Agilent 1200 series system equipped with a UV detector set at 232 nm and a column oven at 29°C. Drugs were eluted with a mixture of 0.01 M phosphate buffer (pH 2.5) and an acetonitrile gradient (20 to 30%) for 3 min at 1 ml/min flow rate. Metronidazole and spiramycin eluted within 5 min and were quantified with the aid of an external calibration curve developed simultaneously. The range of linearity was 0.25 to 50 μg/ml (r2 > 0.999), the inter- and intraday variability, accuracy, and precision were within 15%, the lower limit of quantification was 0.25 μg/ml for metronidazole and spiramycin, the analysis was free of matrix interferences, and the extraction recovery was >75% for each drug (6).
Pharmacokinetic data analysis.
Noncompartmental analysis was used to calculate the main pharmacokinetic parameters. The maximum concentration (Cmax) in plasma and brain and the time to the maximum concentration (Tmax) were determined directly from the concentration-time graphs of each profile. The elimination rate constant (kel) was obtained by log-linear regression of the data in the terminal slope, and the elimination half-life (t1/2) was ln2/kel. The area under the curve (or exposure) from zero to the last concentration, i.e., Clast (AUC0–last) was calculated using the method of the trapezoids. The extrapolated AUC (AUClast–∞) was calculated as Clast/kel, and the total exposure (AUC0–∞) was the addition of both. The mean residence time (MRT) was calculated as the AUMC0–∞ (i.e., the area under the first moment of the curve) divided by the AUC0–∞. Because it is not possible to calculate the bioavailability (F) without intravenous administration of the drug, the clearance and volume of distribution cannot be calculated. Thus, the oral clearance (CL/F) was calculated as D/AUC0–∞, and the apparent volume of distribution at steady-state (VSS/F) was calculated as MRT × CL/F. Finally, the tissue distribution efficiency was estimated at the ratio of tissue to plasma exposure: AUC (brain)/AUC (plasma).
Statistical comparison of the AUC0–∞ obtained from pharmacokinetic profiles generated by sparse or destructive sampling was done using methods developed by Bailer (2) and Yuan (39). This scenario arises in tissue distribution studies because each experimental animal can only provide one sample concentration to the overall profile. Comparison of the Cmax and the concentration at each time point between the control and the study group was done using the Mann-Whitney U test. The analysis were considered significant when P < 0.05.
In vitro culture of T. gondii and chronic toxoplasmosis murine model.
Modified Dulbecco minimal essential culture medium (DMEM) was prepared adding 5 ml of penicillin-streptomycin (Gibco, USA) and 50 ml of heat-deactivated (56°C for 30 min) fetal bovine serum (Gibco) to 500 ml of DMEM (Gibco). T. gondii ME49 was obtained from the American Tissue Culture Collection (ATCC strain 50611). Proliferation of tachyzoites was achieved upon culture of an aliquot of DMEM reconstituted ME46 strain T. gondii in a 25-mm3 flask of confluent Vero E6 cells incubated at 37°C with 5% CO2. After 1 week, the infected Vero E6 cells were washed with phosphate-buffered saline (Calbiochem, USA) and trypsinized, and the tachyzoites were counted and harvested for the in vivo experiments (4).
Chronic toxoplasmosis in mice was established as previously described (5). Fifty randomly selected mice (as described above) were used in the in vivo efficacy experiment. Mice were allocated randomly to either the vehicle control group, the untreated control group or to the three treatment study groups (spiramycin, metronidazole, or coadministration). Mice in the untreated control and study groups were fed 1,000 tachyzoites orally using a 22G feeding needle (Braintree Scientific) to develop the acute-to-chronic toxoplasmosis conversion. Mice in the control group (uninfected) were administered vehicle without of tachyzoites.
In vivo efficacy experiments.
In this murine model, the onset of chronic toxoplasmosis has been established at 4 weeks postinfection (5). The mice that survived the acute infection at 4 weeks were further randomized and allocated to one of the following treatment groups: untreated vehicle control, treatment with spiramycin alone, treatment with metronidazole alone, or treatment with both drugs. Mice in the infected untreated group (control) received only vehicle, mice in the spiramycin group received a 400-mg/kg dose of spiramycin alone, mice in the metronidazole-alone group were given 500 mg of metronidazole/kg and, finally, the combination study group was given 400 mg of spiramycin/kg and 500 mg of metronidazole/kg. All groups received one dose daily for 7 days, the metronidazole was administered 30 min prior to the spiramycin, and the treatment was started at the onset of chronic toxoplasmosis determined previously (5). The experiment was repeated in order to validate the results (n = 9 in experiment 1 and n = 8 in experiment 2 for each group).
Upon the completion of treatment, the mice were euthanized 24 h after the last dose by cervical dislocation, the brains were harvested, rinsed with sterile saline solution, and weighed, and 1 ml of sterile saline was added, followed by homogenization (Omni TH-220) for 5 min. Then, 25 μl of brain homogenate was spread onto a glass slide (four slides per brain sample), air dried, fixed with absolute ethanol (Sigma-Aldrich, Malaysia) for 15 min, further air dried, stained with 10% of Azur-Eosin Giemsa stain (Merck, Germany) for 1 h, washed with water, and dried at 60°C, and coverslips fixed with xylene mounting solution (Merck). Finally, the cysts were counted under a microscope (Nikon Eclipse 80i), and the numbers of cysts in each brain were calculated (15).
In vitro reactivation study.
A 250-μl aliquot of brain homogenate, obtained as described above, was inoculated into a 25-mm3 flask where Vero E6 cells had been cultured to a confluent monolayer with modified DMEM. After 1 week of culture at 37°C and 5% CO2 the cells were washed, trypsinized, and centrifuged at 1,000 rpm for 5 min. The pellet was then collected and resuspended in 1 ml of sterile saline, and the numbers of cysts and tachyzoites were determined.
Statistical analysis of efficacy and in vitro studies.
The means and standard deviations (SD) were calculated, and the test of homogeneity of variances was performed to determine whether the parametric or nonparametric test should be selected for the analysis. The result showed unequal variances, and thus the Kruskal-Wallis test was chosen to analyze each of the groups versus the control group. Differences were considered significant if P < 0.05.
RESULTS
Pharmacokinetic profiles of spiramycin and metronidazole.
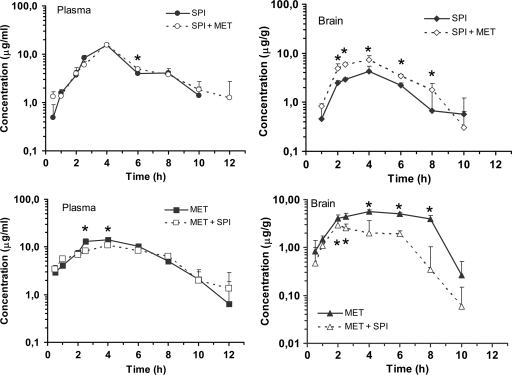
The plasma spiramycin pharmacokinetic profile in the control and study groups are shown in Fig. 1, and the pharmacokinetic parameters calculated using noncompartmental techniques are listed in Table 1. Metronidazole did not affect the plasma pharmacokinetics of spiramycin, and no significant differences were found in the Cmax, the total systemic exposure AUC0–∞, VSS/F, or other disposition parameters. However, metronidazole had a significant effect on the biodistribution of spiramycin to the brain (Fig. 1): there was a 1.7-fold increase in Cmax (P < 0.05), and the AUC0–∞, was 64% greater (P < 0.001) based on the Mann-Whitney U test and Yuan method, respectively. Other disposition parameters were slightly affected, the MRT and in-tissue disposition t1/2 were 13 and 26% shorter, and Tmax remained unchanged (Table 2).
Fig 1.
Pharmacokinetic profiles of spiramycin and metronidazole in plasma and the brain. The data are means ± the SD (n = 4 per time point).
Table 1.
Model independent pharmacokinetic parameters of spiramycin in plasma and brain after oral administration at 400 mg/kg (control group) or coadministered with metronidazole at 500 mg/kg (study group)a
| Parameter | Plasma |
Brain |
||
|---|---|---|---|---|
| Control group | Study group | Control group | Study group | |
| Cmax (μg/ml or μg/g) | 15.29 ± 1.64 | 15.41 ± 1.44 | 4.32 ± 1.11 | 7.45 ± 1.55 |
| Tmax (h) | 4 | 4 | 4 | 4 |
| kel (h−1) | 0.358 | 0.300 | 0.363 | 0.512 |
| t1/2 (h) | 1.9 | 2.3 | 1.9 | 1.4 |
| AUC0–∞ (μg·h/ml) | 60.75 ± 2.13 | 64.19 ± 3.66 | 20.64 ± 1.77 | 34.52 ± 1.70* |
| MRT (h) | 5.1 | 5.7 | 5.2 | 5.4 |
| VSS/F (liters/kg) | 33.8 | 35.6 | NA | NA |
| CL/F (liters/h/kg) | 6.6 | 6.2 | NA | NA |
| AUC0–∞ (brain)/AUC0–∞ (plasma) | 0.34 ± 0.04 | 0.54 ± 0.06 | ||
Values are averages or means ± the SD (where applicable).
, P < 0.001 (39). NA, not applicable.
Table 2.
Model independent pharmacokinetic parameters of metronidazole in plasma and brain after oral administration of 500 mg/kg (control group) or coadministered with 400 mg/kg of spiramycin (study group)a
| Parameter | Plasma |
Brain |
||
|---|---|---|---|---|
| Control group | Study group | Control group | Study group | |
| Cmax (μg/ml or μg/g) | 14.11 ± 0.57 | 10.96 ± 0.86 | 5.62 ± 0.43 | 2.95 ± 0.60 |
| Tmax (h) | 4 | 4 | 4 | 2 |
| kel (h−1) | 0.464 | 0.335 | 1.356 | 0.870 |
| t1/2 (h) | 1.5 | 2.1 | 0.5 | 0.8 |
| AUC0–∞ (μg·h/ml) | 85.15 ± 2.68 | 77.48 ± 3.77* | 36.94 ± 1.04 | 14.00 ± 1.66* |
| MRT (h) | 4.9 | 5.0 | 5.0 | 4.0 |
| VSS/F (liters/kg) | 29.0 | 32.4 | NA | NA |
| CL/F (liters/h/kg) | 5.9 | 6.5 | NA | NA |
| AUC0–∞ (brain)/AUC0–∞ (plasma) | 0.43 ± 0.03 | 0.18 ± 0.03 | ||
Values are averages or means ± the SD (where applicable).
, P < 0.001 (39). NA, not applicable.
The disposition profile of metronidazole was affected by spiramycin (Fig. 1). AUC0–∞ and Cmax were 9% (P < 0.05) and 22% (P < 0.001) lower in the study group than in the metronidazole control group, respectively, but Tmax remained unchanged (Table 2). A similar pattern was observed for metronidazole brain uptake: exposure was 62% lesser than when it was administered alone (P < 0.001), and the Cmax was achieved earlier and was also 47% lower than in the control group (P < 0.05).
In vivo efficacy evaluation.
The in vivo efficacy was assessed twice in order to validate the results. The acute infection survival rate was 90% in the first experiment (experiment 1) and 88% in the repeat experiment (experiment 2), and all mice in the vehicle control (uninfected) survived in both experiments. Chronic toxoplasmosis was well developed at the end of week 4, and brain cysts were established in both sets of experiments without statistical differences between them: 1,071 ± 169 versus 1,198 ± 153 in experiments 1 and 2, respectively (P > 0.05). These rates of survival and the number of brain cysts are in agreement with previous studies (5).
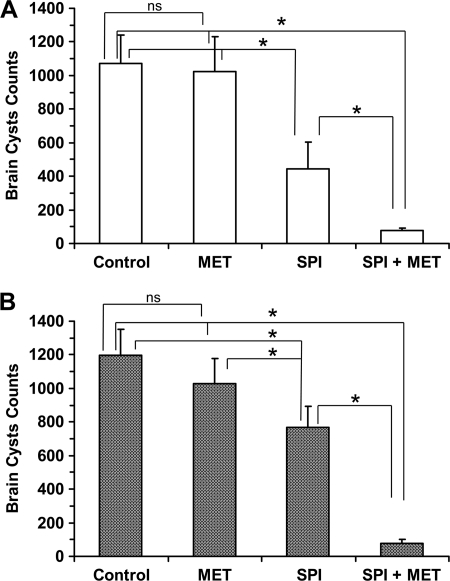
Metronidazole (500 mg/kg) did not have any effect on the brain cyst loads, which were 1,025 ± 207 in experiment 1 and 1,028 ± 148 in the repeat experiment (P > 0.05 between experiments), and this finding was similar to the untreated group of each experiment (P > 0.05). When the mice were treated with 400 mg of spiramycin/kg, the numbers of brain cysts were reduced by 58% (445 ± 156) and 36% (768 ± 125) in experiments 1 and 2, respectively, and this reduction was significantly different from the untreated control group (P < 0.05). In addition, there were no differences between experiments 1 and 2 (P > 0.05). Finally, mice that were administered spiramycin with metronidazole showed a large and significant (P < 0.05) reduction of brain cysts, a finding that was consistent in both experiments (76 ± 15 and 79 ± 23 for experiments 1 and 2, respectively) (Fig. 2). In experiment 1, the reduction of brain cysts was 14-fold compared to the untreated control group (1,071 ± 169; P < 0.05) and 6-fold compared to the spiramycin-treated group (445 ± 156; P < 0.05). The repeat experiment confirmed these results: the reduction of brain cysts was 15-fold upon comparison to the control group (1,198 ± 153; P < 0.05) and 10-fold in comparison to the spiramycin-treated group (768 ± 125; P < 0.05). In addition, no tachyzoites were detected in the brain in any of the control or study group animals in both experiments.
Fig 2.
Amounts of cysts in the brains of mice after treatment with vehicle (untreated control), spiramycin, metronidazole, or spiramycin coadministered with metronidazole. The data are means ± the SD (n = 9 per treatment group). (A) Experiment 1; (B) experiment 2.
In vitro reactivation.
The regeneration of cysts and conversion to tachyzoites were assessed in vitro (Table 3). There were no significant differences in the number of cysts in vitro between the untreated control groups (331 ± 87 and 285 ± 65 for experiments 1 and 2, respectively) and the metronidazole-treated groups (329 ± 80 and 288 ± 70 for experiments 1 and 2, respectively). Furthermore, no differences were found between experiments 1 and 2. After the administration of spiramycin to mice, the numbers of cysts in vitro were reduced to 184 ± 69 in experiment 1 and 166 ± 42 in experiment 2 (44 and 42%, respectively) (P < 0.05). The number of cysts cultured in vitro was lower after coadministration of spiramycin with metronidazole to mice. In experiment 1 the number of cysts was 102 ± 23, and in experiment 2 it was 120 ± 28 (68 and 58% reductions, respectively, compared to the untreated control group).
Table 3.
Cysts in vitro and reactivation of cysts and/or tachyzoites from cultures of brain homogenate obtained from untreated mice or mice treated with 500 mg of metronidazole/kg, 400 mg of spiramycin/kg (control group), or 400 mg of spiramycin/kg coadministered with 500 mg of metronidazole/kg (study group)
| Form | Expt | Mean no. of cysts ± SD |
|||
|---|---|---|---|---|---|
| Untreated | Metronidazole | Spiramycin (control) | Spiramycin + metronidazole | ||
| Cysts | 1 | 331 ± 87 | 329 ± 80 | 184 ± 69 | 102 ± 23 |
| 2 | 285 ± 64 | 288 ± 70 | 166 ± 42 | 120 ± 28 | |
| Tachyzoites | 1 | 695,333 ± 102,292 | 586,667 ± 51,636 | 106,889 ± 12,657 | 250,000 ± 64,185 |
| 2 | 719,125 ± 120,974 | 450,000 ± 73,466 | 140,000 ± 18,768 | 86,200 ± 13,670 | |
The effect of the treatments on the in vitro cyst-to-tachyzoite conversion was also assessed. Spiramycin reduced the number of tachyzoites in experiment 1 from 695,333 ± 102,292 to 106,889 ± 12,657 (P < 0.05) and in experiment 2 from 719,125 ± 120,974 to 140,000 ± 18,768 (P < 0.05). After the coadministration with metronidazole, the results were similar to those of the spiramycin-only group. Metronidazole alone did not have significant effect (Table 3).
DISCUSSION
The plasma pharmacokinetics and brain uptake of spiramycin has been evaluated upon oral coadministration with metronidazole to mice. Both drugs were simultaneously quantified using a validated HPLC assay, and the disposition profiles analyzed using model-independent pharmacokinetic techniques. The activity of spiramycin against T. gondii brain cysts was then studied in a mouse model of chronic toxoplasmosis, and the reactivation ability of the brain cysts was assessed in vitro.
Spiramycin coadministration with metronidazole caused a pharmacokinetic drug-drug interaction that was not manifested in plasma but led to 67% higher spiramycin uptake in the brain. The higher uptake reached efficacious concentrations in the brain to achieve almost the full eradication of T. gondii brain cysts: spiramycin-only treatment reduced the number of brain cysts 3-fold (58%) and 14-fold upon coadministration of spiramycin with metronidazole. The effect was not due to metronidazole, which showed no difference from the untreated control group, and did not contribute to spiramycin effect in vitro. The in vivo efficacy and the in vitro reactivation studies were repeated, the results were confirmed, and the study hypothesis was verified: spiramycin treatment efficacy against chronic cerebral toxoplasmosis may be improved upon a drug-drug interaction at the BBB level.
Differential effects of metronidazole at GIT and BBB levels.
Metronidazole administration did not affect the rate and extent of spiramycin absorption: the plasma Cmax, Tmax, and AUC0–∞ values remained unchanged, suggesting that metronidazole did not influence intestinal wall efflux mechanisms and did not affect spiramycin absorption. In our study, metronidazole failed to affect spiramycin absorption, which is consistent with previous drug-drug interaction studies that showed that metronidazole did not affect the absorption of the P-glycoprotein substrates imatinib (34) and fexofenadine (19). In fact, the role of metronidazole in the inhibition of P-glycoprotein-mediated transport remains inconclusive (28).
The interaction of metronidazole with other drugs has been reported to affect tissue distribution rather than the overall bioavailability. Tan et al. reported that when imatinib was administered with metronidazole to mice, the plasma AUC0–∞ was not affected but brain, liver, and kidney exposures were significantly increased (34). Metronidazole coadministration with spiramycin significantly increased spiramycin brain uptake: the brain AUC0–∞ was 67% greater, and the Cmax increased 72%. This shows that metronidazole affected the permeability of the BBB to allow spiramycin penetration but probably acting on a different transporter than at the intestinal wall membrane. Mechanistic studies in mice have shown that spiramycin bile excretion is mediated by Mrp2 and P-glycoprotein efflux transporters, although P-glycoprotein would play a secondary role (35). Metronidazole may inhibit Mrp2 and P-glycoprotein present at the BBB (14, 34) and prevent the efflux of spiramycin, an Mrp2 substrate (35), leading to spiramycin-enhanced brain uptake and higher accumulation to reach efficacious concentrations. The efficiency of this interaction can further be quantified by using the tissue/plasma AUC0–∞ ratio. The tissue/plasma ratio increased 58% (Table 1) in the coadministration group, which strongly suggests that the higher spiramycin brain exposure is due to metronidazole effects on efflux/transport mechanisms at the BBB rather than changes in plasma exposure.
Finally, it may be worth noting that contrary to other studies in rat (31) or monkey (29) that lack spiramycin brain penetration, we found brain uptake in mice, probably due to species variability, including affinity and localization of the transporters (Fig. 1).
Spiramycin effect on metronidazole.
The pharmacokinetic parameters of metronidazole suggest a slight effect of spiramycin on the plasma disposition profile of metronidazole. The t1/2, MRT, CL/F, and VSS/F values remain unchanged, Cmax was 77% of that found for the control group but was not significant (probably due to variability) (Table 2), and the AUC0–∞ was 9% lower than that of the control (P < 0.05). It is likely that spiramycin has little effect on metronidazole plasma pharmacokinetics. However, metronidazole brain uptake was affected, leading to a 50% lower Cmax and a 62% reduction in the AUC0–∞ (P < 0.05). This reduction of brain uptake cannot be explained by the slight reduction of plasma exposure, but the loss of uptake efficiency: brain/plasma AUC0–∞ ratio was 42% after coadministration with spiramycin (Table 2). This suggests that spiramycin may block the brain uptake transport system that is used by metronidazole. Active transport uptake mechanisms at the BBB have been identified for other drugs (16), but a full conclusion for this drug-drug interaction is beyond the scope of the present study.
Elimination of T. gondii cysts in mouse brain.
The method of inducing chronic toxoplasmosis in mice by oral feeding of T. gondii tachyzoites has been validated. In this model, conversion of tachyzoites to cysts (bradyzoites) in brain tissue begins 2 weeks after acute oral infection and reaches a plateau at 4 weeks, marking the onset of chronic toxoplasmosis (5). Therefore, in the present study, the treatment of chronic toxoplasmosis was started 4 weeks after acute infection.
The drug combination spiramycin-metronidazole showed an extraordinary efficacy against chronic cerebral toxoplasmosis: there was a 10-fold reduction in brain cysts compared to the spiramycin-treated group, and there was a 15-fold reduction compared to the untreated control group (Fig. 2). Metronidazole, which lacks efficacious activity against toxoplasmosis (21), causes a pharmacokinetic interaction to enhance the spiramycin concentration in the brain that is able to eradicate tachyzoites and greatly decrease the cysts in the brain. Clearly, this effect was not associated with the antimicrobial activity of metronidazole, which did not reduce the number of brain cysts (Fig. 2) and have any effect on their in vitro reactivation (Table 3).
However, it should be noted that after the coadministration of spiramycin with metronidazole, the number of in vitro cultured brain cysts after 1 week of incubation was greater than the number of brain cysts obtained from the euthanized mice in the combination treatment group: 102 ± 23 in vitro versus 76 ± 15 in the brain for experiment 1 and 120 ± 28 in vitro versus 79 ± 23 in the brain for experiment 2. This may be due to the early encystment of the reactivated tachyzoites in vitro. The rapid conversion of bradyzoites to tachyzoites and back to bradyzoites in vitro gives a clue regarding the virulence, resistance, and chronicity of T. gondii, especially in immunocompromised hosts, and stresses the importance of an effective treatment regime to eliminate the Toxoplasma brain cysts completely.
In the in vitro reactivation experiments, metronidazole lacked activity and did not significantly reduce the number of cysts or tachyzoites. Spiramycin led to a large reduction of the cyst-to-tachyzoite conversion, but this reduction was similar when the mice were treated with spiramycin alone or after coadministration with metronidazole. Thus, spiramycin in vitro activity was not enhanced when metronidazole was coadministered (Table 3). This provides an additional insight to explain the mechanism for which metronidazole would enhance spiramycin activity in vivo but not in vitro. The efficacy of the coadministration would emerge from the ability of metronidazole to enhance the brain's uptake of spiramycin rather than a direct effect on the tachyzoites or the conversion of tachyzoites to bradyzoites, as shown by the lack of activity in the in vitro experiments.
Clinical translatability.
The synergistic drug combination based on a pharmacokinetic interaction has potential clinical implications for the treatment and prevention of chronic toxoplasmosis. Although the current experiment is developed in a chronic cerebral toxoplasmosis model, the results provide a potential pathway for the treatment of chronic toxoplasmosis, avoiding the risk of reactivation in vivo encephalitis, which could be fatal.
The novel concept of using metronidazole to enhance spiramycin brain uptake was validated in pharmacokinetic terms and translated to significant efficacy against cerebral toxoplasmosis. Further work to optimize the dose ratios between spiramycin and metronidazole and inquiries regarding other efflux inhibitors are needed to realize the full potential of this interaction and its translatability into a novel therapeutic approach.
Conclusions.
Spiramycin, when coadministered with metronidazole, was shown to be effective in the treatment of chronic toxoplasmosis in a mouse model. The combined administration of spiramycin and metronidazole led to increased brain uptake of spiramycin and achieved almost complete elimination of brain cysts. The in vitro cyst-to-tachyzoite-to-cyst conversion occurred within 1 week of incubation, and there was a great inhibition of reactivation of tachyzoites and encystment of tachyzoites in vitro.
ACKNOWLEDGMENT
This study was fully supported by International Medical University grant 163/08.
Footnotes
Published ahead of print 23 January 2012
REFERENCES
- 1. Baggish AL, Hill DR. 2002. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 46:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailer AJ. 1988. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokinet. Biopharm. 16:303–309 [DOI] [PubMed] [Google Scholar]
- 3. Brenier-Pinchart MP, et al. 2004. Infection of human astrocytes and glioblastoma cells with Toxoplasma gondii: monocyte chemotactic protein-1 secretion and chemokine expression in vitro. Acta Neuropathol. 107:245–249 [DOI] [PubMed] [Google Scholar]
- 4. Chatterton JM, Evans R, Ashburn D, Joss AW, Ho-Yen DO. 2002. Toxoplasma gondii in vitro culture for experimentation. J. Microbiol. Methods 51:331–335 [DOI] [PubMed] [Google Scholar]
- 5. Chew WK, Wah MJ, Ambu S, Segarra I. 2012. Toxoplasma gondii: determination of the onset of chronic infection in mice and the in vitro reactivation of brain cysts. Exp. Parasitol. 130:22–25 [DOI] [PubMed] [Google Scholar]
- 6. Chew WK, Segarra I. 2011. Simultaneous HPLC determination of metronidazole and spiramycin in plasma and brain of mouse. Curr. Pharm. Anal. 7:262–267 [Google Scholar]
- 7. Crespo M, et al. 2000. Patterns of sulfadiazine acute nephrotoxicity. Clin. Nephrol. 54:68–72 [PubMed] [Google Scholar]
- 8. Djurkovic-Djakovic O, Nikolic T, Robert-Gangneux F, Bobic B, Nikolic A. 1999. Synergistic effect of clindamycin and atovaquone in acute murine toxoplasmosis. Antimicrob. Agents Chemother. 43:2240–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunay IR, et al. 2004. Atovaquone maintenance therapy prevents reactivation of toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis. Antimicrob. Agents Chemother. 48:4848–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faucher B, Moreau J, Zaegel O, Frank J, Piarroux P. 2011. Failure of conventional treatment with pyrimethamine and sulfadiazine for secondary prophylaxis of cerebral toxoplasmosis in a patient with AIDS. J. Antimicrob. Chemother. 66:1654–1656 [DOI] [PubMed] [Google Scholar]
- 11. Fekadu A, Shibre T, Clere AJ. 2010. Toxoplasmosis as a course for behaviour disorders: overview of evidence and mechanisms. Folia Parasitol. 57:105–113 [DOI] [PubMed] [Google Scholar]
- 12. Ferreira da Silva MF, Barbosa HS, Gross U, Luder CG. 2008. Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol. Biosyst. 8:824–834 [DOI] [PubMed] [Google Scholar]
- 13. Frydman AM, Le Roux Y, Desnottes JF. 1988. Pharmacokinetics of spiramycin in man. J. Antimicrob. Chemother. 22:93–103 [DOI] [PubMed] [Google Scholar]
- 14. Grover A, Benet LZ. 2009. Effects of drug transporters on volume of distribution. AAPS J. 11:250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grujic J, Djurkovic-Djakovic O, Nikilic A, Klun I, Bobic D. 2005. Effectiveness of spiramycin in murine models of acute and chronic toxoplasmosis. J. Antimicrob. Agents 25:226–230 [DOI] [PubMed] [Google Scholar]
- 16. Henthorn TK, Liu Y, Mahapatro M, Ng KY. 1999. Active transport of fentalyl by the blood-brain barrier. J. Pharmacol. Exp. Ther. 289:1084–1089 [PubMed] [Google Scholar]
- 17. Hughes LH, et al. 2011. Design of anti-parasitic and anti-fungal hydroxy-naphthoquinones that are less susceptible to drug resistance. Mol. Biochem. Parasitol. 177:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Israelski DM, Remington JS. 1993. Toxoplasma gondii is an intracellular protozoan parasite: toxoplasmosis in the non-AIDS immunocompromised host. Curr. Clin. Top. Infect. 13:322–356 [PubMed] [Google Scholar]
- 19. Kim KA, Park JY. 2010. Effect of metronidazole on the pharmacokinetics of fexofenadine, a P-glycoprotein substrate, in healthy male volunteers. Eur. J. Clin. Pharmacol. 66:721–725 [DOI] [PubMed] [Google Scholar]
- 20. Luft BJ, Remington JS. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211–222 [DOI] [PubMed] [Google Scholar]
- 21. Mark DG, McLeod R. 1984. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob. Agents Chemother. 26:26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 23. Munoz M, Liesenfeld O, Heimesaat MM. 2011. Immunology of Toxoplasma gondii. Immunol. Rev. 240:269–285 [DOI] [PubMed] [Google Scholar]
- 24. Ortiz-Alegría LB, et al. 2010. Congenital toxoplasmosis: candidate host immune genes relevant for vertical transmission and pathogenesis. Genes Immun. 11:363–373 [DOI] [PubMed] [Google Scholar]
- 25. Prandota J. 2010. Migraine associated with patent foramen ovale may be caused by reactivation of cerebral toxoplasmosis triggered by arterial blood oxygen desaturation. Int. J. Neurosci. 120:81–87 [DOI] [PubMed] [Google Scholar]
- 26. Prandota J. 2009. Mollaret meningitis may be caused by reactivation of latent cerebral toxoplasmosis. Int. J. Neurosci. 119:1655–1692 [DOI] [PubMed] [Google Scholar]
- 27. Prandota J. 2009. The importance of Toxoplasma gondii infection in diseases presenting with headaches: headaches and aseptic meningitis may be manifestations of the Jarisch-Herxheimer reaction. Int. J. Neurosci. 119:2144–2182 [DOI] [PubMed] [Google Scholar]
- 28. Roedler R, Neuhauser MM, Penzak SR. 2007. Does metronidazole interact with cyp3a substrates by inhibiting their metabolism through this metabolic pathway? Or should other mechanisms be considered? Ann. Pharmacother. 41:653–658 [DOI] [PubMed] [Google Scholar]
- 29. Schoondermark-Van de Ven E, et al. 1994. Pharmacokinetics of spiramycin in the rhesus monkey: transplacental passage and distribution in tissue in the fetus. Antimicrob. Agents Chemother. 38:1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serranti- D, Buonsenso D, Valentini P. 2011. Congenital toxoplasmosis treatment. Eur. Rev. Med. Pharmacol. Sci. 15:193–198 [PubMed] [Google Scholar]
- 31. Shi XG, Sun YM, Zhang YF, Zhong DF. 2004. Tissue distribution of bitespiramycin and spiramycin in rats. Acta Pharmacol. Sin. 25:1396–1401 [PubMed] [Google Scholar]
- 32. Shi XG, Fawcett JP, Chen XY, Zhong DF. 2005. Structural identification of bitespiramycin metabolites in rat: a single oral dose study. Xenobiotica 35:343–358 [DOI] [PubMed] [Google Scholar]
- 33. Skariah S, McIntyre MK, Mordue DG. 2010. Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion. Parasitol. Res. 107:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tan SY, et al. 2011. Metronidazole leads to enhanced uptake of imatinib in brain, liver and kidney without affecting its plasma pharmacokinetics in mice. J. Pharm. Pharmacol. 63:918–925 [DOI] [PubMed] [Google Scholar]
- 35. Tian X, et al. 2007. Roles of P-glycoprotein, BcrP, and Mrp2 in biliary excretion of spiramycin in mice. Antimicrob. Agents Chemother. 51:3230–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Webster JP, Lamberton PH, Donnelly CA, Torrey EF. 2006. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer, and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proc. Biol. Sci. 273:1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yazar S, et al. 2003. Investigation of probable relationship between Toxoplasma gondii and cryptogenic epilepsy. Seizure 12:107–109 [DOI] [PubMed] [Google Scholar]
- 38. Yolken RH, Dickerson FB, Torrey EF. 2009. Toxoplasma and schizophrenia. Parasite Immunol. 31:706–715 [DOI] [PubMed] [Google Scholar]
- 39. Yuan J. 1993. Estimation of variance for AUC in animal studies. J. Pharm. Sci. 82:761–763 [DOI] [PubMed] [Google Scholar]