LETTER
In Taiwan, the majority of carbapenem-resistant Enterobacteriaceae (CRE) isolates exhibited low-level carbapenem resistance, and with the exception of a few isolates with VIM or IMP-8 carbapenemase (6, 7, 13), most were due to the production of extended spectrum β-lactamase (ESBL) and/or AmpC β-lactamase plus outer membrane protein porin loss (2, 7, 14). To date, there has been only one case of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae reported in Taiwan from a patient who was hospitalized in China prior to being transferred back to Taiwan. The isolate harbored KPC-2, but the genetic background of the strain was not mentioned (3).
We report the detection of four KPC-2-positive K. pneumoniae isolates from two patients in another hospital in Taiwan. Patient A was a 74-year-old Taiwanese male who was hospitalized in China for emergency medical treatment due to sudden cardiac arrest in 2010. He was transferred back to the coronary care unit (CCU) of a Taiwanese hospital 5 days later. He developed urinary tract infection due to a carbapenem-resistant K. pneumoniae (CRKP) isolate (CRKP1) 5 days after being admitted to the CCU. Two additional CRKP isolates were recovered from the central venous catheter (CRKP3) and from urine (CRKP4) 5 weeks after admission. Unfortunately, he expired due to the occurrence of hepatoma rupture and shock. Patient B was an 87-year-old Taiwanese male who was hospitalized in the same CCU because of congestive heart failure during the same period. He developed pneumonia, and CRKP isolates were recovered from the central venous catheter tip and from a sputum specimen 1 day apart. Only his sputum isolate (CRKP2) was available for further workup. CRKP2 was isolated 19 days after CRKP1. Patient B later accepted hospice care due to the terminal stage of congestive heart failure.
The pulsed-field gel electrophoresis (PFGE) patterns of CRKP1 to CRKP4 isolates were indistinguishable (data not shown). All four isolates had the same antibiogram and were resistant to all tested β-lactams (Table 1), with high ertapenem, imipenem, and meropenem MICs (≥32 μg/ml), and susceptible only to polymyxin B, tigecycline, and trimethoprim-sulfamethoxazole. All four were positive for blaKPC-2, as well as blaSHV-12 and blaCTX-M, and belong to sequence type 11 (ST11) (allelic profile 3-3-1-1-1-4) (5, 8, 9, 11). The plasmids of CRKP1 and CRKP2 were introduced into Escherichia coli DH10B by electroporation. The pCRKP1/DH10B and pCRKP2/DH10B electrotransformants became resistant to all tested β-lactams (Table 1), including carbapenems (MICs of 12 to >32 μg/ml), and were positive for blaKPC-2 and blaSHV-12 but not blaCTX-M. Restriction fingerprinting patterns of plasmid DNAs from the transformants were similar (Fig. 1).
Table 1.
MICs of four KPC-2-positive carbapenem-resistant Klebsiella pneumoniae clinical strains (CRKP1 to CRKP4), Escherichia coli DH10B electrotransformants, and DH10B
| Antimicrobial agent | MIC (μg/ml) of each strain or group of strainsa |
||
|---|---|---|---|
| K. pneumoniae CRKP1 to CRKP4b | Electrotransformants of DH10Bc | E. coli DH10Bd | |
| Amikacin | >128 | 2 | 2 |
| Ampicillin | >256 | >256 | 4 |
| Aztreonam | >256 | >256 | 0.19 |
| Cefepime | 256 | >256 | 0.064 |
| Cefotaxime | >256 | >256 | 0.125 |
| Cefoxitin | >256 | 96 | 6 |
| Ceftazidime | >256 | >256 | 0.38 |
| Cefuroxime | >256 | >256 | 4 |
| Ceftriaxone | >256 | >256 | 0.094 |
| Ciprofloxacin | >32 | 0.004 | 0.004 |
| Ertapenem | >32 | 32 | 0.008 |
| Fosfomycin | 128 | 1 | 1 |
| Gentamicin | >128 | 0.75 | 0.5 |
| Imipenem | 32 | 32 | 0.25 |
| Meropenem | >32 | 12-24 | 0.032 |
| Piperacillin-tazobactam | >256 | >256 | 2 |
| Polymyxin B | 1.5 | 0.5 | 0.19 |
| Tigecycline | 1.0-1.5 | 0.25 | 0.19 |
| TMP/SMX (SXT)e | 0.5 | 0.047 | 0.047 |
MIC data shown are from Etest. All agents except fosfomycin were also tested by a broth microdilution method (4).
In K. pneumoniae CRKP1 to CRKP4, genes encoding SHV-12- and CTX-M-type ESBLs were detected, no AmpC β-lactamase genes were detected, the KPC-2 carbapenemase gene was detected, and the modified Hodge test (MHT) was positive with ertapenem.
pCRKP1/DH10B and pCRKP2/DH10B electrotransformants. In these electrotransformants, the gene encoding SHV-12 ESBL was detected, no AmpC β-lactamase genes were detected, the KPC-2 carbapenemase gene was detected, and the modified Hodge test (MHT) was positive with ertapenem.
In E. coli DH10B, no ESBL, AmpC β-lactamase, and carbapenemase genes were detected, and the modified Hodge test (MHT) with ertapem was negative.
TMP-SMX (SXT), trimethoprim-sulfamethoxazole.
Fig 1.
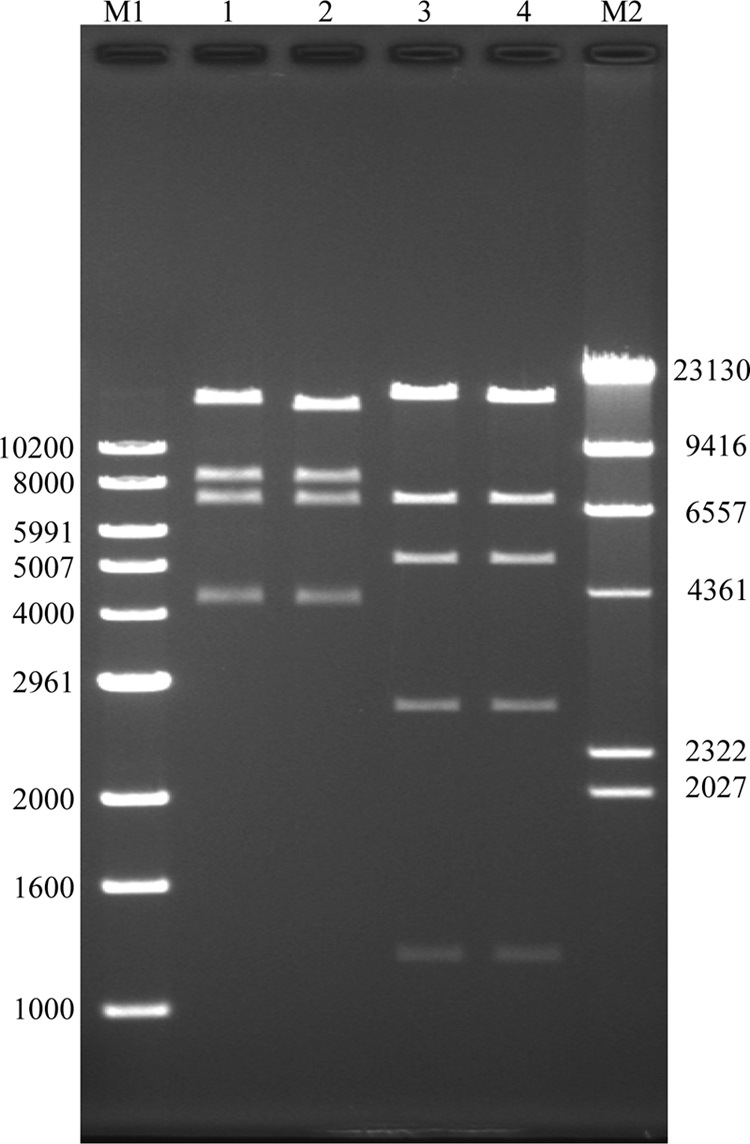
BglII (lanes 1 and 2) and SacI (lanes 3 and 4) restriction digest of plasmid DNAs from E. coli DH10B electrotransformants. M1, 1-kb DNA ladder (numbers are bp); lanes 1 and 3, pCRKP1/DH10B; lanes 2 and 4, pCRKP2/DH10B. CRKP1 and CRKP2 were isolated from 2 patients of the same ward 19 days apart. M2, λ/HindIII ladder.
In Asia, KPC-producing K. pneumoniae was first detected in a 2004 isolate from China (12), where KPC-2-producing Enterobacteriaceae bacteria were subsequently disseminated in different regions (1, 10, 15). ST11 was found to be the predominant KPC-2-producing K. pneumoniae sequence type isolated from multiple cities of China (10). Those ST11 isolates also carried a combination of SHV-type and CTX-M-type ESBLs and AmpC β-lactamases (10).
It is possible that patient A acquired KPC-producing K. pneumoniae during his hospitalization in China and that the strain was then transmitted to patient B in the same ward in Taiwan. Although the prevalence of carbapenem-resistant Enterobacteriaceae in Taiwan has remained low (2, 7, 14), the emergence of KPC-producing K. pneumoniae is worrisome since multidrug-resistant Enterobacteriaceae bacteria are already prevalent in Taiwan, necessitating increased carbapenem use. Careful monitoring systems need to be implemented and should include patients transferred from hospitals abroad.
ACKNOWLEDGMENT
This project was supported by an intramural grant from the National Health Research Institutes, Zhunan, Taiwan (99-A1-CLPP01-014).
Footnotes
Published ahead of print 30 January 2012
Contributor Information
Tsai-Ling Lauderdale, National Institute of Infectious Diseases and Vaccinology National Health Research Institutes Zhunan, Taiwan.
Zhi-Yuan Shi, Division of Infectious Diseases Department of Internal Medicine Taichung Veterans General Hospital Taichung, Taiwan.
Chin-Fu Lin, Department of Laboratory Medicine Taichung Veterans General Hospital Taichung, Taiwan.
Mei-Chen Tan, National Institute of Infectious Diseases and Vaccinology National Health Research Institutes Zhunan, Taiwan.
Shan-Chwen Chang, Division of Infectious Diseases Department of Internal Medicine National Taiwan University Hospital Taipei, Taiwan.
REFERENCES
- 1. Cai JC, Zhou HW, Zhang R, Chen GX. 2008. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob. Agents Chemother. 52:2014–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chia JH, et al. 2010. Development of high-level carbapenem resistance in Klebsiella pneumoniae among patients with prolonged hospitalization and carbapenem exposure. Microb. Drug Resist. 16:317–325 [DOI] [PubMed] [Google Scholar]
- 3. Chung KP, et al. 2011. Arrival of Klebsiella pneumoniae carbapenemase (KPC)-2 in Taiwan. J. Antimicrob. Chemother. 66:1182–1184 [DOI] [PubMed] [Google Scholar]
- 4. CLSI 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee MF, Peng CF, Hsu HJ, Chen YH. 2008. Molecular characterisation of the metallo-β-lactamase genes in imipenem-resistant Gram-negative bacteria from a university hospital in southern Taiwan. Int. J. Antimicrob. Agents 32:475–480 [DOI] [PubMed] [Google Scholar]
- 7. Liu YF, Yan JJ, Ko WC, Tsai SH, Wu JJ. 2008. Characterization of carbapenem-non-susceptible Escherichia coli isolates from a university hospital in Taiwan. J. Antimicrob. Chemother. 61:1020–1023 [DOI] [PubMed] [Google Scholar]
- 8. Monstein HJ, et al. 2007. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 115:1400–1408 [DOI] [PubMed] [Google Scholar]
- 9. Perez-Perez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi Y, et al. 2011. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 66:307–312 [DOI] [PubMed] [Google Scholar]
- 11. Queenan AM, Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei ZQ, et al. 2007. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob. Agents Chemother. 51:763–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan JJ, Ko WC, Tsai SH, Wu HM, Wu JJ. 2001. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying blaIMP-8 in a university medical center in Taiwan. J. Clin. Microbiol. 39:4433–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan JJ, Wu JJ, Lee CC, Ko WC, Yang FC. 2010. Prevalence and characteristics of ertapenem-nonsusceptible Escherichia coli in a Taiwanese university hospital, 1999 to 2007. Eur. J. Clin. Microbiol. Infect. Dis. 29:1417–1425 [DOI] [PubMed] [Google Scholar]
- 15. Zhang R, et al. 2011. Outbreak of Klebsiella pneumoniae carbapenemase 2-producing Klebsiella pneumoniae with high qnr prevalence in a Chinese hospital. J. Med. Microbiol. 60:977–982 [DOI] [PubMed] [Google Scholar]


