Abstract
Elizabethkingia meningoseptica, a Gram-negative rod widely distributed in the environment, is resistant to most β-lactam antibiotics. Three bla genes have been identified in E. meningoseptica, coding for the extended-spectrum serine-β-lactamase CME (class D) and two unrelated wide-spectrum metallo-β-lactamases, BlaB (subclass B1) and GOB (subclass B3). E. meningoseptica is singular in being the only reported microorganism possessing two chromosomally encoded MBL genes. Real-time PCR and biochemical analysis demonstrate that the three bla genes are actively expressed in vivo as functional β-lactamases. However, while CME elicits cephalosporin resistance, BlaB is the only β-lactamase responsible for E. meningoseptica resistance to imipenem, as GOB activity is masked by higher cellular levels of BlaB. On the other hand, we demonstrate that blaBlaB expression is higher in the stationary phase or under conditions that mimic the nutrient-limiting cerebrospinal fluid colonized by E. meningoseptica in human meningitis.
INTRODUCTION
Carbapenemases are the most versatile family of β-lactamases, with an unrivalled substrate spectrum (44). Although known as carbapenemases, most of them recognize almost all hydrolyzable β-lactams and are resilient against inhibition by most clinically useful β-lactamase inhibitors (13). This group of enzymes consists primarily of Zn(II)-dependent metallo-β-lactamases (MBLs) (13).
In many cases MBL genes are carried in mobile genetic elements, thus being able to disseminate among opportunistic pathogens, including members of the Enterobacteriaceae (43, 45). Integrons containing MBL genes together with other resistance cassettes are increasingly common in clinics, resulting in pan-resistance phenotypes (44). Outbreaks of VIM-2- or NDM-1-producing pathogens are rising in incidence all over the world (16). In addition to clinically relevant MBLs, many of these enzymes are produced by a wide variety of environmental bacteria (4, 6, 7, 11, 14, 28, 31, 35). It has been speculated that expression of MBLs by these organisms might confer resistance against natural antibiotics produced by microorganisms living in the same environmental niche (48). Given that natural antibiotics at low concentrations may act as signaling molecules, it has been proposed that MBLs could act as quenching enzymes with these molecules (48). Whatever their role is, environmental bacteria harboring MBL genes provide gene reservoirs for emergent carbapenem resistance (6, 7, 11, 14, 28, 31, 35).
Elizabethkingia meningoseptica (formerly Flavobacterium meningosepticum and Chryseobacterium meningosepticum, from the Bacteroidetes phylum) is a Gram-negative rod that is widely distributed in the environment (9, 10, 20, 24, 25, 27). This organism generally acts as an opportunistic pathogen, causing meningitis in premature and newborn infants and pneumonia, postoperative bacteremia, and meningitis in adults, usually associated with severe underlying illness (9, 10, 20, 24, 25, 27). E. meningoseptica is usually multiresistant to antibiotics typically prescribed for Gram-negative bacterial infections, including most extended-spectrum β-lactams (5, 10, 20, 24, 25, 27). E. meningoseptica is the only known microorganism with two chromosome-borne intrinsic MBL genes (5).
MBLs (also known as class B β-lactamases) have been classified into three subclasses according to sequence homology: B1 (which includes most of the broad-spectrum, clinically relevant MBLs), B2 (the smallest subclass, comprising exclusive carbapenemases), and B3 (the more distant group in terms of phylogeny) (17, 18). Three β-lactamase genes have been identified in E. meningoseptica: blaCME, coding for the class D serine-β-lactamase (SBL) CME (5, 8, 36), and blaBlaB and blaGOB, coding for two unrelated MBLs, BlaB (subclass B1) and GOB (subclass B3), respectively (5, 29, 32, 37, 41).
Reported catalytic efficiencies obtained from pure recombinant enzymes suggest that both MBLs are likely responsible for E. meningoseptica resistance to carbapenems (CME is incapable of hydrolyzing these antibiotics) (5, 8, 29, 32, 36, 37, 41). Resistance to penicillins and cephalosporins could be due to expression of any of the three β-lactamases, as they show similar catalytic efficiencies (5, 8, 20, 29, 32, 36, 37, 41).
MBL-producing clinical pathogens, in general, escape the action of carbapenems by expressing a single MBL whose catalytic efficiency is similar to that of BlaB or GOB (4). The expression of two MBL genes is particularly puzzling for a bacterium not highly exposed to β-lactam antibiotics. It is interesting to elucidate then the roles of BlaB and GOB in E. meningoseptica resistance to carbapenems.
Here we report a biochemical, microbiological, and transcriptional study on a clinical strain of E. meningoseptica (32) which was aimed to assess the role of both MBLs in the carbapenem resistance of this organism. We show that while cephalosporin resistance is mainly due to CME, BlaB is the only β-lactamase responsible for E. meningoseptica resistance to carbapenems. GOB is actively expressed and functional in E. meningoseptica, although its activity is hindered by higher cellular levels of BlaB. In addition, we demonstrate that blaBlaB is induced in stationary phase, which is the first evidence of a growth-regulated MBL gene.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A previously identified E. meningoseptica clinical strain from Hospital Clemente Alvarez (Rosario, Argentina) was used as the source of protein and genomic DNA (32). E. meningoseptica was grown aerobically at 37°C in Luria-Bertani (LB) medium or in M9 minimal medium supplemented with 0.5% yeast extract and/or 0.5% Casamino Acids. Escherichia coli DH5α (Gibco-BRL, Gaithersburg, MD) was used as the host for the E. meningoseptica genomic library. E. coli clones were grown aerobically at 37°C in LB medium supplemented with the appropriate antibiotics when necessary.
Recombinant DNA methodology.
Recombinant DNA procedures were performed as described by Sambrook et al. (37a). β-Lactamase genes present in E. meningoseptica were determined by PCR followed by sequencing. Genomic DNA from E. meningoseptica was extracted as previously described (36) and subjected to PCR using primers CMEf and CMEr (Table 1) for amplification of the blaCME gene and primers 1 and 2 (8) for amplification of the blaBlaB gene; the blaGOB sequence from this strain was previously determined (32). For construction of an E. meningoseptica genomic library, extracted DNA was partially digested with restriction endonuclease Sau3AI and fragments preferentially in the 5- to 20-kb size range were purified and ligated to a BamHI-linearized and dephosphorylated pACYC184 vector (36). The ligation mixture was electroporated in E. coli DH5α, and transformants selected on LB agar plates containing 85 μg/ml chloramphenicol. The library was washed from the plates and stored at −80°C. The library was screened for blaBlaB in LB-chloramphenicol medium containing 50 μg/ml ampicillin. Isolated clones were tested for production of carbapenemase activity on plates by facing disks containing imipenem and dipicolinic acid (DPA) as described below (39). DNA constructions were verified by sequencing at the DNA Sequencing Service of the University of Maine (Orono, ME).
Table 1.
Primers for amplification of genomic blaCME (primers 1 and 2) and of blaBlaB, blaGOB, blaCME, and 16S rRNA from E. meningoseptica cDNA (primers 3 to 10)
| Primer no. and name | Primer sequence |
|---|---|
| 1, CMEf | AAGAAAGCCACAGTAGCTGTTTC |
| 2, CMEr | ACTGCAATTGCATAATGTTTACC |
| 3, CMEfw | ACATGGTCACCACTTCGTGAGA |
| 4, CMErv | GTCGCATCCGTTGTTGTCACTT |
| 5, BlaBfw | TTGTGGTTATAGACTGTCCGTGGG |
| 6, BlaBrv | TATTCAAGACCTCCGGCACGAT |
| 7, GOBfw | GGAACGGCAGAATCGTTTCCAA |
| 8, GOBrv | AGTGAGCCTGAGTAAGCAGCAA |
| 9, rRNA16Sfw | GCGGTGGAGCATGTGGTTTAAT |
| 10, rRNA16Srv | GACAACCATGCAGCACCTTGAA |
Microbiological and crude extract assays.
Resistance of E. meningoseptica to ceftazidime and imipenem was analyzed on plates as follows. An LB agar plate was inoculated with a liquid culture of E. meningoseptica adjusted to an optical density (OD) of 0.1. Commercial disks of 10 μg imipenem and 30 μg ceftazidime (BBL) were placed on top of the agar surface. To test the contribution of MBLs, filter disks containing 40 μl of 100 mM DPA were applied at the periphery of the antibiotic disks (39) in the expected zone of sensitivity. For detection of CME action, commercial disks of ceftazidime-clavulanic acid (CLA) and ceftazidime (at the periphery of an imipenem disk) were applied (26, 46). Hydrolytic activity of crude extracts was measured as follows. A 10-ml portion of mid-log-phase liquid culture of E. meningoseptica was harvested, sonicated in phosphate-buffered saline (PBS), and then clarified by centrifugation. Initial rates of hydrolysis were measured in a JASCO V550 spectrophotometer at 30°C with 300 μM cefotaxime or imipenem. Crude extracts were preincubated for 20 min at 25°C with and without addition of 25 mM EDTA. No metal was added to the reaction medium. The total protein concentration was estimated by the Bradford assay, using bovine serum albumin (BSA) as standard (12).
MBL protein levels were measured as follows. The products from SDS-PAGE of crude extracts, obtained as described above, were transferred to a nitrocellulose membrane for Western blot analysis. Membrane-purified polyclonal rabbit anti-BlaB or anti-GOB antiserum, obtained after inoculating a rabbit with a mixture of recombinant GOB or BlaB and Freund's adjuvant, and anti-rabbit immunoglobulin G–alkaline phosphatase conjugate were used to detect BlaB or GOB protein independently. Protein band intensities were quantified with Gel-Pro Analyzer 4.0 software and total protein concentrations normalized as before. For induction experiments, early-log-phase E. meningoseptica cells (OD, 0.4) were harvested and resuspended in 0.2-μm-filtered medium from a 1-day E. meningoseptica stationary-phase culture, 0.2-μm-filtered medium from a 1-day E. meningoseptica stationary-phase culture supplemented with 0.5% yeast extract and 0.5% Casamino Acids, or M9 supplemented with 1% Casamino Acids. Samples were taken at different times and MBL protein levels and imipenem activity measured as before.
RNA expression analysis.
Expression levels of the blaCME, blaBlaB, and blaGOB genes were measured by quantitative real-time PCR (qRT-PCR). Total RNA was purified from cells with TRIzol reagent (Sigma) according to the manufacturer's instructions, treated with RNase-free DNase (Promega), and then converted to cDNA using a mixture containing SuperScript III reverse transcriptase (Invitrogen), random hexanucleotides, and RNaseOUT inhibitor (Invitrogen). cDNA samples were appropriately diluted and subjected to qRT-PCR with a reaction mixture containing 1× reaction buffer, 0.4× SYBR green, 3 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), 1 U Platinum Taq (Invitrogen), and 1 μM primers (Table 1, primers 3 to 10). The cycling parameters were as follows: 1 cycle of 95°C for 1 min; 40 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 40 s; and 1 cycle (melting curve) of 95°C for 15 s, 60°C for 15 s, a gradient for 20 min, and 95°C for 15 s. Changes in gene expression were determined by the comparative threshold cycle (CT) method (30). For this purpose, 16S rRNA expression was measured under identical conditions and used to normalize values obtained for blaGOB, blaBlaB, and blaCME expression. DNase-treated RNAs were employed as negative controls for testing genomic DNA remnants.
For expression analysis of bla genes in the presence of sub-MICs of imipenem, cefotaxime, and ampicillin, two 100-ml cultures of E. meningoseptica, the experimental and control samples, treated identically, were grown to an OD of 0.6. After addition of each antibiotic (1-μg/ml final concentration) to the experimental sample, duplicate aliquots from both cultures were taken at 0, 1, 15, 30, 45, and 90 min and treated with TRIzol for total RNA extraction. RNA samples were converted to cDNA and subjected to qRT-PCR. Each sample was amplified twice with CME, BlaB, GOB, and 16S rRNA primers. The expression level of 16S rRNA was assumed to be unaffected by antibiotic treatment.
Gene expression at distinct phases of growth was measured in the following way. Samples were taken in duplicate from a single 100-ml LB culture of E. meningoseptica at 20, 150, 300, 400, and 1,500 min after inoculation. Expression values for bla genes were determined relative to 16S rRNA and early-log-phase levels (at 20 min) for comparison.
Immunoprecipitation assays.
BlaB and GOB were separately immunoprecipitated from E. meningoseptica crude extracts with protein A-Sepharose resin (Sigma) previously incubated with anti-BlaB and anti-GOB rabbit antisera. As a negative control, the same procedure was followed but using protein A-Sepharose resin previously incubated with preimmune antiserum. Immunocomplexes and supernatants were subjected to SDS-PAGE and analyzed by Western blot analysis. Initial rates of carbapenem hydrolysis were measured in duplicate in a JASCO V550 instrument at 30°C, using the following reaction medium: 50 μl of supernatant, 150 μl of PBS, and 2 μl of 20 mM imipenem or 20 mM meropenem (1-cm-path-length cuvette).
Separation of MBLs.
BlaB, pre-BlaB, and GOB were partially purified from 2 liters of E. meningoseptica culture. Crude extracts, obtained by cell sonication and ultracentrifugation, were precipitated with 60 to 90% ammonium sulfate, dialyzed against 20 mM Bis-Tris–propane (pH 7), and passed through Q-Sepharose resin (Pharmacia Biotech). The washed fraction was dialyzed against 50 mM sodium phosphate (pH 7), concentrated, and injected into a MonoS chromatograph (Pharmacia Biotech). Proteins were eluted with a salt gradient (0 to 200 mM NaCl), and fractions containing the different MBL species, analyzed by Western blotting and EDTA-inhibitable β-lactamase activity as explained above, were eventually purified by Superdex 75 chromatography (GE).
Nucleotide sequence accession number.
The blaBlaB sequence has been deposited in the GenBank/EMBL/DDBJ database under accession number JN635697.
RESULTS AND DISCUSSION
E. meningoseptica bla genes.
The β-lactamases from a clinical isolate of E. meningoseptica were studied (MICs of β-lactams are shown in Table 2) (32). The blaCME, blaBlaB, and blaGOB genes were amplified by PCR from the E. meningoseptica genome and sequenced. The GOB and CME alleles correspond to the previously reported GOB-18 and CME-2, respectively (8, 32). E. meningoseptica BlaB, however, is identical to the previously reported B-11 in its mature form (http://www.ncbi.nlm.nih.gov/protein/ABO21420.1), while presenting a different leader peptide sequence.
Table 2.
MICs of β-lactams for the E. meningoseptica strain used in this study
| β-Lactam | MIC (μg/ml) |
|---|---|
| Ceftazidime | 256 |
| Cefotaxime | 64 |
| Imipenem | 64 |
| Meropenem | 64 |
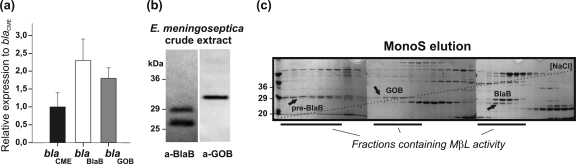
The relative expression levels of the blaCME, blaBlaB, and blaGOB genes were determined by quantitative real-time PCR (qRT-PCR). All bla genes were actively transcribed (Fig. 1a). MBL protein production in E. meningoseptica crude extracts was also confirmed (Fig. 1b). Western blot analysis for BlaB or GOB identified three soluble species: mature GOB of ∼31 kDa and mature and precursor BlaBs of ∼26 and ∼29 kDa, respectively.
Fig 1.
(a) Relative expression of bla genes with respect to blaCME, measured by qRT-PCR. (b) SDS-PAGE and Western blotting of BlaB, precursor BlaB, and GOB present in the soluble fraction of E. meningoseptica crude extract. (c) SDS-PAGE of MonoS salt gradient elution. The first 37 fractions in which all the MBL species elute are shown spanning through the three gels. Arrows show soluble precursor BlaB (pre-BlaB), BlaB, and GOB as determined by Western blot analysis. Fractions containing MBL activity, i.e., imipenem or nitrocefin activity inhibited by EDTA, are marked.
The β-lactam activities of the identified species were further assayed. Fractions enriched with GOB, BlaB, and precursor BlaB were separately obtained from E. meningoseptica crude extracts by means of ammonium sulfate precipitation and Q-Sepharose, MonoS, and Superdex 75 chromatographies (Fig. 1c). Activity assays against nitrocefin and imipenem showed that all enzyme species possess β-lactam activity that was inhibited by EDTA. However, cellular levels of mature GOB were 3-fold lower than those of mature BlaB.
Involvement of MBLs in carbapenem resistance.
First we assessed the relative contributions of the SBL (CME) and the MBLs (BlaB and/or GOB) in the profile of resistance of E. meningoseptica to different β-lactam antibiotics. The in vivo resistance of E. meningoseptica to ceftazidime and imipenem was determined in the presence of clavulanic acid (CLA) and dipicolinic acid (DPA), which are inhibitors of SBLs and MBLs, respectively (39). Disks containing the antibiotics and β-lactamase inhibitors were placed on top of an LB agar plate inoculated with E. meningoseptica. As shown in Fig. 2a, halos of inhibition developed when ceftazidime was combined with clavulanic acid (right) or imipenem (a competitive inhibitor of CME) (46) (left) and not when challenge was with DPA (center). This suggests that CME is mainly responsible for E. meningoseptica resistance to ceftazidime, as inhibition of MBLs does not affect bacterial growth. The opposite is observed for imipenem (left). In this case, inhibition of MBLs by DPA prevents cell growth when combined with imipenem, suggesting that MBLs are responsible for imipenem resistance. These results are in agreement with the hydrolytic activities of crude extracts against ceftazidime, imipenem, cefotaxime, and nitrocefin measured in the presence and absence of 20 mM EDTA (Fig. 2b). Addition of EDTA (an MBL inhibitor) causes an ∼20% decrease in cephalosporin hydrolysis while totally inhibiting imipenemase activity.
Fig 2.
Contributions of CME and MBLs (BlaB and GOB) to resistance. (a) Disks containing imipenem (IMI), ceftazidime (CAZ), and the inhibitors dipicolinic acid (DPA) (for MBLs) and clavulanic acid (CLA) (for CME), applied to an E. meningoseptica culture. (b) Crude extract activity against cefotaxime, ceftazidime, nitrocefin, and imipenem with and without preincubation with EDTA [EDTA and DPA chelate Zn(II) ions, which are essential for MBL activity). Initial rates of hydrolysis were measured at 30°C in PBS medium. Antibiotic and EDTA concentrations were 300 μM and 25 mM, respectively. (c) Anti-GOB and anti-BlaB Western blots of immunoprecipitates and supernatants. (d) Carbapenemase activity of supernatants before and after removal of GOB and BlaB. IP, immunoprecipitate; SN, supernatant; pIS, treated with preimmune serum; aS, treated with antiserum.
We then analyzed the involvement of each MBL (BlaB and GOB) in carbapenem resistance. BlaB and GOB were immunoprecipitated in separate batches from a previously ultracentrifuged mid-log-phase E. meningoseptica crude extract. The carbapenemase activity of the extracts was measured before and after removal of each enzyme. We carried out two immunoprecipitation experiments in parallel: (i) to remove BlaB or GOB from a mid-log-phase crude extract using a specific antiserum and (ii) by the same procedure as in but inducing immunoprecipitation with a preimmune serum as a negative control. Western blot analysis of the immunocomplexes and supernatants (Fig. 2c) showed that soluble GOB and BlaB were quantitatively and selectively removed from the crude extracts. The activities of the supernatants against 200 μM imipenem and meropenem are shown in Fig. 2d. Removal of BlaB caused an almost 100% reduction in imipenemase activity and an 85% reduction in meropenemase activity, revealing that, at least under these experimental conditions, the MBL activity present in E. meningoseptica crude extracts is mostly due to BlaB. Given the large difference in the Km values of meropenem toward BlaB (>1,650 μM) (41) and GOB (40 μM) (32), the contribution of GOB to meropenem resistance is expected to be more relevant at low (submicromolar) substrate concentrations. In this scenario, GOB would act then as an accessory mechanism to reinforce the high-Km BlaB.
MBL gene regulation.
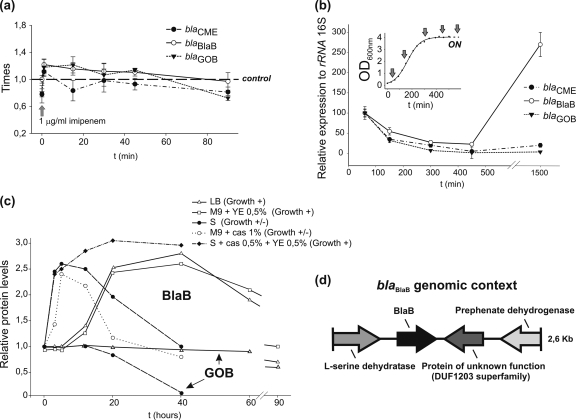
To test whether expression of any of the E. meningoseptica bla genes is affected by β-lactam compounds, transcript levels of the blaCME, blaBlaB, and blaGOB genes were measured in E. meningoseptica cells cultured with subinhibitory concentrations (1 μg/ml) of imipenem, cefotaxime, or ampicillin. No significant changes in β-lactamase transcripts or MBL protein levels were observed under these experimental conditions (Fig. 3a).
Fig 3.
(a) Changes in RNA expression levels of blaCME, blaBlaB, and blaGOB upon addition of 1 μg/ml of imipenem to a mid-log-phase E. meningoseptica culture. (b) Transcript levels of bla genes at different stages of growth relative to 16S rRNA. Inset, E. meningoseptica growth curve showing times of sample extraction (c) E. meningoseptica BlaB and GOB (marked with arrows) protein levels after early-log-phase cells were placed in different media (see text). (d) Sequence of a blaBlaB-containing E. meningoseptica 2.6-kb genomic fragment. Arrows represent predicted open reading frames (ORFs). S, 1-day stationary-phase depleted medium, YE, yeast extract; cas, Casamino Acids.
Expression levels of the bla genes were measured at different stages of bacterial growth in rich LB medium. Aliquots were taken from a single culture of E. meningoseptica at different times of growth, and transcript levels of the blaCME, blaBlaB, and blaGOB genes relative to 16S rRNA were measured (Fig. 3b). All β-lactamase transcripts experienced a decrease throughout the growth curve. However, a 3-fold increase of blaBlaB transcripts was observed in the stationary phase, as well as an increase in BlaB protein levels, relative to early-log-phase levels. The same behavior was observed in M9 minimal medium supplemented with 0.5% yeast extract, the simplest medium in which E. meningoseptica can grow vigorously.
BlaB production was additionally tested at different times after culturing early-log-phase cells in different media supporting growth but under stress conditions: (i) a 1-day stationary-phase E. meningoseptica depleted medium (slow growth), (ii) the same but supplemented with 0.5% Casamino Acids and 0.5% yeast extract (vigorous growth), and (iii) M9 minimal medium supplemented with 1% Casamino Acids (slow growth). As shown in Fig. 3c, levels of BlaB similar to those achieved in stationary phase were observed at 5 h of incubation. These results indicate that blaBlaB is activated either under suboptimal growth conditions, such as in M9 plus 1% Casamino Acids, or by signaling molecules accumulating in stationary-phase medium, such as in stationary-phase E. meningoseptica depleted medium supplemented with Casamino Acids and yeast extract. In all cases, changes in BlaB levels were accompanied by equal variations in the imipenemase activity of crude extracts. No variation in the production of GOB was observed either in stationary phase or in the restrictive media, suggesting that the two MBL genes are independently regulated.
In an attempt to find clues about blaBlaB regulation, the genomic context of the blaBlaB gene was investigated. A genomic library of E. meningoseptica was constructed in the pACYC184 plasmid vector and transformed into the E. coli DH5α strain. Approximately 6,000 transformants, grown on LB-chloramphenicol medium, were obtained. This library was screened in LB-chloramphenicol medium containing 50 μg/ml ampicillin. We isolated eight clones which were able to grow in the presence of ampicillin and produced a carbapenemase activity sensitive to inhibition by dipicolinic acid. One of them, containing the blaBlaB gene, was sequenced and analyzed. As shown in Fig. 3d, the blaBlaB gene is flanked by two putative amino acid metabolism genes (encoding l-serine dehydratase and prephenate dehydrogenase) and an open reading frame coding for a protein of unknown function. No apparent −7/−33 consensus sequence typical of σABfr Bacteroidetes housekeeping gene promoters was found upstream of the blaBlaB sequence (42).
Studies on MBL regulation are scarce, as many clinically relevant MBL genes are constitutively transcribed from mobile genetic element promoters. However, like blaBlaB and blaGOB, most MBL genes produced by environmental bacteria are transcribed from endogenous promoters (4, 6, 7, 11, 14, 28, 31, 35). Two cases of regulation of MBL expression have been reported. Subclass B3 L1 from Stenotrophomonas maltophilia (21, 22, 34) and subclass B2 AsbM1 from Aeromonas jandaei (1) are induced by β-lactam compounds and coregulated with SBL genes (blaL2 in S. maltophilia and blaAsbA1 and blaOXA-12 in A. jandaei). Transcription of blaL1 and blaL2 is controlled by the ampG-ampN-ampDI-ampR network, which senses metabolic intermediates accumulating during cell wall degradation in the presence of β-lactam compounds (22). In the case of E. meningoseptica, qRT-PCR and Western blotting experiments revealed that bla genes are insensitive to β-lactam exposure. However, blaBlaB transcripts and protein levels increase approximately 3-fold once the cells are in the stationary phase. Similar induction levels were observed in early-log-phase cells cultivated under sudden nutrient-limiting conditions or in suboptimal growth media. To the best of our knowledge, there are no previous reports of a growth-regulated MBL gene. blaGOB, instead, is independently regulated, as GOB levels remained unaltered under all assayed conditions. These results suggest that E. meningoseptica increases BlaB production after sensing stress conditions of growth, probably resembling those encountered when causing human meningitis (the cerebrospinal fluid is a nutrient-limiting medium) or in its natural habitats. Cases of complex growth-dependent regulation were observed for SBLs such as E. coli AmpC and Ralstonia pickettii OXA-22 and OXA-60 (19, 38). The gene bolA, a regulator of cell wall biosynthetic enzymes and ampC expression, is induced in stationary phase or under sudden carbon starvation. In R. pickettii, the blaOXA-22 and blaOXA-60 SBL genes are inducible by β-lactam molecules (19). Disruption of the regulatory protein RP3 abolishes induction of both β-lactamases, together with resistance to pH and osmolarity, and survival in the stationary phase. ampR, which plays a clear role in cell wall integrity protection, was shown to be involved in virulence, quorum sensing, and expression of proteases in Pseudomonas aeruginosa (23, 33). Furthermore, recent studies linked ampR expression to the σ22 alternative sigma factor, which is responsible for exopolysaccharide alginate production, an important virulence factor in P. aeruginosa (3). The lack of an obvious −7/−33 consensus sequence in the blaBlaB promoter may suggest that its transcription depends on an alternative sigma factor. Unfortunately, studies of molecular regulatory mechanisms in bacteria of the phylum Bacteroidetes are scarcely developed due to the lack of genetic tools and the fact that genetic elements are poorly characterized.
Concluding remarks.
The present series of experiments allow us to reach some relevant conclusions: (i) resistance to cephalosporins is mainly due to the serine lactamase CME, (ii) both MBL genes are actively transcribed in E. meningoseptica, and (iii) both MBLs are functional β-lactamases and display carbapenemase activity. Immunoprecipitation studies strongly suggest that resistance to imipenem (and probably meropenem) is conferred mostly by BlaB. These results call into question the cellular role of an efficient carbapenemase such as GOB. Similar concerns have been raised for other B3 subclass enzymes, such as BJP-1 and CAU-1 (14, 15, 40), despite their lower catalytic efficiencies (32). Recent experiments from our laboratory suggest that another portion of GOB, lacking β-lactam activity, would be associated with the E. meningoseptica inner membrane (unpublished data).
It still remains to be elucidated whether BlaB participates in some complex cellular process in the stationary phase or whether it confers resistance to natural β-lactam compounds produced by competing microorganisms in nutrient-limiting media. Given that the first clinical strains of BlaB-producing E. meningoseptica were isolated in the 1950s (decades before the use of carbapenems), more efforts are needed to understand the actual role of endogenous MBLs in bacterial fitness (47). Functional metagenomics studies have disclosed the presence of GOB homologues from bacteria in an Alaskan soil never inhabited by humans (2). Regardless of the role of these MBLs (which may be a matter of speculation), environmental bacteria such as E. meningoseptica are of clinical concern as reservoirs of resistance genes.
ACKNOWLEDGMENTS
L.J.G. was the recipient of a doctoral fellowship from CONICET. A.J.V. is a staff member of CONICET and an International Research Scholar of the Howard Hughes Medical Institute. This work was supported by grants from ANPCyT and HHMI to A.J.V.
We acknowledge A. Viale and F. Soncini for critical reading of the manuscript and helpful suggestions.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Alksne LE, Rasmussen BA. 1997. Expression of the AsbA1, OXA-12, and AsbM1 beta-lactamases in Aeromonas jandaei AER 14 is coordinated by a two-component regulon. J. Bacteriol. 179:2006–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen HK, Moe LA, Rodbumrer J, Gaarder A, Handelsman J. 2009. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 3:243–251 [DOI] [PubMed] [Google Scholar]
- 3. Balasubramanian D, et al. 2011. Co-regulation of β-lactam resistance, alginate production and quorum sensing in Pseudomonas aeruginosa. J. Med. Microbiol. 60:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bebrone C. 2007. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 74:1686–1701 [DOI] [PubMed] [Google Scholar]
- 5. Bellais S, Aubert D, Naas T, Nordmann P. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellais S, Girlich D, Karim A, Nordmann P. 2002. EBR-1, a novel Ambler subclass B1 β-lactamase from Empedobacter brevis. Antimicrob. Agents Chemother. 46:3223–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellais S, Naas T, Nordmann P. 2002. Genetic and biochemical characterization of CGB-1, an Ambler class B carbapenem-hydrolyzing β-lactamase from Chryseobacterium gleum. Antimicrob. Agents Chemother. 46:2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellais S, Poirel L, Naas T, Girlich D, Nordmann P. 2000. Genetic-biochemical analysis and distribution of the Ambler class A β-lactamase CME-2, responsible for extended-spectrum cephalosporin resistance in Chryseobacterium (Flavobacterium) meningosepticum. Antimicrob. Agents Chemother. 44:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernardet JF, Hugo C, Bruun B. 2006. The genera Chryseobacterium and Elizabethkingia. Prokaryotes 7:638–676 [Google Scholar]
- 10. Bloch KC, Nadarajah R, Jacobs R. 1997. Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine (Baltimore) 76:30–41 [DOI] [PubMed] [Google Scholar]
- 11. Boschi L, et al. 2000. The Legionella (Fluoribacter) gormanii metallo-β-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 β-lactamases. Antimicrob. Agents Chemother. 44:1538–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 13. Crowder MW, Spencer J, Vila AJ. 2006. Metallo-β-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 39:721–728 [DOI] [PubMed] [Google Scholar]
- 14. Docquier JD, et al. 2002. CAU-1, a subclass B3 metallo-β-lactamase of low substrate affinity encoded by an ortholog present in the Caulobacter crescentus chromosome. Antimicrob. Agents Chemother. 46:1823–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Docquier JD, et al. 2010. High-resolution crystal structure of the subclass B3 metallo-β-lactamase BJP-1: rational basis for substrate specificity and interaction with sulfonamides. Antimicrob. Agents Chemother. 54:4343–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falagas ME, Karageorgopoulos DE, Nordmann P. 2011. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 6:653–666 [DOI] [PubMed] [Google Scholar]
- 17. Galleni M, et al. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garau G, et al. 2004. Update of the standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 48:2347–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Girlich D, Kolb A, Naas T, Nordmann P. 2009. Characterization of regulatory element Rp3 of regulation of β-lactamases from Ralstonia pickettii. FEMS Microbiol. Lett. 301:50–56 [DOI] [PubMed] [Google Scholar]
- 20. Horsfall LE, et al. 2011. Broad antibiotic resistance profile of the subclass B3 metallo-β-lactamase GOB-1, a di-zinc enzyme. FEBS J. 278:1252–1263 [DOI] [PubMed] [Google Scholar]
- 21. Hu RM, Huang KJ, Wu LT, Hsiao YJ, Yang TC. 2008. Induction of L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 52:1198–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang YW, et al. 2010. AmpN-AmpG operon is essential for expression of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 54:2583–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong KF, et al. 2005. Pseudomonas aeruginosa AmpR is a global transcriptional factor that regulates expression of AmpC and PoxB β-lactamases, proteases, quorum sensing, and other virulence factors. Antimicrob. Agents Chemother. 49:4567–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CC, et al. 2006. Fatal case of community-acquired bacteremia and necrotizing fasciitis caused by Chryseobacterium meningosepticum: case report and review of the literature. J. Clin. Microbiol. 44:1181–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CH, et al. 2008. Community-acquired osteomyelitis caused by Chryseobacterium meningosepticum: case report and literature review. Diagn. Microbiol. Infect. Dis. 60:89–93 [DOI] [PubMed] [Google Scholar]
- 26. Lee K, et al. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 7:88–91 [DOI] [PubMed] [Google Scholar]
- 27. Lee SW, Tsai CA, Lee BJ. 2008. Chryseobacterium meningosepticum sepsis complicated with retroperitoneal hematoma and pleural effusion in a diabetic patient. J. Chin. Med. Assoc. 71:473–476 [DOI] [PubMed] [Google Scholar]
- 28. Lin XH, Xu YH, Cheng J, Li T, Wang ZX. 2008. Heterogeneity of blaIND metallo-β-lactamase-producing Chryseobacterium indologenes isolates detected in Hefei, China. Int. J. Antimicrob. Agents. 32:398–400 [DOI] [PubMed] [Google Scholar]
- 29. Lisa MN, Hemmingsen L, Vila AJ. 2010. Catalytic role of the metal ion in the metallo-β-lactamase GOB. J. Biol. Chem. 285:4570–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Mammeri H, Bellais S, Nordmann P. 2002. Chromosome-encoded β-lactamases TUS-1 and MUS-1 from Myroides odoratus and Myroides odoratimimus (formerly Flavobacterium odoratum), new members of the lineage of molecular subclass B1 metalloenzymes. Antimicrob. Agents Chemother. 46:3561–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morán-Barrio J, et al. 2007. The metallo-β-lactamase GOB is a mono-Zn(II) enzyme with a novel active site. J. Biol. Chem. 282:18286–18293 [DOI] [PubMed] [Google Scholar]
- 33. Normark S. 1995. β-Lactamase induction in gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb. Drug Resist. 1:111–114 [DOI] [PubMed] [Google Scholar]
- 34. Okazaki A, Avison MB. 2008. Induction of L1 and L2 β-lactamase production in Stenotrophomonas maltophilia is dependent on an AmpR-type regulator. Antimicrob. Agents Chemother. 52:1525–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossolini GM, et al. 2001. Metallo-β-lactamase producers in environmental microbiota: new molecular class B enzyme in Janthinobacterium lividum. Antimicrob. Agents Chemother. 45:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rossolini GM, et al. 1999. Cloning of a Chryseobacterium (Flavobacterium) meningosepticum chromosomal gene (blaACME) encoding an extended-spectrum class A β-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER β-lactamases. Antimicrob. Agents Chemother. 43:2193–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossolini GM, et al. 1998. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B beta-lactamase showing a broad substrate profile. Biochem. J. 332:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Santos JM, Lobo M, Matos AP, De Pedro MA, Arraiano CM. 2002. The gene bolA regulates dacA (PBP5), dacC (PBP6) and ampC (AmpC), promoting normal morphology in Escherichia coli. Mol. Microbiol. 45:1729–1740 [DOI] [PubMed] [Google Scholar]
- 39. Shin KS, Son BR, Hong SB, Kim J. 2008. Dipicolinic acid-based disk methods for detection of metallo-β-lactamase—producing Pseudomonas spp. and Acinetobacter spp. Diagn. Microbiol. Infect. Dis. 62:102–105 [DOI] [PubMed] [Google Scholar]
- 40. Stoczko M, Frère JM, Rossolini GM, Docquier JD. 2006. Postgenomic scan of metallo-β-lactamase homologs in rhizobacteria: identification and characterization of BJP-1, a subclass B3 ortholog from Bradyrhizobium japonicum. Antimicrob. Agents Chemother. 50:1973–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vessillier S, et al. 2002. Overproduction and biochemical characterization of the Chryseobacterium meningosepticum BlaB metallo-β-lactamase. Antimicrob. Agents Chemother. 46:1921–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vingadassalom D, et al. 2005. An unusual primary sigma factor in the Bacteroidetes phylum. Mol. Microbiol. 56:888–902 [DOI] [PubMed] [Google Scholar]
- 43. Walsh TR. 2005. The emergence and implications of metallo-β-lactamases in Gram-negative bacteria. Clin. Microbiol. Infect. 11:2–9 [DOI] [PubMed] [Google Scholar]
- 44. Walsh TR. 2010. Emerging carbapenemases: a global perspective. Int. J. Antimicrob. Agents 36:8–14 [DOI] [PubMed] [Google Scholar]
- 45. Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weldhagen GF, Poirel L, Nordmann P. 2003. Ambler class A extended-spectrum β-lactamases in Pseudomonas aeruginosa: novel developments and clinical impact. Antimicrob. Agents Chemother. 47:2385–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woodford N, Palepou MF, Babini GS, Holmes B, Livermore DM. 2000. Carbapenemases of Chryseobacterium (Flavobacterium) meningosepticum: distribution of blaB and characterization of a novel metallo-β-lactamase gene, blaB3, in the type strain, NCTC 10016. Antimicrob. Agents Chemother. 44:1448–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yim G, Wang HH, Davies J. 2007. Antibiotics as signalling molecules. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]