Abstract
This study examines in vitro antimicrobial resistance data from Escherichia coli isolates obtained from urine samples of U.S. outpatients between 2000 and 2010 using The Surveillance Network (TSN). Antimicrobial susceptibility results (n = 12,253,679) showed the greatest increases in E. coli resistance from 2000 to 2010 for ciprofloxacin (3% to 17.1%) and trimethoprim-sulfamethoxazole (TMP-SMX) (17.9% to 24.2%), whereas nitrofurantoin (0.8% to 1.6%) and ceftriaxone (0.2% to 2.3%) showed minimal change. From 2000 to 2010, the antimicrobial resistance of urinary E. coli isolates to ciprofloxacin and TMP-SMX among outpatients increased substantially.
TEXT
Antimicrobial resistance significantly increases patient morbidity, costs of treatment, rates of hospitalization, and use of broad-spectrum agents (7). Resistant Escherichia coli isolates are associated with decreases in clinical cure rates and higher risk of recurrence (17, 20).
Several studies have described the in vitro susceptibility of E. coli isolates among outpatients in the United States, and most of these studies have focused on women. The most-recent published data available for U.S. outpatients were collected from April 2003 to June 2004 and suggested the levels of antimicrobial resistance of urinary E. coli isolates to be 39.3% for ampicillin, 22.6% for trimethoprim-sulfamethoxazole (TMP-SMX), 6.8% for ciprofloxacin, and 1.4% for nitrofurantoin (22). Other, smaller regional studies suggested a continued trend of rising resistance in the outpatient setting (8, 13, 16).
Limited data are available to describe long-term trends in antimicrobial resistance of E. coli isolates among outpatients in the United States. The objective of this study was to examine trends of antimicrobial resistance of urinary E. coli isolates among outpatients in the United States from 2000 to 2010.
Antimicrobial susceptibility test results were obtained from The Surveillance Network (TSN) Database—USA (Eurofins Medinet, Chantilly, VA). This surveillance database collects data from over 200 institutions in the United States, and antimicrobial susceptibility testing is performed on-site by each laboratory in accordance with FDA-approved testing methods and interpreted using Clinical and Laboratory Standards Institute (CLSI)-recommended breakpoints. TSN data have been used before to evaluate trends in antimicrobial resistance, and further details of quality control have been described previously (5, 9, 18, 19).
The present study included antimicrobial susceptibility data for urinary E. coli isolates obtained from U.S. outpatients between 2000 and 2010. Outpatients are defined as individuals who visited emergency departments, hospital-based outpatient clinics, and physicians' offices. E. coli isolates with intermediate susceptibility were not classified as being resistant. The outcomes of interest in this study were the changes in and most-recent prevalence of antimicrobial resistance to commonly prescribed oral agents used to treat urinary tract infections (UTIs). Cephalothin and ceftriaxone, both intravenously administered agents, were selected as narrow- and broad-spectrum cephalosporin surrogates, respectively, for their orally administered formulations.
A chi-square test was performed for each antimicrobial agent to determine whether a significant difference existed between resistance rates observed in 2000 and those observed in 2010. An alpha level of 0.05 was used. Analyses were performed using Statistical Analysis Software (SAS) version 9.1.
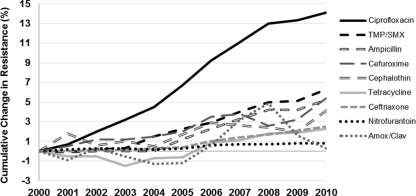
Antimicrobial susceptibility results (n = 12,253,679) for urinary E. coli isolates obtained from outpatients in the United States from 2000 to 2010 were examined (Table 1). The greatest increases in resistance among isolates obtained from all outpatients from 2000 to 2010 were observed for ciprofloxacin (from 3% in 2000 to 17.1% in 2010) and TMP-SMX (17.9% to 24.2%) (Fig. 1). Conversely, over the same time period, nitrofurantoin (0.8% to 1.6%), amoxicillin-clavulanate (5% to 5.3%), and ceftriaxone (0.2% to 2.3%) demonstrated only small changes in resistance. In 2010, ampicillin, tetracycline, cephalothin, and cefuroxime showed antimicrobial resistance rates of 43.4%, 24.9%, 18.1%, and 5.0%, respectively.
Table 1.
Annual rates of resistance of urinary Escherichia coli isolates to select antimicrobials among all outpatients from 2000 to 2010a
| Antimicrobial agent | No. of test results | Antimicrobial resistance rate (%) for indicated yr |
Total change (%) from 2000–2010b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |||
| Ciprofloxacin | 1,836,598 | 3 | 3.7 | 5 | 6.2 | 7.5 | 9.7 | 12.2 | 14.1 | 16 | 16.3 | 17.1 | 14.1 |
| TMP-SMX | 2,034,254 | 17.9 | 17.8 | 18.1 | 18.2 | 19.4 | 20.2 | 20.8 | 21.9 | 22.9 | 23 | 24.2 | 6.3 |
| Ampicillin | 2,002,221 | 38.2 | 38.1 | 38.2 | 38.2 | 38.2 | 39.4 | 40.5 | 41.5 | 42.4 | 42.4 | 43.4 | 5.2 |
| Cefuroxime | 806,659 | 1.5 | 1.9 | 2.1 | 2.3 | 2.4 | 2.3 | 2.9 | 3.2 | 3.7 | 4 | 5 | 4.5 |
| Cephalothin | 502,231 | 14 | 15.8 | 14.6 | 15 | 14.5 | 15.7 | 16.9 | 16.6 | 16.4 | 16 | 18.1 | 4.1 |
| Tetracycline | 580,328 | 22.6 | 22.1 | 22.1 | 21.1 | 21.9 | 22 | 23.5 | 23.7 | 24.4 | 24.5 | 24.9 | 2.3 |
| Ceftriaxone | 1,759,006 | 0.2 | 0.2 | 0.3 | 0.3 | 0.5 | 0.6 | 0.9 | 1.2 | 1.6 | 1.9 | 2.3 | 2.1 |
| Nitrofurantoin | 1,972,633 | 0.8 | 1 | 1.1 | 1.1 | 1 | 1.1 | 1.4 | 1.5 | 1.5 | 1.6 | 1.6 | 0.8 |
| Amox-Clav | 759,749 | 5 | 4.1 | 5.3 | 4.5 | 3.7 | 3.8 | 5.6 | 8.2 | 9.9 | 6.6 | 5.3 | 0.3 |
Isolates demonstrating intermediate susceptibility were not counted as resistant. Amox-Clav, amoxicillin-clavulanate; TMP-SMX, trimethoprim-sulfamethoxazole.
All antimicrobial agents demonstrated statistically significant changes from 2000 to 2010.
Fig 1.
Cumulative annual change in E. coli antimicrobial resistance in outpatient urinary E. coli isolates from 2001 to 2010. Amox/Clav, amoxicillin-clavulanate.
Emerging antimicrobial resistance of E. coli in the outpatient setting is well documented (2, 3, 7, 12, 14). In the early 2000s, quinolones surpassed sulfa drugs as the most common class of antimicrobials prescribed by clinicians to treat uncomplicated UTIs (10). This increase in provider use of fluoroquinolones may account for the rapid rise in antimicrobial resistance of E. coli to ciprofloxacin, as resistance to this agent has been shown to correlate with the level of use (8, 15, 21). Due to the propensity of E. coli to acquire resistance to this agent, use of ciprofloxacin for empirical treatment of UTIs in outpatients should be used sparingly and only where local antimicrobial resistance rates remain low (4).
Antimicrobial resistance of urinary E. coli isolates to TMP-SMX continued to increase from 2000 to 2010, a trend that has continued for decades (1, 6, 11). In the 2010 IDSA guidelines for treating acute uncomplicated cystitis in women, TMP-SMX is recommended as the second-line antimicrobial agent (4). Our data are consistent with previous reports regarding increases in antimicrobial resistance of urinary E. coli isolates to TMP-SMX and potential subsequent decreases in its efficacy as empirical therapy among U.S. outpatients.
Levels of antimicrobial resistance of E. coli to cephalothin, the narrow-spectrum oral cephalosporin surrogate, were higher than those for expanded-spectrum (cefuroxime) and broad-spectrum (ceftriaxone) cephalosporins. It is important to note that while the absolute change in antimicrobial resistance to ceftriaxone was small (0.2% in 2000 to 2.3% in 2010), there was a 10-fold increase in resistance of E. coli to this agent over the study time period. Though these changes do not bear immediate clinical significance, future surveillance of antimicrobial resistance to this agent is warranted.
The in vitro antimicrobial resistance rates among E. coli isolates in our investigation were consistent with those reported previously (6, 11, 22). For example, antimicrobial resistance of E. coli to nitrofurantoin demonstrated little change over our study time period, a finding that is consistent with the resistance prevalence reported in the NAUTICA study (22).
It is important to note the strengths and limitations of our data. The strengths of our study include the large number of isolates, the variety of antimicrobial agents studied, the large number of reporting institutions within the United States, the long time period for which data were reported, and the geographically representative distribution of isolates from TSN. The limitations of our study include a lack of central laboratory testing, the use of multiple susceptibility test methods, and an assumed underrepresentation of isolates from those for whom empirical treatment was successful. These data should be interpreted with caution. Although traditional in vitro surveillance systems are well designed to provide insight into overall trends and prevalence of antimicrobial resistance, they are not meant to guide antimicrobial therapy in the management of individual clinical cases.
In summary, our study shows that from 2000 to 2010, antimicrobial resistance of urinary E. coli isolates to ciprofloxacin and TMP-SMX increased substantially but that resistance to nitrofurantoin and ceftriaxone remained low. Given the frequency with which UTIs are treated empirically, compounded with the speed that E. coli acquires resistance, prudent use of antimicrobial agents remains crucial.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Diekema DJ, et al. 2004. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin. Infect. Dis. 38:78–85 [DOI] [PubMed] [Google Scholar]
- 2. Gordon KA, Jones RN. 2003. Susceptibility patterns of orally administered antimicrobials among urinary tract infection pathogens from hospitalized patients in North America: comparison report to Europe and Latin America. Results from the SENTRY Antimicrobial Surveillance Program (2000). Diagn. Microbiol. Infect. Dis. 45:295–301 [DOI] [PubMed] [Google Scholar]
- 3. Gupta K, Hooton TM, Stamm WE. 2001. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 135:41–50 [DOI] [PubMed] [Google Scholar]
- 4. Gupta K, et al. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:e103–e120 [DOI] [PubMed] [Google Scholar]
- 5. Gupta K, Sahm DF, Mayfield D, Stamm WE. 2001. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin. Infect. Dis. 33:89–94 [DOI] [PubMed] [Google Scholar]
- 6. Hooton TM. 2003. The current management strategies for community-acquired urinary tract infection. Infect. Dis. Clin. North Am. 17:303–332 [DOI] [PubMed] [Google Scholar]
- 7. Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. 2004. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin. Infect. Dis. 39:75–80 [DOI] [PubMed] [Google Scholar]
- 8. Johnson L, et al. 2008. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am. J. Med. 121:876–884 [DOI] [PubMed] [Google Scholar]
- 9. Jones ME, et al. 2004. Antibiotic susceptibility of bacteria most commonly isolated from bone related infections: the role of cephalosporins in antimicrobial therapy. Int. J. Antimicrob. Agents 23:240–246 [DOI] [PubMed] [Google Scholar]
- 10. Kallen AJ, Welch HG, Sirovich BE. 2006. Current antibiotic therapy for isolated urinary tract infections in women. Arch. Intern. Med. 166:635–639 [DOI] [PubMed] [Google Scholar]
- 11. Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. 2002. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob. Agents Chemother. 46:2540–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kashanian J, et al. 2008. Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int. 102:1634–1637 [DOI] [PubMed] [Google Scholar]
- 13. Khawcharoenporn T, Vasoo S, Ward E, Singh K. 2010. High rates of quinolone resistance among urinary tract infections in the ED. Am. J. Emerg. Med. [Epub ahead of print.] doi:10.1016/j.ajem.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 14. Lautenbach E, et al. 2004. Longitudinal trends in fluoroquinolone resistance among Enterobacteriaceae isolates from inpatients and outpatients, 1989–2000: differences in the emergence and epidemiology of resistance across organisms. Clin. Infect. Dis. 38:655–662 [DOI] [PubMed] [Google Scholar]
- 15. MacDougall C, Powell JP, Johnson CK, Edmond MB, Polk RE. 2005. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin. Infect. Dis. 41:435–440 [DOI] [PubMed] [Google Scholar]
- 16. Olson RP, Harrell LJ, Kaye KS. 2009. Antibiotic resistance in urinary isolates of Escherichia coli from college women with urinary tract infections. Antimicrob. Agents Chemother. 53:1285–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raz R, et al. 2002. Empiric use of trimethoprim-sulfamethoxazole (TMP-SMX) in the treatment of women with uncomplicated urinary tract infections, in a geographical area with a high prevalence of TMP-SMX-resistant uropathogens. Clin. Infect. Dis. 34:1165–1169 [DOI] [PubMed] [Google Scholar]
- 18. Sahm DF, Marsilio MK, Piazza G. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database—U. S. A. Clin. Infect. Dis. 29:259–263 [DOI] [PubMed] [Google Scholar]
- 19. Sahm DF, Brown NP, Yee YC, Evangelista AT. 2008. Stratified analysis of multidrug-resistant Escherichia coli in US health care institutions. Postgrad. Med. 120:53–59 [DOI] [PubMed] [Google Scholar]
- 20. Talan DA, et al. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA 283:1583–1590 [DOI] [PubMed] [Google Scholar]
- 21. Zervos MJ, et al. 2003. Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991–2000. Clin. Infect. Dis. 37:1643–1648 [DOI] [PubMed] [Google Scholar]
- 22. Zhanel GG, et al. 2006. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 27:468–475 [DOI] [PubMed] [Google Scholar]