Abstract
To date, no outbreak of carbapenemase-producing bacteria has been reported for Austria. While outbreaks of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae have been increasingly reported, no outbreak caused by KPC-producing Klebsiella oxytoca has been described yet, to the best of our knowledge. We report an outbreak of KPC-producing K. oxytoca. In 5 months, 31 KPC-producing Klebsiella oxytoca strains were isolated from five patients. All patients were admitted to the same medical intensive care unit in Austria.
TEXT
Carbapenems are considered the agents of choice for treatment of serious infections caused by resistant Gram-negative bacteria. Resistance to carbapenems is therefore the major threat for treatment of these infections, and production of carbapenemases is the most important molecular mechanism, both epidemiologically and clinically.
Carbapenemases in Enterobacteriaceae are represented by three molecular classes of beta-lactamase: A, B, and D (3). Klebsiella pneumoniae carbapenemase (KPC) is a class A beta-lactamase that poses a serious clinical challenge, as KPC-producing Klebsiella pneumoniae isolates are rapidly disseminating worldwide (1, 4).
KPC-type enzymes in carbapenem-resistant Klebsiella pneumonia were first detected in 2001 in North Carolina and since then have spread all over the United States (14, 18, 24). Sizeable outbreaks of KPC-producing K. pneumoniae have also occurred in Israel, Greece, South America, and China (6, 12, 15, 22, 27, 28). The emergence of KPC-producing K. pneumoniae, often associated with smaller outbreaks, has recently been reported also from several European countries, including Germany, Italy, Poland, Switzerland, and France (9, 10, 13, 17, 23). In contrast, reports of KPC-producing Klebsiella oxytoca are rare (5, 11, 19, 25). Most of these reports cover one or two isolates and, to the best of our knowledge, no outbreak caused by KPC-producing K. oxytoca has been described yet. Even non-carbapenemase-producing K. oxytoca has rarely been reported as the cause of nosocomial outbreaks (7, 20).
We recently reported the emergence of New Dehli metallo-beta-lactamase 1 (NDM-1) strains in Austria (26). To date, no outbreak of carbapenemase-producing bacteria has been reported for Austria. In this study, we describe an outbreak of a KPC-producing K. oxytoca, which affected five patients. All of them had stayed in the same room of a medical intensive care unit (ICU).
The study.
A retrospective observational study of patients infected or colonized with KPC-producing K. oxytoca was conducted. Thirty-one strains (from five patients) were isolated within 5 months at the Medical University Hospital Graz, Austria, a hospital with a total of 1,600 beds. All strains had been stored at −70°C. For microbiology studies, one isolate per patient (either the first colonizing or the first pathogenic isolate identified) was thawed and retested. Three of these strains had been isolated in the medical ICU (15 beds) and one each at the Division of Hematology (28 beds) and the respiratory care unit (RCU; 4 beds) that is spatially divided from the medical ICU. Identification and antimicrobial susceptibility profiles were determined using a Vitek II Instrument (bioMérieux Vitek, Inc., Hazelwood, MO) and the Etest method (AB bioMérieux, Solna, Sweden) and interpreted according to the CLSI guidelines (3a). For detection of KPC, primers and PCR protocols were used as previously described (8). Automated repetitive PCR (rep-PCR) was performed with a DiversiLab Instrument to determine clonal relationships (7).
Medical records of the five patients colonized or infected with KPC-producing K. oxytoca were retrospectively reviewed. For the clinical and microbiological diagnosis of infections, previously published criteria were used (2). Treatment outcome was evaluated on day 7. Successful outcome was defined as cure or improvement (partial resolution of signs and symptoms and improvement of laboratory parameters) while on anti-infective therapy.
From October 2010 through February 2011, five patients were found to have been colonized (n = 2) or infected (n = 3) by KPC-producing K. oxytoca. The first isolate was introduced to the medical ICU by a patient who was already hospitalized in various regional ICUs for 31 days due to an ischemic stroke and consecutively developed a urinary tract infection and ventilator-associated pneumonia (VAP) due to KPC-producing K. oxytoca. The strain was horizontally transmitted to at least two additional patients in the same four-bed room of the medical ICU (patient 2 and patient 3). While patient 2 died due to staphylococcal scalded skin syndrome before identification of the carbapenemase-producing pathogen, KPC-producing K. oxytoca was repeatedly isolated from the other two patients for 49 and 15 days, respectively. The same KPC-producing K. oxytoca strain was isolated from the fourth patient in mid-December 2010 at the division of hematology. This patient had stayed in the medical ICU simultaneously with the three other patients in mid-October, making cross-transmission likely. Thereafter, the strain disappeared for 2 months. A strain belonging to the same clonal group was detected at the RCU in February 2011 in bronchoalveolar lavage fluid from patient 5. This patient had been transferred from the medical ICU 10 days before isolation of the multiresistant strain and had stayed in the same room as all other patients with KPC-producing K. oxytoca. The strain may have continuously circulated at the medical ICU while remaining undetected for more than 2 months. The source, however, remained unidentified. Endoscope-related transmission was ruled out, as environmental cultures of bronchoscopes and equipment used with them remained negative in repeated tests.
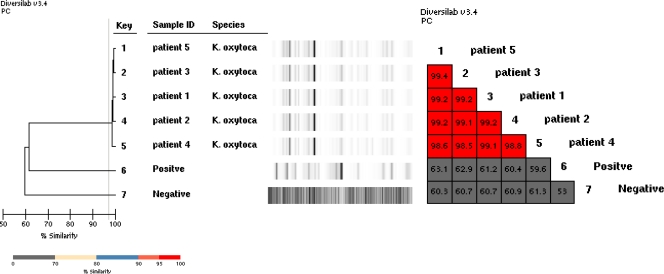
Rep-PCR with the DiversiLab instrument showed that all strains were indistinguishable from one another, with a similarity index of >98.5% (Fig. 1).
Fig 1.
Dendrogram and similarity matrix for K. oxytoca for five patients. The K. oxytoca isolates of this outbreak were indistinguishable, with a similarity of between 98.6% and 99.4% (and no band difference) in the DiversiLab system.
KPC-producing K. oxytoca was causative for infection in three patients (VAP in all three patients and also a urinary tract infection in one patient). Systemic anti-infective treatment for KPC-producing K. oxytoca infection was started for two of the patients and was successful for both. The third patient with VAP died before adequate anti-infective treatment could be started.
Travel history was unremarkable for all of the patients. Patients 1 and 2 had not even been outside Austria for the last 10 years. This is of particular interest, as carbapenem-resistant K. pneumoniae isolates have rarely been reported for Austria in the European Antimicrobial Resistance Surveillance Network (http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net/database/Pages/table_reports.aspx). All detected KPC-producing K. oxytoca strains were multiresistant, and they exhibited susceptibility to colistin, fosfomycin, tigecyclin, and amikacin only. Details of the outbreak are given in Table 1.
Table 1.
Clinical data, strains, and detected carbapenemases in KPC Klebsiella oxytoca outbreak, Austria, 2010-2011a
| Patient | Age (yr)/sex | Date of first detection | Comorbidities | LOS before detection (days) | Total LOS (days) | Site of first detection | Other sites detected | No. of KPC K. oxytoca isolates detected | Duration of colonization (days) | Date of infection caused by KPC K. oxytoca | Site of infection caused by KPC K. oxytoca | Treatment outcome (final outcome) | Antimicrobial therapy before isolation of KPC K. oxytoca | Antimicrobial therapy for KPC K. oxytoca infection | Susceptibility phenotype of KPC K. oxytoca (MIC; mg/liter)b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43/f | 12 Oct 2010 | Ischemic stroke, DIC, ARF, AH | 31 | 82 | Urine | Tracheostoma, tracheal aspirate, nasal, axilla | 19 | 49 | 12 Oct 2010 | UTI, VAP | Successful (death) | Meropenem, gentamicin, linezolid, moxifloxacin | COL, FOS | AMK (2.0), COL (1.0), FOS (2.0), TIG (0.5) |
| 2 | 76/f | 19 Oct 2010 | SSSS, ARF, AH, DM | 11 | 12 | Surveillance swab (throat) | None | 1 | 1 | NA | NA | NA (death) | Ceftriaxone | NA | AMK (2.0), COL (0.125), FOS (2.0), TIG (0.5) |
| 3 | 43/m | 27 Oct 2010 | CAP (Legionella pneumphila) | 9 | 30 | BAL | Throat, nasal, sputum, groin | 9 | 15 | 27 Oct 2010 | VAP | Successful (discharge) | Ceftriaxone, moxifloxacin | FOS, TIG | AMK (4.0), COL (1.0), FOS (4.0), TIG (0.5) |
| 4 | 70/m | 14 Dec 2010 | Secondary AML, COPD | 106 | 115 | Pressure ulcer from urinary catheter | None | 1 | 1 | NA | NA | NA (discharge) | Meropenem, levofloxacin | NA | AMK (2.0), COL (0.125), FOS (4.0), TIG (0.5) |
| 5 | 89/f | 16 Feb 2011 | UTI, CAD, heart failure, CMP, ARF | 26 | 29 | BAL | None | 1 | 1 | 16 Feb 2011 | VAP | NA (death) | Ciprofloxacin, Amox/Clav | NA | AMK (2.0), COL (1.5), FOS (4.0), TIG (0.25) |
AH, arterial hypertension; AMK, amikacin; AML, acute myeloid leukemia; Amox/Clav, amoxicillin-clavulanic acid; ARF, acute renal failure; BAL, broncoalvelar lavage fluid; CAD, coronary artery disease; CAP, community-acquired pneumonia; CMP, cardiomyopathy; COL, colistin; Dec, December; DIC, disseminated intravascular coagulation; DM, diabetes mellitus; f, female; Feb, February; FOS, fosfomycin; GM, gentamicin; LOS, length of stay; m, male; NA, not applicable; Oct, October; SSSS, staphylococcal scalded skin syndrome; TIG, tigeclycline; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
MICs were determined by the Etest method (AB bioMérieux, Solna, Sweden).
The emergence and worldwide spread of carbapenem-resistant Enterobacteriaceae is a challenge for both clinicians and clinical microbiologists. We report a clonal outbreak of KPC-producing K. oxytoca in Austria involving five patients and lasting for 5 months. While outbreaks of KPC-producing K. pneumoniae have been described frequently, no outbreak of KPC-producing K. oxytoca has yet been described, to the best of our knowledge.
Previous studies have identified poor functional status, ICU stay, transplantation, mechanical ventilation, prolonged hospitalization, and receipt of antibiotics as risk factors for acquisition of KPC-producing organisms (16, 21). The observational design of our study did not allow us to make any conclusions regarding risk factors for KPC-producing K. oxytoca acquisition at our institution. All of these risk factors except transplantation, however, were present in three or more of the patients described here.
The susceptibility profile of the isolates recovered during the present outbreak underscores the extremely limited therapeutic options available for the treatment of infected patients. Similar results have also been reported in previous studies (22). Surprisingly, fosfomycin was active in vitro in all of the isolated strains.. In vivo efficacy of this bactericidal agent has not yet been evaluated. In our study, however, fosfomycin combination therapy with either tigecycline or colistin was associated with a successful outcome.
In conclusion, we describe a nosocomial outbreak of KPC-producing K. oxytoca. These observations provide some insight into the epidemiology and clinical importance of KPC carbapenemases that also pose a serious clinical threat when produced by K. oxytoca.
ACKNOWLEDGMENTS
We thank Christina Strempfl and Bernadette Neuhold for their support in the microbiologic laboratory and Katharina Seeber for her editorial assistance.
Martin Hoenigl received a research grant from Merck. All other authors declare no conflicts of interest.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Bonomo RA. 2011. New Delhi metallo-beta-lactamase and multidrug resistance: a global SOS? Clin. Infect. Dis. 52:485–487 [DOI] [PubMed] [Google Scholar]
- 2. Calandra T, et al. 2005. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 33:1538–1548 [DOI] [PubMed] [Google Scholar]
- 3. Chu YW, et al. 2011. Carbapenemases in enterobacteria, Hong Kong, China, 2009. Emerg. Infect. Dis. 17:130–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Cuzon G, et al. 2010. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gootz TD, et al. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53:1998–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gregory CJ, et al. 2010. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect. Control Hosp. Epidemiol. 31:476–484 [DOI] [PubMed] [Google Scholar]
- 7. Grisold AJ, et al. 2010. Use of automated repetitive-sequence-based PCR for rapid laboratory confirmation of nosocomial outbreaks. J. Infect. 60:44–51 [DOI] [PubMed] [Google Scholar]
- 8. Grobner S, et al. 2009. Emergence of carbapenem-non-susceptible extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates at the university hospital of Tubingen, Germany. J. Med. Microbiol. 58:912–922 [DOI] [PubMed] [Google Scholar]
- 9. Grundmann H, et al. 2010. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. 15:19711. [DOI] [PubMed] [Google Scholar]
- 10. Kassis-Chikhani N, et al. 2010. Outbreak of Klebsiella pneumoniae producing KPC-2 and SHV-12 in a French hospital. J. Antimicrob. Chemother. 65:1539–1540 doi:10.1093/jac/dkq132 [DOI] [PubMed] [Google Scholar]
- 11. Li B, et al. 2011. First report of Klebsiella oxytoca strain coproducing KPC-2 and IMP-8 carbapenemases. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marchaim D, et al. 2011. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob. Agents Chemother. 55:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marchese A, Coppo E, Barbieri R, Debbia E. 2010. Emergence of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains and spread of an isolate of sequence type 258 in the neuro-rehabilitation unit of an Italian hospital. J. Chemother. 22:212–214 [DOI] [PubMed] [Google Scholar]
- 14. Nadkarni AS, Schliep T, Khan L, Zeana CB. 2009. Cluster of bloodstream infections caused by KPC-2 carbapenemase-producing Klebsiella pneumoniae in Manhattan. Am. J. Infect. Control 37:121–126 [DOI] [PubMed] [Google Scholar]
- 15. Navon-Venezia S, et al. 2009. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob. Agents Chemother. 53:818–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106 [DOI] [PubMed] [Google Scholar]
- 17. Poirel L, et al. 2011. Plasmid-mediated carbapenem-hydrolysing β-lactamase KPC-2 in a Klebsiella pneumoniae isolate from Switzerland. J. Antimicrob. Chemother. 66:675–676 [DOI] [PubMed] [Google Scholar]
- 18. Pope J, Adams J, Doi Y, Szabo D, Paterson DL. 2006. KPC type beta-lactamase, rural Pennsylvania. Emerg. Infect. Dis. 12:1613–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasheed JK, et al. 2008. Detection of the Klebsiella pneumoniae carbapenemase type 2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J. Clin. Microbiol. 46:2066–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sardan YC, et al. 2004. A cluster of nosocomial Klebsiella oxytoca bloodstream infections in a university hospital. Infect. Control Hosp. Epidemiol. 25:878–882 [DOI] [PubMed] [Google Scholar]
- 21. Schwaber MJ, et al. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Souli M, et al. 2010. An outbreak of infection due to beta-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek university hospital: molecular characterization, epidemiology, and outcomes. Clin. Infect. Dis. 50:364–373 [DOI] [PubMed] [Google Scholar]
- 23. Wendt C, et al. 2010. First outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 29:563–570 [DOI] [PubMed] [Google Scholar]
- 24. Woodford N, et al. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yigit H, et al. 2003. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing beta-lactamase KPC-2. Antimicrob. Agents Chemother. 47:3881–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zarfel G, et al. 2011. Emergence of New Delhi metallo-beta-lactamase, Austria. Emerg. Infect. Dis. 17:129–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zavascki AP, et al. 2010. KPC-2-producing Klebsiella pneumoniae in Brazil: a widespread threat in waiting? Int. J. Infect. Dis. 14:e539–e540 [DOI] [PubMed] [Google Scholar]
- 28. Zhang R, et al. 2011. Outbreak of KPC-2-producing Klebsiella pneumoniae with high qnr prevalence in a Chinese hospital. J. Med. Microbiol. 60:977–982 [DOI] [PubMed] [Google Scholar]