Abstract
Antiretrovirals that reach higher concentrations in cerebrospinal fluid (CSF) are associated with better control of HIV in CSF and possibly better neurocognitive performance. The objective of this study was to determine whether amprenavir (APV) concentrations in CSF are in the therapeutic range. Individuals were selected based on the use of regimens that included fosamprenavir (FPV), a prodrug of APV, and the availability of stored CSF and matched plasma. Total APV was measured in 119 matched CSF-plasma pairs from 75 subjects by high-performance liquid chromatography (HPLC) (plasma) or liquid chromatography tandem mass spectrometry (LC/MS/MS) (CSF). Concentrations were compared to the 50% inhibitory concentration (IC50) for wild-type HIV (5.6 ng/ml). Subjects were predominantly middle-aged (median 44 years) white (57%) men (78%) with AIDS (77%). APV was detected in all but 4 CSF specimens, with a median concentration of 24.8 ng/ml (interquartile range [IQR], 16.2 to 44.0). The median CSF-to-plasma ratio was 0.012 (IQR, 0.008 to 0.018). CSF concentrations correlated with plasma concentrations (rho = 0.61; P < 0.0001) and with postdose sampling interval (rho = −0.29; P = 0.0019). APV concentrations in CSF exceeded the median IC50 for wild-type HIV in more than 97% of CSF specimens with detectable APV by a median of 4.4-fold (IQR, 2.9 to 7.9). We conclude that administration of fosamprenavir should contribute to control of HIV replication in the central nervous system (CNS) as a component of effective antiretroviral regimens.
INTRODUCTION
The central nervous system (CNS) is infected early in the course of HIV disease, and HIV RNA is often detected in the cerebrospinal fluid (CSF) of untreated individuals with chronic disease. CNS infection can lead to HIV-associated neurocognitive disorders (HAND), which remain common despite potent combination antiretroviral therapy (ART) (8). HIV and the resulting immune and glial responses are likely to be important factors in the pathogenesis of HAND, leading to structural and functional synaptodendritic changes (15). Supporting the importance of the virus in the pathogenesis of HAND, HIV RNA concentrations in CSF are higher in patients with cognitive impairment than in those without impairment in cross-sectional and longitudinal studies (6, 7, 16), although the association has weakened in the highly active ART (HAART) era (20).
Antiretroviral drugs differ in their distribution—or penetration—into the CNS, with some drugs penetrating at concentrations similar to those in plasma and others penetrating at less than 1% of those in plasma. Only antiretrovirals that reach therapeutic concentrations in the CNS should be able to reduce HIV replication in that compartment. Factors such as molecular weight, liposolubility, protein binding, and transmembrane transport all affect CNS penetration of antiretrovirals. Antiretrovirals that reach higher levels in CSF are associated with better control of HIV replication (10, 14) and often with better neurocognitive performance (4, 12, 13, 21), although not all reports agree (14).
HIV protease inhibitors are highly bound to plasma proteins and are substrates for P-glycoprotein (9). Perhaps as a result, protease inhibitors have the lowest fractional penetrance (i.e., the amount of drug reaching the CSF compartment) into CSF of the antiretroviral drug classes. The limited distribution of protease inhibitors into the CNS may be mitigated by the substantial potency of these drugs. One protease inhibitor, fosamprenavir (FPV), a phosphorylated prodrug of amprenavir (APV), has low plasma protein binding (90%) relative to other drugs in its class. The objectives of this study were to measure APV concentrations in CSF, to compare them with matched plasma concentrations, and to estimate the efficacy of APV in the CNS by comparing the drug's concentrations in CSF to the in vitro 50% inhibitory concentration (IC50) range for wild-type HIV-1.
MATERIALS AND METHODS
CSF-plasma specimen pairs were selected from subjects who had HIV-1 infection and who had enrolled in parent observational cohort studies conducted at or coordinated by the University of California, San Diego, between July 2004 and January 2009. An optional lumbar puncture was part of the design of those parent studies. These cohort studies included the CNS HIV AntiRetroviral Therapy Effects Research (CHARTER) and the California NeuroAIDS Tissue Network (CNTN) projects. Selection criteria included use of FPV and availability of stored CSF and matched plasma obtained within 16 h (for FPV twice-daily [BID] dosing) and 24 h (for FPV once-daily dosing) of self-reported dosing. Oral FPV was dosed at 700 mg twice daily with ritonavir (RTV) 100 mg twice daily (n = 62), 1,400 mg once daily with ritonavir 200 mg once daily (n = 34), or 1,400 mg twice daily without RTV (n = 23). The UCSD Human Research Protections Program approved this research. Informed consent was obtained from all subjects.
CSF was obtained by lumbar puncture performed with an aseptic technique using a 22-gauge pencil-point needle by experienced operators. Blood was obtained within 1 h of CSF by routine phlebotomy. All specimens were stored at −70°C until analysis. Total APV was measured by high-performance liquid chromatography (HPLC) (in plasma) and liquid chromatography tandem mass spectrometry (LC/MS/MS) (in CSF). The dynamic ranges of these assays were 3.9 to 2,000 ng/ml (plasma) and 0.39 to 200 ng/ml (CSF). APV concentrations in CSF were compared to the in vitro IC50 range for wild-type HIV, including the median (5.6 ng/ml) and the 99th percentile (16.7 ng/ml) of the IC50 range (17). HIV RNA was quantified by reverse transcription-PCR using a Roche TaqMan RealTime assay (Roche Diagnostics) with a lower limit of detection of 50 copies/ml (1.7 log10 copies/ml). Peripheral blood CD4+ T cells were counted by flow cytometry. Adequate adherence was defined as subjects taking >95% of their scheduled antiretroviral drugs over 4 days preceding the CSF and blood specimen collection and was based on self-reporting. Data were analyzed with descriptive, bivariable, and multivariable statistics using standard methods (JMP; SAS Institute, Cary, NC). Spearman's correlation coefficient was used to assess the relationship between plasma and CSF APV concentrations. Descriptive statistics were calculated using all data available. To eliminate bias due to interindividual differences in the numbers of CSF-plasma pairs, certain statistical analyses, such as the analysis comparing dosing schedule to APV concentrations, were limited to one time point per subject. By convention, we selected the earliest time point for each subject for these analyses.
RESULTS
A total of 119 CSF-plasma pairs were obtained from 75 subjects. Most subjects provided either one (n = 52) or two (n = 14) specimen pairs. The remaining 9 subjects provided 3 (n = 5), 4 (n = 1), 5 (n = 1), 6 (n = 1), and 9 (n = 1) pairs. Subjects were predominantly middle-aged (median age of 44 years; interquartile range [IQR], 39 to 51) white (57%) men (78%) with AIDS (77%). The median CD4+ cell count at the time of sampling was 413/mm3 (IQR, 279 to 542), with 11% of values falling below 200/mm3. The median nadir CD4+ cell count was 105/mm3 (IQR, 15 to 227). Disease severity based on the 1993 Centers for Disease Control and Prevention classification system was categorized as C for 56%, B for 30%, and A for 14%. HIV RNA was at or below the detection limit (1.70 log10 copies/ml) in 57% of plasma and 88% of CSF specimens.
The median duration of FPV use was 9.5 months (IQR, 3.1 to 20.1). Concurrent antiretrovirals included nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) in all subjects, a non-NRTI in 13%, an additional protease inhibitor other than ritonavir in 11%, and one fusion inhibitor in 1%. The self-reported FPV dose before sampling was taken with food at 83% of the sampling time points. Adherence information was available for 85 sampling time points, and adequate adherence was reported for 75 (87%).
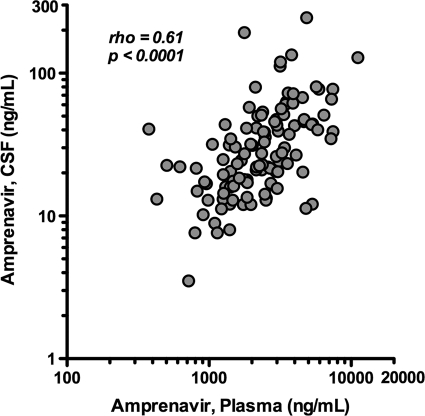
APV concentrations in plasma and CSF are displayed in Fig. 1, and aggregate data are summarized in Table 1, including both overall results and results by FPV dosing schedule. APV was not detected in four CSF specimens, while the corresponding plasma had detectable APV. The median CSF APV concentrations with and without RTV (including both FPV dosing schedules) were 26.1 ng/ml (IQR, 16.9 to 45.8) (not shown in table) and 23.4 ng/ml (IQR, 10.7 to 41.5), respectively. The data for the timing of CSF sampling were evenly distributed over the dosing intervals, and the median postdose sampling intervals (± standard deviation [SD]) were 7.2 ± 5.2 h for CSF and 6.9 ± 5.2 h for plasma. APV was detectable in all but four CSF specimens. CSF fractional penetrance (i.e., the amount of drug reaching the CSF compartment) was 1.2% of the median total plasma APV concentration (IQR, 0.8% to 1.8%). APV concentrations in CSF correlated with those in plasma (rho = 0.61; P < 0.0001) (Fig. 2) with persistent statistical significance in subgroups with (rho = 0.63; P < 0.0001) and without (rho = 0.57; P = 0.005) RTV. Further, correlations remained consistently strong when correlative analyses were performed using postdose sampling interval quartiles ranging from 0.5 to 5, 5 to 7, 7 to 12, and 12 to 23 h. The correlation weakened slightly for the shortest postdose sampling interval range of 0.5 to 5 h, but the coefficient (rho = 0.30) was greater than 0.25 although not statistically significant (P = 0.123). APV concentrations in plasma and CSF correlated with the postdose sampling interval (rho = −0.25 [P = 0.0054] and −0.29 [P = 0.0019], respectively). The CSF-to-plasma ratio did not change significantly across the dosing interval (rho = −0.12; P = 0.22). RTV-boosted FPV regimens were associated with higher plasma concentrations of APV compared to FPV without RTV (t = 1.61; P = 0.058) but not with higher CSF concentrations (t = 1.07; P = 0.15). These results were not substantially different when the analyses were performed with inclusion of a single time point for subjects with multiple CSF-plasma pairs.
Fig 1.
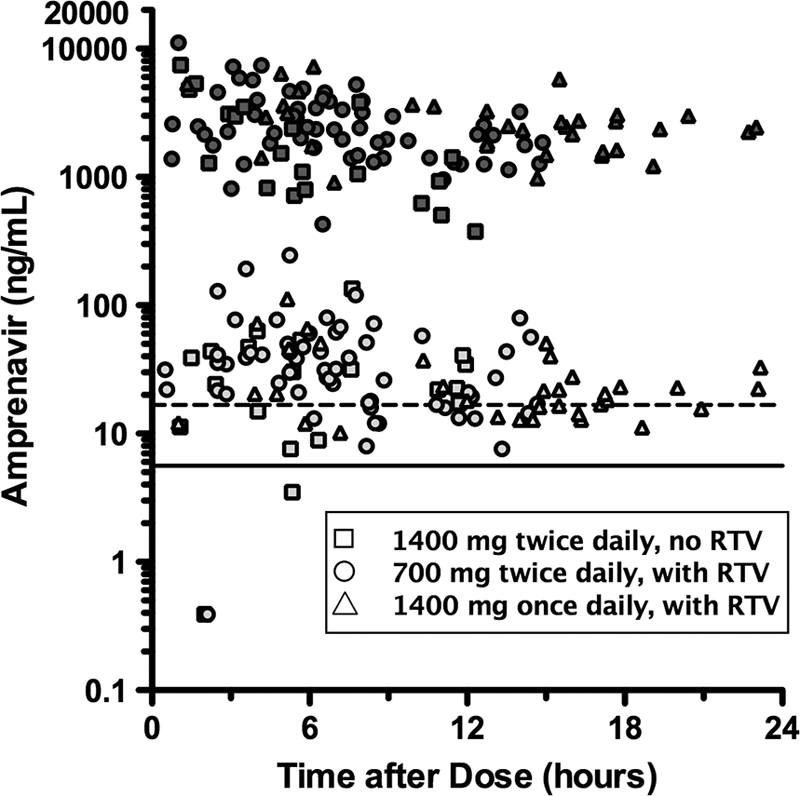
Amprenavir concentrations by fosamprenavir dosing schedule in plasma (dark shading) and CSF (light shading) by postdose sampling time. The horizontal solid line represents the median IC50 (5.6 ng/ml) and the horizontal dashed line the IC50 99th percentile (16.7 ng/ml). Plasma concentrations below 300 ng/ml and corresponding CSF data were excluded from the graph.
Table 1.
Summary of amprenavir concentrations and sampling by fosamprenavir dosing schedule
| Parameter | Amprenavir concn (ng/ml) or IQR |
CSF-to-plasma ratio or IQR | CSF-to-IC50 ratio or IQR | |
|---|---|---|---|---|
| CSF | Plasma (overall) | |||
| Regimen | ||||
| FPV 700 mg BID, with RTV | 60 | 62 | 60 | 60 |
| FPV 1,400 mg once a day, with RTV | 33 | 34 | 33 | 33 |
| FPV 1,400 mg BID, no RTV | 22 | 23 | 22 | 22 |
| Total | 115 | 119 | 115 | 115 |
| Median | ||||
| FPV 700 mg BID, with RTV | 31.6 | 2,230 | 0.014 | 4.2 |
| FPV 1,400 mg once a day, with RTV | 20.5 | 2,460 | 0.010 | 3.7 |
| FPV 1,400 mg BID, no RTV | 23.4 | 1282 | 0.018 | 5.6 |
| Overall | 24.8 | 2,261 | 0.012 | 4.4 |
| IQR | ||||
| FPV 700 mg BID, with RTV | 18.3–51 | 1,459–3,559 | 0.009–0.018 | 1.9–7.4 |
| FPV 1,400 mg once a day, with RTV | 14.9–35.2 | 1,556–3,185 | 0.007–0.012 | 2.7–6.3 |
| FPV 1,400 mg BID, no RTV | 10.7–41.5 | 718–3,108 | 0.008–0.031 | 3.3–9.1 |
| Overall | 16.2–44.0 | 1,397–3,353 | 0.008–0.018 | 2.9–7.9 |
Fig 2.
Correlation between amprenavir CSF and plasma concentrations.
APV concentrations in CSF exceeded the median IC50 for wild-type HIV-1 in 97.3% of specimens with detectable APV by a median of 4.4-fold (IQR, 2.9 to 7.9), while 73.9% of the same specimens had concentrations above the 99th percentile of the IC50 range. Higher APV concentrations in plasma were not associated with undetectable HIV RNA levels in plasma (t = 0.42; P > 0.34). Overall, higher APV concentrations in CSF were not associated with undetectable HIV RNA in CSF (t = 1.5; P = 0.15). Of note, all HIV RNA levels in CSF were undetectable when HIV RNA levels in plasma were undetectable. In the subgroup of subjects who had detectable HIV RNA levels in plasma, detectable HIV RNA levels in CSF trended toward an association with lower APV concentrations in CSF (mean, 24.5 versus 37.5 ng/ml [t = −1.8; P = 0.08]). This association was present (β = 0.04; P = 0.08) in multivariable analyses that accounted for APV concentrations in plasma, duration of FPV therapy, and postdose sampling time. Classification and regression trees showed that all of the detectable HIV RNA levels in CSF occurred in subjects who had APV concentrations in CSF below 53.5 ng/ml. This equated to a CSF-to-IC50 ratio of 9.6. In other words, all detectable HIV RNA levels in CSF occurred in subjects whose APV concentrations in CSF exceeded the 50% inhibitory concentration by less than 9.6-fold. This categorical expression of CSF APV concentrations was statistically significantly associated with detectable HIV RNA levels in CSF (β = −12.5; P = 0.01) in multivariable analyses that accounted for APV concentrations in plasma, duration of FPV therapy, and postdose sampling time.
FPV dosing schedules were associated with different CSF and plasma APV concentrations as well as CSF-to-plasma and CSF-to-IC50 ratios as shown in Table 1 and Fig. 3. While FPV at 700 mg BID with RTV resulted in the highest median CSF APV concentration, the other FPV dosing schedules did not substantially differ. However, FPV at 1,400 mg BID without RTV had the highest CSF-to-plasma ratio, with trends toward significance (Fig. 3). FPV at 1,400 mg BID without RTV appeared to be associated with lower plasma APV concentrations, although the differences compared to other dosing schedules were not statistically significant.
Fig 3.
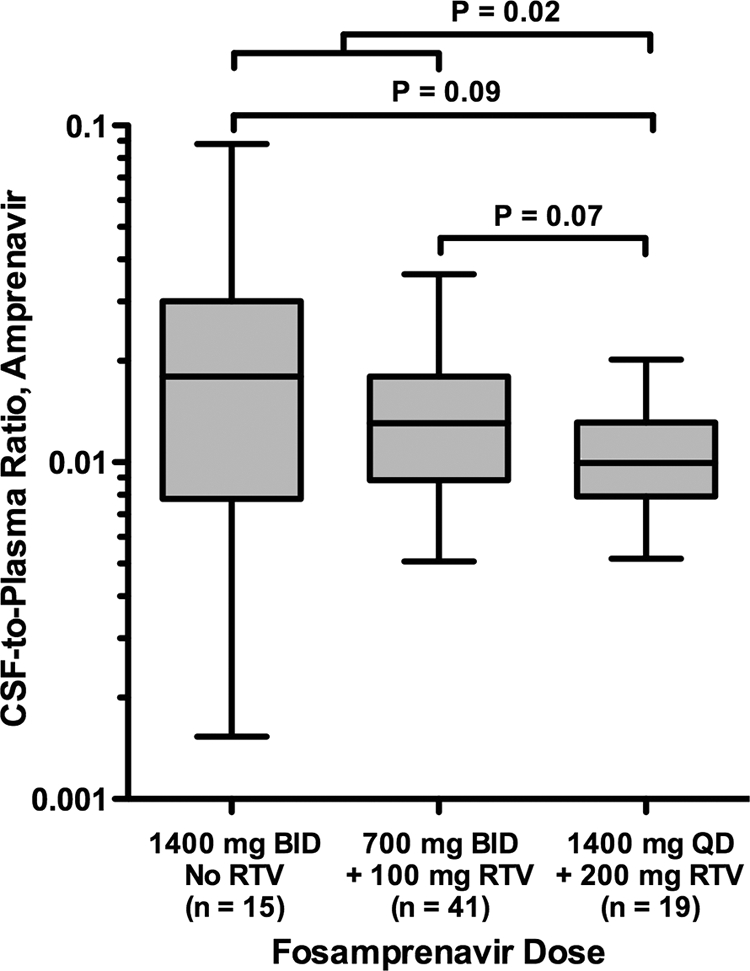
CSF-to-plasma ratios of amprenavir concentrations by fosamprenavir dosing schedule, including only the first time point for subjects with multiple time points. QD, once daily.
DISCUSSION
APV CSF concentrations have not been reported previously other than in a much smaller series with a median CSF APV concentration comparable to the one reported here (19). In this study, APV concentrations in plasma were somewhat variable whereas APV concentrations in CSF were more stable and exceeded the median concentration required to inhibit wild-type HIV in vitro in nearly all specimens regardless of dosing schedules (median, 4.4-fold higher). In addition, the majority of specimens exceeded the 99th percentile of the IC50 range. The concentrations of APV in CSF were lower than expected based on the drug's physicochemical characteristics. For example, APV has a relatively low binding (90%) to plasma proteins such as albumin and alpha-1 acid glycoprotein compared to other drugs in its class (18). Therefore, assuming all unbound drug penetrates into the CSF, one would expect the CSF concentrations to be approximately 10% of those in plasma. The lower-than-expected CSF fractional penetrance of 1.2% indicates that limiting factors restrict distribution of APV into the CSF, which is similar to what has been seen previously with other protease inhibitors (2, 3, 11). APV affinity for active efflux transporters such as P-glycoprotein may be one of those barriers (1, 22). Despite this limitation, the CSF fractional penetrance still compares well to that of other protease inhibitors.
The moderate-to-strong correlation between APV concentrations in CSF and plasma suggests that APV concentrations in plasma would be useful for estimating concentrations in the CNS and suggests that interventions focused on increasing APV concentrations in plasma might also result in higher concentrations in the CNS. The weakening of the correlation coefficient (although it was still greater than 0.25) observed for the shortest postdose sampling interval quarter may indicate the presence of some system hysteresis for the CSF compartment, particularly at the beginning of the dosing interval, perhaps suggesting achievement of minimum concentrations in CSF while plasma concentrations are rising. The finding of a low proportion of CSF specimens with detectable HIV RNA further supports the idea of the effectiveness of APV in the CNS. However, the latter should not necessarily be attributed to APV, since other factors, particularly the presence of other antiretroviral agents in each drug regimen, may have accounted for the finding. Disease-related variables may have also accounted for the finding. For example, participants had relatively good immune reconstitution (89% had CD4+ T-cell counts > 200/mm3), which has been linked to HIV in CSF being more likely to originate from migrating lymphocytes (i.e., a predominant blood source) than from macrophages and microglia in brain tissue (i.e., a predominant CNS source) (5, 23).
Analyses of FPV dosing schedules indicated that coadministration of RTV may increase APV concentrations in plasma to a greater extent than it increases APV concentrations in CSF, resulting in lower CSF-to-plasma APV ratios. Multiple explanations for this finding are possible. Active efflux transporters at the level of the blood-brain and blood-CSF barriers may not be saturated under conditions of lower APV concentrations in plasma, with the result that higher concentrations are more efficiently pumped out of the CNS. A non-mutually exclusive explanation is that active efflux transporters may become upregulated in response to higher concentrations of substrate or as result of RTV induction. One possible conclusion from this finding is that higher doses of unboosted FPV may result in higher APV concentrations in CSF, which may better ensure suppression of HIV replication in the CNS. Four specimens had APV below the lower limit of quantitation in CSF, and all had a relatively short postdose sampling interval (postdose sampling intervals of 2.0, 2.1, 6.2, and 7.8 h), possibly suggesting that those may represent the minimum CSF concentration ([Cmin] as opposed to the trough concentration. This may be the result of system hysteresis pertaining to the CSF compartment), which may explain the discrepancy with respect to plasma concentrations. Subjects with these low levels of APV in CSF early in the postdose interval were no more likely to use one dosing schedule of FPV than another.
Compared to other protease inhibitors, FPV appears to have intermediate distribution into the CNS based on fractional penetrance (higher than atazanavir penetrance and lower than indinavir or darunavir penetrance). APV's CSF-to-IC50 ratio is numerically lower than that of lopinavir, darunavir, or indinavir and higher than that of atazanavir, but the clinical value of this measure is unproven (2, 3, 11). Even though APV has the lowest molecular weight of the HIV protease inhibitors currently in use and a relatively high unbound fraction—characteristics that should favor excellent CSF distribution—these characteristics may be mitigated by APV's average liposolubility relative to those of other protease inhibitors and the P-glycoprotein active efflux transporter's affinity for APV. Despite these findings, more than 97% of CSF specimens had APV concentrations that exceeded the median IC50 for wild-type HIV, almost 75% of concentrations were above the 99th percentile of the IC50 range, and nearly 90% of CSF specimens had undetectable HIV RNA (often when HIV RNA was detectable in plasma), supporting the idea of APV's antiviral effectiveness in the CNS, although other antiretrovirals may have been contributing to those findings.
In summary, our findings provide supporting evidence that APV reaches therapeutic concentrations in the CNS and, therefore, that FPV should be an effective component of antiretroviral regimens that aim to control HIV replication in this pharmacologically restricted compartment. The data presented in this report support an intermediate position for fosamprenavir relative to other protease inhibitors in terms of CNS penetration effectiveness, but this conclusion requires validation with a clinical trial that would directly compare FPV to another protease inhibitor(s).
ACKNOWLEDGMENTS
This study was supported by an investigator-initiated research grant from GlaxoSmithKline and by the National Institutes of Health via the following awards: N01 MH22005, HHSN271201000027C, and HHSN271201000030C.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Bachmeier CJ, Spitzenberger TJ, Elmquist WF, Miller DW. 2005. Quantitative assessment of HIV-1 protease inhibitor interactions with drug efflux transporters in the blood-brain barrier. Pharm. Res. 22:1259–1268 [DOI] [PubMed] [Google Scholar]
- 2. Best BM, et al. 2009. Low atazanavir concentrations in cerebrospinal fluid. AIDS 23:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Capparelli EV, et al. 2005. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS 19:949–952 [DOI] [PubMed] [Google Scholar]
- 4. Cysique LA, et al. 2009. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology 73:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellis RJ, et al. 2000. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology 54:927–936 [DOI] [PubMed] [Google Scholar]
- 6. Ellis RJ, et al. 1997. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann. Neurol. 42:679–688 [DOI] [PubMed] [Google Scholar]
- 7. Ellis RJ, et al. 2002. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch. Neurol. 59:923–928 [DOI] [PubMed] [Google Scholar]
- 8. Heaton RK, et al. 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kis O, Robillard K, Chan GNY, Bendayan R. 2010. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharm. Sci. 31:22–35 [DOI] [PubMed] [Google Scholar]
- 10. Letendre S, et al. 2008. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 65:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Letendre SL, Capparelli EV, Ellis RJ, McCutchan JA. 2000. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. The HIV Neurobehavioral Research Center Group. Antimicrob. Agents Chemother. 44:2173–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Letendre SL, et al. 2004. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann. Neurol. 56:416–423 [DOI] [PubMed] [Google Scholar]
- 13. Letendre SL, et al. 2007. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin. Infect. Dis. 54:1511–1517 [Google Scholar]
- 14. Marra CM, et al. 2009. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 23:1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masliah E, Heaton RK, Marcotte, et al. 1997. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 42:963–972 [DOI] [PubMed] [Google Scholar]
- 16. McArthur JC, et al. 1997. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann. Neurol. 42:689–698 [DOI] [PubMed] [Google Scholar]
- 17. Parkin NT, et al. 2004. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadler B, et al. 2001. Pharmacokinetic study of human immunodeficiency virus protease inhibitors used in combination with amprenavir. Antimicrob. Agents Chemother. 45:3663–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saumoy M, et al. 2011. Viral response in stable patients switching to fosamprenavir/ritonavir monotherapy (the FONT Study). HIV Med. 12:438–441 [DOI] [PubMed] [Google Scholar]
- 20. Sevigny JJ, et al. 2004. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 63:2084–2090 [DOI] [PubMed] [Google Scholar]
- 21. Tozzi V, et al. 2009. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J. Acquir. Immune Defic. Syndr. 52:56–63 [DOI] [PubMed] [Google Scholar]
- 22. Varatharajan L, Thomas SA. 2009. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 82:A99–A109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiley C, Schrier R, Nelson J, Lampert PW, Oldstone MB. 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. U. S. A. 83:7089–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]