Abstract
Because studies showed similar viral suppression with lower raltegravir doses and because Asians usually have high antiretroviral concentrations, we explored low-dose raltegravir therapy in Thais. Nineteen adults on raltegravir at 400 mg twice daily (BID) with HIV RNA loads of <50 copies/ml were randomized to receive 400 mg once daily (QD) or 800 mg QD for 2 weeks, followed by the other dosing for 2 weeks. Intensive pharmacokinetic analyses were performed, and HIV RNA was monitored. Two patients were excluded from the 400-mg QD analysis due to inevaluable pharmacokinetic data. The mean patient weight was 58 kg. Mean pharmacokinetic values were as follows: for raltegravir given at 400 mg BID, the area under the concentration-time curve from 0 to 12 h (AUC0-12) was 15.6 mg/liter-h and the minimum plasma drug concentration (Ctrough) was 0.22 mg/liter; for raltegravir given at 800 mg QD, the AUC0-24 was 33.6 mg/liter-h and the Ctrough was 0.06 mg/liter; and for raltegravir given at 400 mg QD, the AUC0-24 was 18.6 mg/liter-h and the Ctrough was 0.08 mg/liter. The HIV RNA load was <50 copies/ml at each dose level. Compared to the adjusted AUC0-24 for Westerners on raltegravir at 400 mg BID, Thais on the same dose had double the AUC0-24 and those on raltegravir at 400 mg QD had a similar AUC0-24. More patients had a Ctrough of <0.021 mg/liter on raltegravir at 400 mg QD (9/17 patients) than on raltegravir at 800 mg QD (1/19 patients) or 400 mg BID (0/19 patients). Seventeen patients used raltegravir at 400 mg QD for a median of 35 weeks; two had confirmed HIV RNA loads between 50 and 200 copies/ml, and both had low Ctrough values. Low-dose raltegravir could be a cost-saving option for maintenance therapy in Asians or persons with low body weight. However, raltegravir at 400 mg QD was associated with a low Ctrough and with a risk for HIV viremia. Raltegravir at 200 or 300 mg BID should be studied, but new raltegravir formulations will be needed.
INTRODUCTION
Raltegravir is an HIV-1 integrase strand inhibitor with potency against wild-type and multidrug-resistant viruses (5, 19). It is approved for use as part of first-line and salvage therapy in treatment guidelines, at a dosage of 400 mg twice daily (BID) (7, 9, 21), based on large phase III trials showing favorable efficacy and safety profiles in both treatment-naïve (12) and treatment-experienced (20) patients. Phase II studies have shown raltegravir dosages ranging from 200 mg to 600 mg BID to be no different in antiviral activity in treatment-naïve and -experienced patients (10, 14, 15, 19). Worldwide, raltegravir is most needed for salvage therapy, but even in this setting, it is rarely available, due mainly to its high cost (11). Antiretroviral dose reduction has been one of the options used in Thailand to decrease cost and increase access. Several studies have shown that Thai adults and children on low-dose antiretroviral regimens achieve comparable pharmacokinetic parameters and treatment outcomes to those of Westerners on standard doses (2, 3, 6, 13, 17). Therefore, we conducted this study to evaluate the pharmacokinetics of low-dose, once-daily raltegravir (400 mg QD) in HIV-1-infected Thai adults. In addition, we evaluated the pharmacokinetics of raltegravir at 800 mg QD, which would provide a more convenient once-daily regimen with the same total daily raltegravir dose as the standard dosing of 400 mg BID. We assessed the virologic response by using a raltegravir minimum plasma drug concentration (Ctrough) threshold of 0.021 mg/liter, which was reported in the QDMRK study to be associated with virologic failure risk (8, 23).
(This study was presented as an oral discussion and poster presentation at the 18th Conference on Retroviruses and Opportunistic Infections, 27 February to 2 March 2011, Boston, MA.)
MATERIALS AND METHODS
This was a single-center, open-label, crossover design study conducted at the HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT) in Bangkok, Thailand (www.clinicaltrials.gov study no. NCT01159132). The study was approved by the Chulalongkorn University Institutional Review Board, and all patients gave written informed consent before enrollment.
HIV-1-infected adult Thai patients (over 18 years of age) who were on raltegravir at 400 mg BID for at least 3 months and had HIV RNA loads of <50 copies/ml were enrolled. After screening, all were monitored on days 1, 15, and 29. A medical history evaluation, physical examination, and laboratory analyses were performed at all visits. The laboratory analyses included clinical chemistry, hematology, urinalysis, pregnancy test, CD4 cell count, and HIV RNA load determination. All patients underwent 3 intensive raltegravir pharmacokinetic evaluations for dosing of 400 mg BID, 400 mg QD, and 800 mg QD, but in different orders according to randomization. A block randomization was used, stratifying for whether the subject's regimen included atazanavir. After sample collection for the first intensive pharmacokinetic evaluation on day 1, all patients were randomized to either arm A or arm B. Patients in arm A received raltegravir at 400 mg QD, and those in arm B received raltegravir at 800 mg QD, for 14 days. On day 15, a second intensive pharmacokinetic evaluation was carried out, after which the patients crossed over to the other study arm. Patients in arm A received raltegravir at 800 mg QD, and those in arm B received raltegravir at 400 mg QD, for another 14 days. On day 29, a third intensive pharmacokinetic evaluation was carried out, after which all patients switched back to the initial regimen of raltegravir at 400 mg BID.
Pharmacokinetics.
The intensive pharmacokinetic evaluations were performed on days 1, 15, and 29. On all 3 pharmacokinetic study days, patients took their medicine after breakfast, with direct observation from the study nurse. On day 1 (BID dose), 6 ml of blood was collected into a lithium heparin tube predose and at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 10.0, and 12.0 h postdosing, and on days 15 and 29 (QD dose), an additional sample was collected at 24 h postdosing. Blood samples were then centrifuged at 3,000 rpm for 10 min at 20°C. Plasma was transferred to a polypropylene tube and stored at −20°C until analysis (no longer than 1 month), after which all samples were transferred to −80°C for long-term storage. Raltegravir concentrations were determined by using a validated high-performance liquid chromatography method with fluorescence detection (22). The raltegravir calibration curve was linear over the concentration range of 0.014 to 10.0 mg/liter, and the lower limit of quantification was 0.014 mg/liter.
Pharmacokinetic parameters were calculated using WinNonlin software, version 5.2 (Pharsight Corporations, Mountain View, CA). The following pharmacokinetic parameters were determined using noncompartmental analysis: area under the plasma concentration-time curve from 0 to 12 h (AUC0-12), AUC0-24, the maximum plasma drug concentration (Cmax), Ctrough at 12 h (C12) and 24 h (C24), the time to reach the maximum plasma drug concentration (Tmax), and the apparent elimination half-life (t1/2).
Procedures after completion of pharmacokinetic study.
After completing the pharmacokinetic evaluations, all patients were followed in the HIV-NAT 006 long-term cohort study (www.clinicaltrials.gov study no. NCT00411983) and continued on standard raltegravir at 400 mg BID as part of their antiretroviral regimens. Patients whose pharmacokinetic values for raltegravir at 400 mg QD fulfilled the protocol-defined target AUC of ≥4.0 h-mg/liter, an exposure deemed to be adequate for viral suppression (4), were offered raltegravir at 400 mg QD. Patients had adherence counseling and HIV RNA monitoring every 3 months. They were informed about the QDMRK results, when they became available, showing raltegravir at 800 mg QD to be associated with more virologic failure than raltegravir at 400 mg BID in antiretroviral-naïve adults (8), and all elected to continue raltegravir at 400 mg QD. More intensive HIV RNA monitoring every 1 to 2 months was performed, and Ctrough determinations were repeated. Patients were switched back to raltegravir at 400 mg BID immediately for confirmed HIV RNA loads above 50 copies/ml.
Statistical analysis.
Statistical analyses were performed using Stata 11.0 (Statacorp, College Station, TX). Comparison of pharmacokinetic parameters was assessed using a random effects regression model adjusting for patient and order of regimen, with maximum likelihood estimation. The geometric mean of each pharmacokinetic parameter was used as the outcome variable in all regression models. In univariate models, dose regimens were modeled with the BID regimen as the reference group. Other potential explanatory covariates included gender, age, weight, estimated creatinine clearance by the Cockroft-Gault formula, plasma alanine aminotransferase (ALT) concentration, and whether the patient was taking tenofovir, darunavir-ritonavir, lopinavir-ritonavir, or atazanavir. The exponentiated regression coefficients presented in our paper correspond to changes in the ratio of the expected geometric means of the outcome variable. Parameters that were significant in the univariate analysis, with P values of ≤0.1, were included in multivariate models, and a backwards stepwise selection procedure was used to select the final model, retaining covariates at a significance level of 0.05. In making comparisons of the AUC between BID and QD regimens, the AUC0-12 for the BID regimens was first converted to an AUC0-24. Ctrough refers to C12 for BID regimens and to C24 for QD regimens. Formal comparisons with the pharmacokinetic parameters for Caucasians taking raltegravir at 400 mg BID reported by Markowitz et al. (14) were made by using the ttesti command in Stata and estimating the geometric standard deviation (SD) from the 95% confidence interval (CI) around the geometric mean, which were provided by Merck. The number of patients with HIV RNA loads of >50 copies/ml after treatment with raltegravir at 400 mg QD was summarized according to the Ctrough value during the intensive pharmacokinetic study.
RESULTS
Baseline characteristics.
Nineteen patients (13 men [68%]) were enrolled in the study. The median (interquartile range [IQR]) age was 44 (38 to 65) years, body weight was 58 (44 to 73) kg, body surface area was 1.65 (1.51 to 1.75) m2, and CD4 cell count was 453 (102 to 681) cells/mm3. All patients had HIV RNA loads of <50 copies/ml. The medians (IQR) for other laboratory values were as follows: hemoglobin, 13 (12 to 14.6) g/dl; total cholesterol (TC), 217 (190 to 246) mg/dl; triglycerides (TG), 202 (147 to 274) mg/dl; high-density lipoprotein (HDL), 42 (31 to 51) mg/dl; ALT, 25 (17 to 38) U/liter; and creatinine clearance, 68 (61 to 93) ml/min/1.73 m2. The median (IQR) time on raltegravir at 400 mg BID at enrollment was 90 (66 to 110) weeks. All patients were on raltegravir as part of their third-line regimen with a protease inhibitor (PI). The antiretroviral drugs coadministered with raltegravir were lamivudine (n = 18), tenofovir (n = 9), darunavir-ritonavir (n = 11), lopinavir-ritonavir (n = 6), and atazanavir without ritonavir (n = 2). None of the patients was on a nonnucleoside reverse transcriptase inhibitor (NNRTI). No patients dropped out of the study.
Pharmacokinetics.
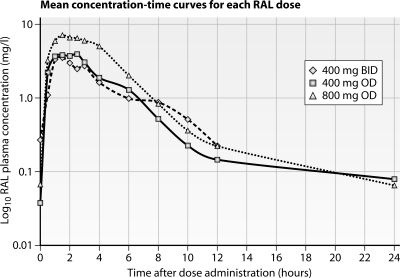
Two patients were excluded from the analysis of raltegravir at 400 mg QD, one due to fever on the pharmacokinetic study day and the other because the patient took the medication at the incorrect time before the intensive pharmacokinetic study day. The mean log plasma raltegravir concentration-time curves for all patients on all three dosing regimens are plotted in Fig. 1. The geometric mean and arithmetic mean pharmacokinetic parameters are summarized in Table 1. There were significant differences in pharmacokinetic parameters between the three dosing regimens. Compared to the standard dosing of 400 mg BID, the 800-mg QD dosing had significantly higher values for AUC0-24, Cmax, and t1/2 but a lower Ctrough (P < 0.001 for all). Reduction of the raltegravir dose to 400 mg QD led to a significant reduction in the time-adjusted AUC0-24, Ctrough, and t1/2 values compared to those for the standard recommended dose of 400 mg BID (P < 0.001 for all). For all dosing regimens, no patient had an AUC below 4.0 mg-h/liter, with the exception of two patients with AUCs of 3.5 h-mg/liter and 3.96 h-mg/liter while on raltegravir at 400 mg BID. Ctrough was below 0.021 mg/liter in 9/17, 1/19, and 0/19 patients on raltegravir at 400 mg QD, 800 mg QD, and 400 mg BID, respectively.
Fig 1.
Mean log10 raltegravir concentration-time curves for three raltegravir dosing regimens. RAL, raltegravir. Raltegravir dosing was as follows: diamonds with dashed line, raltegravir at 400 mg BID; squares with solid line, raltegravir at 400 mg QD; triangles with dotted line, raltegravir at 800 mg QD.
Table 1.
Pharmacokinetic parameters of raltegravir in Thai patients receiving each of three different dosing regimens
| Parametera | Result formatb | Value for regimenc |
||
|---|---|---|---|---|
| Raltegravir at 400 mg BID (n = 19) | Raltegravir at 800 mg QD (n = 19) | Raltegravir at 400 mg QD (n = 17) | ||
| AUC (h-mg/liter) | Geometric mean (%CV) | 13.0 (76.2) | 29.8 (50.8) | 16.1 (60.3)** |
| Mean (SD) | 15.6 (8.8) | 33.6 (19.3) | 18.6 (10.8) | |
| Half-life (h) | Geometric mean (%CV) | 2.6 (67.4) | 5.8 (45.9)** | 4.8 (35.5)** |
| Mean (SD) | 3.1 (2.1) | 6.3 (2.7) | 5.0 (1.8) | |
| Clearance (liters/h) | Geometric mean (%CV) | 30.9 (76.2) | 24.8 (60.3) | 26.8 (50.8)* |
| Mean (SD) | 39.0 (30.1) | 29.7 (13.8) | 28.7 (16.8) | |
| Cmax (mg/liter) | Geometric mean (%CV) | 4.5 (116.6) | 10.2 (65.5)** | 5.59 (83.4) |
| Mean (SD) | 6.1 (3.9) | 11.9 (6.4) | 6.7 (3.5) | |
| Ctrough (mg/liter) | Geometric mean (%CV) | 0.14 (125.4) | 0.03 (169.9)** | 0.05 (69.2)** |
| Mean (SD) | 0.22 (0.24) | 0.06 (0.04) | 0.08 (0.19) | |
| Tmax (h) | Median (IQR) | 1.5 (1.5–6) | 2 (1–2.5) | 2 (1–3) |
The AUC for twice-daily (BID) dosing is the AUC0-12; the AUC for once-daily (QD) dosing is the AUC0-24. Statistical comparisons of AUC were performed against the calculated AUC0-24 for dosing at 400 mg BID (i.e., 26 h-mg/liter). The Ctrough for BID dosing is the C12, and that for QD dosing is the C24.
CV, coefficient of variance; SD, standard deviation; IQR, interquartile range.
Statistical comparisons were made for geometric mean values, using paired data.
, P < 0.05;
, P < 0.001.
Table 2 shows the univariate analysis of factors affecting the raltegravir AUC, Cmax, Ctrough, and t1/2 for the three dosing regimens. There was a 36% decrease in the AUC0-24 when the dose was reduced from 400 mg BID to 400 mg QD (P < 0.001). A similar decrease was found for Ctrough (80%; P < 0.001). However, t1/2 showed a significant increase of 79% (P < 0.001). When 800 mg QD was compared to 400 mg BID, there was a significant increase in Cmax (127%; P < 0.001) and t1/2 (121%; P < 0.001) and a decrease in Ctrough (62%; P < 0.001).
Table 2.
Univariate and multivariate analyses of factors affecting raltegravir pharmacokinetic parameters at standard and reduced doses
| Parameter and variablea | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |
| AUC0-24 | ||||
| Dose of 400 mg QD (vs 400 mg BID) | 0.64 (0.50–0.80) | <0.001 | 0.63 (0.50–0.80) | <0.001 |
| Dose of 800 mg QD (vs 400 mg BID) | 1.15 (0.92–1.44) | 0.22 | 1.15 (0.92–1.45) | 0.23 |
| Atazanavir comedication | 2.13 (1.15–3.96) | 0.02 | 2.16 (1.18–3.25) | 0.01 |
| Darunavir comedication | 0.67 (0.45–1.01) | 0.054 | ||
| Half-life | ||||
| Dose of 400 mg QD (vs 400 mg BID) | 1.79 (1.42–2.25) | <0.001 | 1.80 (1.42–2.29) | <0.001 |
| Dose of 800 mg QD (vs 400 mg BID) | 2.21 (1.77–2.76) | <0.001 | 2.20 (1.75–2.77) | <0.001 |
| Tenofovir comedication | 1.36 (0.99–1.86) | 0.055 | 1.35 (1.03–1.77) | 0.03 |
| Darunavir comedication | 1.34 (0.97–1.84) | 0.076 | 1.34 (1.02–1.76) | 0.04 |
| Clearance | ||||
| Dose of 400 mg QD (vs 400 mg BID) | 0.78 (0.62–0.99) | 0.04 | 0.79 (0.62–1.00) | 0.05 |
| Dose of 800 mg QD (vs 400 mg BID) | 0.87 (0.69–1.09) | 0.22 | 0.87 (0.69–1.09) | 0.23 |
| Atazanavir comedication | 0.46 (0.25–0.84) | 0.01 | 0.46 (0.25–0.85) | 0.01 |
| Darunavir comedication | 1.53 (1.04–2.26) | 0.03 | ||
| Cmax | ||||
| Dose of 400 mg QD (vs 400 mg BID) | 1.28 (0.86–1.91) | 0.22 | ||
| Dose of 800 mg QD (vs 400 mg BID) | 2.27 (1.54–3.33) | <0.001 | ||
| Ctrough | ||||
| Dose of 400 mg QD (vs 400 mg BID) | 0.20 (0.13–0.31) | <0.001 | ||
| Dose of 800 mg QD (vs 400 mg BID) | 0.38 (0.25–0.59) | <0.001 | ||
AUC comparisons between twice-daily (BID) and once-daily (QD) dosing were made by first converting the AUC0-12 to the AUC0-24. The Ctrough for BID dosing is C12, and that for QD dosing is C24. Only covariates significant in univariate analysis and doses are presented.
Age, gender, body weight, calculated creatinine clearance, ALT, and tenofovir or lopinavir-ritonavir comedication did not affect the AUC of raltegravir in the univariate analysis. As expected, atazanavir comedication was associated with a higher AUC of raltegravir (113%) and a lower raltegravir clearance rate. The use of darunavir-ritonavir significantly increased the clearance of raltegravir.
In the multivariate analyses of changes in AUC, after adjusting for atazanavir comedication, there was a significant decrease in the geometric mean of the AUC (37%; P < 0.001) in the 400-mg QD dosing group compared to the standard 400-mg BID group, whereas there was no difference in the geometric mean of the AUC when patients were given 800 mg QD compared to 400 mg BID. The geometric mean of the AUC for patients treated with atazanavir was approximately 116% higher after adjusting for the raltegravir dosing regimen. There were significant increases in t1/2 and decreases in clearance with the nonstandard raltegravir doses and with tenofovir and darunavir-ritonavir comedications.
Table 3 compares the geometric mean ratios of AUC, Cmax, and Ctrough values for the three raltegravir dosing regimens in Thais in our study with those reported for Westerners taking raltegravir at 400 mg BID (14). Using the same 400-mg BID dosing, our Thai patients had double the values for AUC, Cmax, and Ctrough compared to Westerners. Thais on raltegravir at 400 mg QD had an AUC that was 1.2 times that of Westerners on raltegravir at 400 mg BID, with an almost tripled Cmax and half the Ctrough. Raltegravir at 800 mg QD in Thais resulted in 2 times the AUC, 5 times the Cmax, and a similar Ctrough compared to those for raltegravir at 400 mg BID in Westerners.
Table 3.
Geometric mean ratios of AUC0-12, Cmax, and Ctrough for raltegravir dosing regimens used in our study compared to those in Western subjects taking raltegravir at 400 mg BID
| Parameter | Value for Western subjects receiving raltegravir at 400 mg BIDa | Value for Thais in this study |
||
|---|---|---|---|---|
| 400 mg BID | 400 mg QD | 800 mg QD | ||
| n | 6 | 19 | 17 | 19 |
| Mean (SD) age (yr) | 41 (10) | 47 (8.1) | 47 (8.1) | 47 (8.1) |
| No. (%) of males | 3 (50) | 13 (68) | 11 (65) | 13 (68) |
| % Ethnicity | 50% White, 50% others | 100% Asian | 100% Asian | 100% Asian |
| AUC0-12 (h-mg/liter)b | 6.89 (3.09–15.28) | 1.9 (1.2–3.1)* | 1.2 (0.7–1.9) | 2.2 (1.4–3.4)* |
| Cmax (mg/liter)b | 2.17 (0.77–6.17) | 2.1 (1.1–4.0)* | 2.6 (1.4–4.7)* | 4.7 (2.6–8.4)** |
| Ctrough (mg/liter)b | 0.069 (0.037–0.126) | 2.1 (1.2–3.5)** | 0.4 (0.2–0.8)* | 0.8 (0.5–1.2) |
Merck provided data for Westerners who were enrolled in the published study of Markowitz et al. (14).
Data are geometric means with 95% confidence intervals (CI) for Western subjects and geometric mean ratios with 95% CI for Thai subjects compared to Western subjects receiving 400 mg BID. Statistical comparisons were based on an unpaired t test of the geometric means, with standard deviations estimated from the reported 95% CI. AUC comparisons between twice-daily (BID) and once-daily (QD) dosing were made by first converting the AUC0-12 to the AUC0-24. The Ctrough is C12 for BID regimens and C24 for QD regimens.
, P < 0.05;
, P < 0.001.
Short-term virologic response and safety.
All patients had plasma HIV RNA loads of <50 copies/ml at the end of all dosing regimens, and no serious adverse events were seen. There were no significant differences in ALT, creatinine clearance, TG, TC, and HDL after each dosing.
After a median (IQR) of 35 (33 to 39) weeks on raltegravir at 400 mg QD, 2 of 17 patients had confirmed HIV RNA loads of >50 copies/ml. The first patient had an HIV RNA rise 19 weeks after starting low-dose raltegravir, with an HIV RNA load of 95 copies/ml, and had a load of 126 copies/ml 10 weeks later. His raltegravir Ctrough at the time of full pharmacokinetic evaluation was 0.015 mg/liter. The second patient had an HIV RNA load of 239 copies/ml after 34 weeks of low-dose raltegravir, which was confirmed by an HIV RNA load of 123 copies/ml 4 weeks later. His Ctrough at the time of full pharmacokinetic analysis was 0.014 mg/liter. An additional 3 patients had a single HIV RNA value above 50 copies/ml (51, 79, and 129 copies/ml), followed by HIV RNA suppression. Their raltegravir Ctrough concentrations at the time of full pharmacokinetic evaluation of low-dose raltegravir were 0.028, 0.060, and 0.006 mg/liter, respectively.
The outcomes for patients with Ctrough values of <0.021 and ≥0.021 mg/liter at the time of the 400-mg QD pharmacokinetic study of raltegravir are summarized in Table 4. There were approximately equal numbers of patients in each group, with slightly more females, persons with higher body weight, and persons who used PIs other than darunavir-ritonavir in the low-Ctrough group. The geometric mean for Ctrough was 0.014 mg/liter for the low-Ctrough group and 0.066 mg/liter for the high-Ctrough group. After about 9 months on low-dose raltegravir, virologic failure was seen only in the low-Ctrough group (22% versus 0% in the high-Ctrough group). The geometric mean Ctrough value continued to be lower in the low (n = 8)- than in the high (n = 6)-Ctrough group at the last follow-up visit.
Table 4.
Short-term virologic response to raltegravir at 400 mg once daily, based on raltegravir Ctrough
| Parameter | Value |
|
|---|---|---|
| Subjects with Ctrough of <0.021 mg/liter at time of pharmacokinetic study (n = 9) | Subjects with Ctrough of ≥0.021 mg/liter at time of pharmacokinetic study (n = 8) | |
| Baseline parametersa | ||
| Median (IQR) age (yr) | 42 (39–54) | 49 (44–52) |
| No. (%) of females | 4 (44) | 2 (25) |
| Median (IQR) body wt (kg) | 60.4 (55.9–67.8) | 55.8 (49.9–60.0) |
| Median (SD) CD4 count (cells/mm3) | 387 (213–525) | 434 (286–533) |
| No. of subjects receiving coadministered antiretrovirals | 4 subjects on 5 drugs, 5 subjects on 4 drugs | 4 subjects on 5 drugs, 4 subjects on 4 drugs |
| No. (%) of patients receiving coadministered antiretroviral(s)b | ||
| Lamivudine | 9 (100) | 8 (100) |
| Tenofovir | 4 (44) | 4 (50) |
| Darunavir-ritonavir | 4 (44) | 5 (63) |
| Other protease inhibitors | 5 (56) | 3 (37) |
| Parameters at time of pharmacokinetic study of raltegravir at 400 mg once dailya | ||
| Geometric mean (95% CI) AUC0-24 | 13.6 (9.7–19.0) | 19.5 (11.4–33.3) |
| Geometric mean (95% CI) Cmax | 6.1 (3.7–9.8) | 5.1 (2.5–10.4) |
| Geometric mean (95% CI) Ctrough | 0.014 (0.011–0.018) | 0.066 (0.024–0.184) |
| Parameters at time of last follow-up while on raltegravir at 400 mg once daily | ||
| Median (SD) duration on raltegravir at 400 mg once daily (wk) | 35 (34–39) | 37 (31–39) |
| No. of patients with confirmed HIV RNA load of >50 copies/ml/total no. of patients (%) | 2/9 (22) | 0/0 |
| Geometric mean (95% CI) Ctrough at last follow-up (n = 14) | 0.013 (0.005–0.035) | 0.046 (0.021–0.993) |
| No. of subjects with Ctrough of <0.021 mg/liter/total no. of subjects | 4/8 | 1/6 |
All subjects had HIV RNA loads of <50 copies/ml at baseline and at the time of pharmacokinetic study.
Other antiretrovirals were administered at standard doses.
DISCUSSION
Our study demonstrated that Thais had double the pharmacokinetic values of Westerners when the same 400-mg BID standard raltegravir dosing regimen was used. Importantly, low-dose raltegravir at 400 mg QD achieved similar AUC and Cmax values to those observed in Westerners using the standard raltegravir dosing of 400 mg BID. For each of the 3 dosing regimens (raltegravir at 400 mg BID, 400 mg QD, and 800 mg QD), all subjects achieved the protocol-defined target AUC of ≥4.0 h-mg/liter and were able to maintain viral suppression below 50 copies/ml over the duration of the 6-week pharmacokinetic study. However, with a longer follow-up time of about 9 months on raltegravir at 400 mg QD, 2 of 17 patients had confirmed low-level viremia of 50 to 200 copies/ml, which corresponded with having a Ctrough value below 0.021 mg/liter—a threshold extrapolated from the QDMRK study as showing an association with virologic failure risk (8, 23).
Higher pharmacokinetic values in Thais than in Westerners have been shown for nucleoside reverse transcriptase inhibitors (NRTIs), NNRTIs, and PIs and are reported for the first time for raltegravir in this study. This is possibly due to differences in body weight, diet, and genetics between populations (2, 3, 6, 13, 17). Our findings show that Thais need less raltegravir to achieve similar pharmacokinetic values to those of Westerners, suggesting that low-dose raltegravir as maintenance therapy for Thais could be possible. Whether this is the case for other Asians or persons of low body weight is unknown.
The design of our study allowed for an unbiased and systematic pharmacokinetic comparison of three raltegravir dosing regimens in each individual. With the same total daily dosing, dosing at 800 mg QD had a similar adjusted AUC0-24 but double the Cmax of dosing at 400 mg BID, which suggests a linear absorption. When the dose was halved to 400 mg QD, the adjusted AUC0-24 was lower than that observed with the standard 400-mg BID dosing but was still above the protocol-defined AUC threshold. The main differences between the two QD dosing groups and the standard BID dosing group were the longer t1/2 and the lower Ctrough with once-daily raltegravir. The longer t1/2 could be due to better estimation of the terminal elimination phase and/or the inclusion of a circadian rhythm of slower drug metabolism during the night, which was not studied for the BID regimen, whereas the lower Ctrough could be from the longer terminal elimination phase. The result of t1/2 estimation for BID and QD dosing is a composite of the initial and 2nd elimination phases, with a longer t1/2 after QD dosing because more of the 2nd phase contributes to the t1/2 estimation. Taking more samples in the 12- to 24-hour part of the curve would be ideal but is unpractical because it would require subjects to stay overnight. Consistent with published literature, age, gender, liver and renal function, and tenofovir and lopinavir-ritonavir comedication did not affect pharmacokinetic values (24), but atazanavir significantly increased the raltegravir AUC (4, 7), likely owing to its inhibitory effect on UDP-glucuronosyl-transferase-1A1, which metabolizes raltegravir (16). Darunavir-ritonavir has been shown to decrease the raltegravir AUC by 29% (7). Such a trend was seen in our study, too, but the only significant finding was the longer raltegravir t1/2 with concomitant darunavir-ritonavir therapy.
Recently, data from the QDMRK study indicated that treatment-naïve patients on raltegravir at 800 mg QD plus two NRTIs, with baseline HIV RNA loads above 100,000 copies/ml, had significantly lower antiviral responses than patients on raltegravir at 400 mg BID plus two NRTIs (8). A pharmacokinetic/pharmacodynamic analysis showed that the majority of failures could be explained by high baseline HIV RNA loads and low raltegravir Ctrough values (23). Although no formal cutoff for raltegravir Ctrough was given by the investigators, patients with Ctrough values in the lowest quartile (0.003 to 0.021 mg/liter) had significantly lower antiviral responses than patients with values in the other quartiles. In our study patients, this was the case in 9/17, 1/19, and 0/19 patients while on raltegravir at 400 mg QD, 800 mg QD, and 400 mg BID, respectively. Notably, our study differs in two major aspects from the QDMRK study, namely, (i) maintenance versus initial therapy and (ii) QD dosing of raltegravir with a PI and 1 or 2 NRTIs versus QD dosing of raltegravir with two NRTIs. Our data suggest that low-level viremia with low-dose raltegravir (400 mg QD) was likely due to the low Ctrough rather than the low total daily dosing. At the last follow-up visit, about 9 months after beginning raltegravir at 400 mg QD, one-fifth of patients in the low-Ctrough (<0.021 mg/liter) group had confirmed virologic failure, while none of the patients with Ctrough values of ≥0.021 mg/liter had failure. The QDMRK study showed an association between a low raltegravir Ctrough and virologic failure only for the QD, not BID, raltegravir regimens (8). We would have chosen to study raltegravir at 200 mg BID or 300 mg BID if these formulations were available, but this was not the case. This forced us to study a 50% dose reduction in both daily dose and dose frequency. Although low-level viremia of <200 copies/ml may not be clinically significant (18), we resumed raltegravir at 400 mg BID immediately in such patients, as their future drug options are limited.
Raltegravir is not available as part of the Thai government universal health care program, and the monthly cost of raltegravir is around $530, 3 times the average Thai household monthly income. Our patients faced the prospect of interrupting raltegravir therapy due to a lack of funds. With dose selection studies consistently showing good virologic efficacy with doses as low as 100 mg BID in treatment-naïve patients (10, 14, 15), coupled with the adequate AUC0-24 obtained using raltegravir at 400 mg QD, we felt that low-dose raltegravir at 400 mg QD could be an option for our patients and those in similar settings. A copayment system of the HIV-NAT drug fund was used to ensure an uninterrupted supply of raltegravir for these patients (1). Even with monthly HIV RNA monitoring at $70 per test and transportation reimbursement at $20 per visit from the HIV-NAT drug fund, significant savings are gained with low-dose raltegravir maintenance therapy, allowing for more patients to be treated.
In summary, Thai patients had higher raltegravir pharmacokinetic values than Westerners, and low-dose raltegravir could be a cost-saving maintenance therapy option. However, once daily, low-dose raltegravir at 400 mg QD is associated with a low raltegravir Ctrough and subsequent risk for low-level viremia. Therefore, such a regimen should not be used in settings where raltegravir therapeutic drug monitoring and frequent HIV RNA monitoring are not possible, nor should it be attempted in patients with detectable HIV viremia. However, if low-dose raltegravir for maintenance therapy is needed to help more patients access raltegravir, Ctrough monitoring could assist in dose selection and determining the frequency of HIV RNA monitoring. In addition, further work should be done to evaluate the relationship of Ctrough and HIV RNA load in a greater number of Thai patients on raltegravir therapy at 400 mg QD. Since Ctrough is generally higher with BID than QD regimens, a reduced raltegravir dosing regimen of 200 mg or 300 mg BID would be preferred, and such formulations should be manufactured and studied. The ability to safely reduce the raltegravir dose by half could allow more people in low- and middle-income countries to receive this drug when their initial regimens fail.
ACKNOWLEDGMENTS
We are in debt to the patients who participated in this study. We thank Alexandra Calmy for her input in the development of the protocol and Piraporn June Ohata for her help in preparing the manuscript. We are grateful to Larissa Wenning and Marian Iwamoto from Merck for providing pharmacokinetic data from the P004 study.
This work was funded by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, Thailand (grant RA 49/53), and The Office of the National Research Council of Thailand (grant from fiscal year 2010). The Thai National Health Security Office provided support for antiretrovirals, except for raltegravir.
The HIV-NAT 127 Study Team is comprised of the following: members at HIV-NAT, Reshmie Ramautarsing, Chatsuda Auchieng, Tulathip Suwanlerk, Chavalun Ruengpanyathip, Somporn Chatbuddhiwet, Tawan Mengthaisong, Supalak Klungklang, Siriporn Saeloo, Supaporn Pengsuma, Wanida Thiansanguankul, and Bussara Krasaeboot; and members at the Radboud University Nijmegen Medical Centre, Angela Colbers, Corrien Verwey-van Wissen, and Noor van Ewijk-Beneken Kolmer.
D.M.B. performed data collection. M.G., J.A., S.J.K., and D.M.B. drafted the manuscript, and all authors gave input.
J. Ananworanich has received honoraria or speaker's fees from Merck, the manufacturer of raltegravir, as well as from Abbott and Gilead. D. M. Burger has received honoraria for serving on advisory boards, speaker's fees, and educational grants for clinical research from Merck. All other authors declare no conflict of interest.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Ananworanich J, et al. 2004. Creation of a drug fund for post-clinical trial access to antiretrovirals. Lancet 364:101–102 [DOI] [PubMed] [Google Scholar]
- 2. Autar RS, et al. 2005. Interindividual variability of once-daily ritonavir boosted saquinavir pharmacokinetics in Thai and UK patients. J. Antimicrob. Chemother. 56:908–913 [DOI] [PubMed] [Google Scholar]
- 3. Avihingsanon A, et al. 2009. A low dose of ritonavir-boosted atazanavir provides adequate pharmacokinetic parameters in HIV-1-infected Thai adults. Clin. Pharmacol. Ther. 85:402–408 [DOI] [PubMed] [Google Scholar]
- 4. Cattaneo D, et al. 2010. Exposure-related effects of atazanavir on the pharmacokinetics of raltegravir in HIV-1-infected patients. Ther. Drug Monit. 32:782–786 [DOI] [PubMed] [Google Scholar]
- 5. Cooper DA, et al. 2008. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N. Engl. J. Med. 359:355–365 [DOI] [PubMed] [Google Scholar]
- 6. Cressey TR, et al. 2006. Intensive pharmacokinetics of zidovudine 200 mg twice daily in HIV-1-infected patients weighing less than 60 kg on highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 42:387–389 [DOI] [PubMed] [Google Scholar]
- 7. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents 10 January 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 13 January 2011
- 8. Eron JJ, Jr, et al. 2011. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect. Dis. 11:907–915 [DOI] [PubMed] [Google Scholar]
- 9. Freedberg KA, et al. 2001. The cost effectiveness of combination antiretroviral therapy for HIV disease. N. Engl. J. Med. 344:824–831 [DOI] [PubMed] [Google Scholar]
- 10. Grinsztejn B, et al. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261–1269 [DOI] [PubMed] [Google Scholar]
- 11. Hill A, Ananworanich J, Calmy A. 2010. Dose optimisation: a strategy to improve tolerability and lower antiretroviral drug prices in low and middle income countries. Open Infect. Dis. J. 4:85–91 [Google Scholar]
- 12. Lennox JL, et al. 2009. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 374:796–806 [DOI] [PubMed] [Google Scholar]
- 13. Manosuthi W, et al. 2005. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS 19:1481–1486 [DOI] [PubMed] [Google Scholar]
- 14. Markowitz M, et al. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509–515 [DOI] [PubMed] [Google Scholar]
- 15. Markowitz M, et al. 2007. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46:125–133 [DOI] [PubMed] [Google Scholar]
- 16. Neely M, et al. 2010. Pharmacokinetics and pharmacogenomics of once-daily raltegravir and atazanavir in healthy volunteers. Antimicrob. Agents Chemother. 54:4619–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramautarsing R, Ananworanich J. 2010. Generic and low dose antiretroviral therapy in adults and children: implication for scaling up treatment in resource limited settings. AIDS Res. Ther. 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ribaudo H, et al. 2009. Virologic failure endpoint definition in clinical trials: is using HIV-1 RNA threshold <200 copies/mL better than <50 copies/mL? An analysis of ACTG studies, abstr 580. Sixteenth Conf. Retrovir. Opportun. Infect., 8 to 11 February 2009, Montreal, Canada [Google Scholar]
- 19. Steigbigel RT, et al. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339–354 [DOI] [PubMed] [Google Scholar]
- 20. Steigbigel RT, et al. 2010. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin. Infect. Dis. 50:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson MA, et al. 2010. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 304:321–333 [DOI] [PubMed] [Google Scholar]
- 22. van Luin M, et al. 2009. The effect of raltegravir on the glucuronidation of lamotrigine. J. Clin. Pharmacol. 49:1220–1227 [DOI] [PubMed] [Google Scholar]
- 23. Wenning L, et al. 2011. Pharmacokinetic/PD analyses for QDMRK, a phase III study of the safety and efficacy of once versus twice daily raltegravir in treatment-naïve patients, abstr O_09. Twelfth Int. Workshop Clin. Pharmacol. HIV Ther., Miami, FL, 13 to 15 April 2011 [Google Scholar]
- 24. Wenning LA, et al. 2008. Lack of a significant drug interaction between raltegravir and tenofovir. Antimicrob. Agents Chemother. 52:3253–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]