Abstract
A patient with septic shock due to extensively drug resistant (XDR) Pseudomonas aeruginosa was cured by optimizing the meropenem (MEM) regimen to obtain at least 40% of the time between two administrations in which drug levels were four times higher than the MIC of the pathogen. As the standard drug dose did not achieve these optimal concentrations, the MEM regimen was progressively increased up to 12 g/day (3 g every 6 h in a 3-h extended infusion), which eventually resulted in sepsis resolution. High MEM dosage may represent a valuable therapeutic option for infection due to multidrug-resistant (MDR) strains, and drug monitoring would allow rapid regimen adjustment in clinical practice.
TEXT
Pseudomonas aeruginosa infections are associated with increased morbidity and mortality in critically ill patients. Moreover, the increasing frequency of extensively drug resistant (XDR) strains of this pathogen is a considerable therapeutic challenge for clinicians (5, 11). Broad-spectrum β-lactams are the first therapeutic option for the treatment of P. aeruginosa infections, but in the case of XDR strains, colistin or aminoglycosides remain the last therapeutic option; however, their effectiveness has been poorly demonstrated in this setting (6). The epidemic spread of XDR bacteria and the lack of development of new drugs active against these pathogens have forced clinicians to optimize the antimicrobial activity of the available antibiotics (14). We report herein a case of septic shock due to P. aeruginosa that was successfully treated by adapting the meropenem (MEM) regimen to the serum drug concentrations and the in vitro pathogen susceptibility.
Case report.
A 70-year-old obese man (body weight, 120 kg; body mass index, 35) was transferred to the intensive care unit (ICU) from another hospital for ventilator-associated pneumonia, developed a few days after an elective tracheostomy. The patient had prolonged mechanical ventilation (MV) after pulmonary edema complicating an episode of acute heart failure 1 month before, and his tracheal aspirates were colonized by P. aeruginosa.
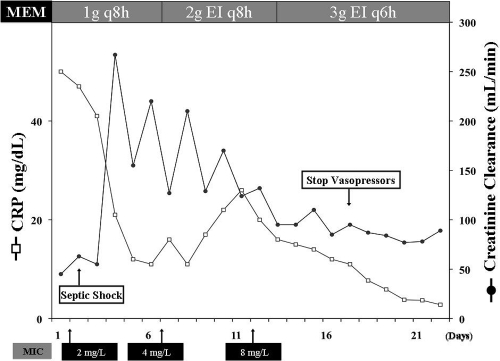
At ICU admission, controlled-volume MV was initiated and norepinephrine titrated to achieve a mean arterial pressure of at least 70 mm Hg; initial serum creatinine levels were 2.7 mg/dl and, because of concomitant oliguria, fluid resuscitation consisted of 5,200 ml during the first day of therapy. Blood cultures, as well as cultures from endotracheal aspirate, showed P. aeruginosa which was resistant to several antibiotics (including aztreonam, ceftazidime, cefepime, and piperacillin-tazobactam), except MEM (MIC = 2 mg/liter), amikacin, ciprofloxacin, and colistin. The patient received MEM (1 g every 8 h [q8h]) and ciprofloxacin (400 mg q8h). The MIC of P. aeruginosa for MEM was determined by the Etest method (bioMérieux, Marcy l'Etoile, France). Creatinine levels rapidly decreased below 1 mg/dl, and creatinine clearance measured on 24-h urinary excretion went up to more than 200 ml/min, while no improvement of respiratory and hemodynamic status was remarked after the first 5 days of treatment. On days 2 and 5 of therapy, two samples were taken for serum MEM level monitoring during the elimination phase after a 30-min IV administration. The method and validation for the MEM assay have been previously published (16). The adequacy of MEM therapy was assessed by calculating the time that drug levels remained above 4 times the MIC of the isolate (T > 4× MIC) for an extensive period between two doses; T > 4× MIC was considered to be optimal if it was >40% of the dose interval (13). In both measurements, drug concentrations were below this threshold (Table 1). Because of persistent septic shock, a bronchoalveolar lavage was performed at day 6 and again yielded P. aeruginosa, which in vitro was intermediate to MEM (MIC = 4 mg/liter), resistant to ciprofloxacin, and only susceptible to colistin (MIC = 2 mg/liter) and amikacin (MIC = 8 mg/liter). The dose of MEM was then increased to 2 g q8h in a 3-h extended infusion (EI) and combined with colistin (6 × 106 IU q12h). The C-reactive protein levels initially decreased, but the patient still needed MV and norepinephrine infusion. With this treatment, MEM serum concentrations remained below the threshold of efficacy. A few days later, P. aeruginosa was recovered in another endotracheal aspirate and was now resistant to MEM (MIC = 8 mg/liter). The meropenem regimen was then increased to 3 g q6h as a 3-h extended infusion (total daily dose of 12 g), while colistin was discontinued. The T > 4× MIC then increased to nearly 50%. The patient's clinical status improved thereafter with resolution of signs of sepsis and reduction of inflammatory parameters (Fig. 1). No adverse events were observed; an electroencephalogram performed at day 16 showed no abnormalities. The patient was discharged from the ICU 10 days after the end of therapy (33 days) to the floor.
Table 1.
Meropenem regimens, concentrations, and pharmacodynamics during therapya
| Day of therapy | MEM dose | Time of sampling | MEM concn (mg/liter) | MIC (mg/liter) | % T > 4× MIC |
|---|---|---|---|---|---|
| 1 | 1 g q8h | ||||
| 2 | 1 g q8h | 2 h | 12.3 | 2 | 37 |
| 8 h | <2.0 | ||||
| 5 | 1 g q8h | 2 h | 13.4 | 2 | 39 |
| 8 h | <2.0 | ||||
| 9 | 2 g EI q8h | 3 h | 17 | 4 | 39 |
| 8 h | 3 | ||||
| 15 | 3 g EI q6h | 3 h | 43 | 8 | 51 |
| 6 h | 19 |
MEM, meropenem; q8h and q6h, every 8 h and 6 h; EI, extended infusion (over 3-h period); 2 h, 3 h, 6 h, and 8 h, 2, 3, 6, and 8 h after the onset of MEM administration; T > 4× MIC, time above 4 times the MIC.
Fig 1.
Evolution of C-reactive protein (CRP) and creatinine clearance measured on urinary excretion during the ICU stay. MEM, meropenem.
Discussion.
We described the case of successful treatment of XDR P. aeruginosa septic shock with an antimicrobial strategy using a higher-than-recommended regimen of MEM. The daily dose of MEM was adapted using repeated drug concentration monitoring and considering the increasing MIC of the pathogen to optimize the antimicrobial activity of the drug. This case illustrates the difficult task in antibiotic prescription for critically ill patients, as several factors that may alter drug concentrations were concomitantly present. First, sepsis alters the pharmacokinetic (PK) parameters of antibiotics, such as volume of distribution and elimination and degradation processes, so that standard regimens derived from patients with less severe infections or healthy volunteers may not be applicable in this setting (14). Second, the increased cardiac output and the large amount of fluid needed during the infectious episode can result in an increased renal blood flow and glomerular hyperfiltration, leading to an increased antibiotic clearance and potentially subtherapeutic drug concentrations (18). Third, obesity may have a significant impact on antimicrobials' PKs and further alter drug concentrations when standard regimens are administered (7). Moreover, achieving therapeutic drug concentrations is particularly difficult when infections are caused by some pathogens, such as P. aeruginosa and Gram-negative rods, with naturally reduced susceptibility to antimicrobials, and the presence of a XDR strain resulted in another reason for inadequate MEM concentrations during initial therapy (6).
Experimental studies have demonstrated that β-lactams have a slow continuous kill characteristic that is almost entirely related to the time during which serum concentrations exceed the MIC for the infecting organism (1); in these models, maximal bacterial killing was obtained with drug concentrations of 4 to 5 times the MIC (19). For human infections, the optimal β-lactam strategy (T>MIC or T > 4× to 5× MIC) has not yet been identified. Although it has been shown that, in patients treated with cephalosporins, T>MIC of 100% had significantly greater clinical cure and bacteriological eradication than T>MIC of less than 100% (12), carbapenems have different PK properties and, because of a postantibiotic effect, do not need such a prolonged time of concentrations exceeding the MIC to be effective (13). Interestingly, the MEM regimens resulted in a T>MIC of almost 100% for the different regimens in our patient but clinical success was obtained only when drug concentrations exceeded 4 times the MIC for at least 40% of the dosing interval.
Human studies on serum concentrations of broad-spectrum β-lactams, such as cephalosporins or piperacillin, have already reported that drug levels in patients with severe infections are insufficient to treat less-susceptible strains, while serum MEM concentrations were found to be adequate in most of the critically ill patients with sepsis (17). Nevertheless, all of these studies considered only strains susceptible to the drug (MIC < 2 mg/liter), while for less-susceptible pathogens, a higher-than-recommended regimen using extended infusion would be necessary to optimize the efficacy of the drug (8). Importantly, as shown in the present case, the low serum concentrations obtained with recommended doses may have induced the emergence of resistant strains, and a favorable outcome can be obtained only when serum concentrations reach levels corresponding to PK properties of β-lactams (4). We did not measure colistin levels and cannot exclude any synergistic effect of meropenem and colistin in the treatment of this XDR P. aeruginosa strain; however, the patient did not improve with this combination therapy and only the increase of the meropenem regimen to 12 g/day allowed the resolution of the septic process.
According to population modeling simulation, continuous (CI) or extended β-lactam infusions are required to optimize pathogen exposure to bactericidal concentrations of β-lactams (15). There are still some limitations to this strategy. First, clinical data that have shown a better outcome using this strategy have come only from retrospective studies in critically ill populations with pneumonia (9, 10). Second, when high doses are used to cure less-susceptible strains, overdosing and toxicity of β-lactams could also be a concern, so that drug monitoring is mandatory in this setting to correctly adjust the dose (3). However, higher-than-recommended carbapenem regimens have already been used in other diseases, such as cystic fibrosis, to treat XDR pathogens, resulting in clinical success and being well tolerated (2). Large prospective cohorts are needed to assess the influence on morbidity and mortality of CI/EI administration, especially in patients with sepsis and infections caused by XDR pathogens.
ACKNOWLEDGMENT
The authors do not have any conflicts to declare related to the manuscript.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bulik CC, Quintiliani R, Jr, Pope JS, Kuti JL, Nicolau DP. 2010. Pharmacodynamics and tolerability of high-dose, prolonged infusion carbapenems in adults with cystic fibrosis—a review of 3 cases. Respir. Med. CME. 3:146–149 [Google Scholar]
- 3. Chapuis TM, et al. 2010. Prospective monitoring of cefepime in intensive care unit adult patients. Crit. Care 14:R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi SH, et al. 2008. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrob. Agents Chemother. 52:995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falagas ME, Bliziotis JMIA. 2007. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 29:630–636 [DOI] [PubMed] [Google Scholar]
- 6. Giamarellou H. 2010. Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int. J. Antimicrob. Agents 36(Suppl 2):S50–S54 [DOI] [PubMed] [Google Scholar]
- 7. Hanley MJ, Abernethy DR, Greenblatt DJ. 2010. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 49:71–87 [DOI] [PubMed] [Google Scholar]
- 8. Jaruratanasirikul S, Sriwiriyajan S, Punyo J. 2005. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob. Agents Chemother. 49:1337–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lodise TP, Jr, Lomaestro B, Drusano GL. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 44:357–363 [DOI] [PubMed] [Google Scholar]
- 10. Lorente L, et al. 2007. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart review. Clin. Ther. 29:2433–2439 [DOI] [PubMed] [Google Scholar]
- 11. Magiorakos AP, et al. 7 May 2011. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. [Epub ahead of print.] doi:10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 12. McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 31:345–351 [DOI] [PubMed] [Google Scholar]
- 13. Ong CR, Tessier PR, Li C, Nightingale CH, Nicolau DP. 2007. Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diagn. Microbiol. Infect. Dis. 57:153–161 [DOI] [PubMed] [Google Scholar]
- 14. Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840–851 [DOI] [PubMed] [Google Scholar]
- 15. Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. 2009. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit. Care Med. 37:2071–2078 [DOI] [PubMed] [Google Scholar]
- 16. Seyler L, et al. 2011. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care. 15:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taccone FS, et al. 2010. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care. 14:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Udy AA, Roberts JA, Lipman J. 2011. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 7:539–543 [DOI] [PubMed] [Google Scholar]
- 19. Vogelman B, et al. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy. J. Infect. Dis. 158:831–847 [DOI] [PubMed] [Google Scholar]