Abstract
T-2307, a novel arylamidine, has been shown to exhibit broad-spectrum antifungal activities against clinically significant pathogens. Here, we evaluated the in vitro and in vivo antimalarial activity of T-2307. The 50% inhibitory concentrations (IC50s) of T-2307 against Plasmodium falciparum FCR-3 and K-1 strains were 0.47 and 0.17 μM, respectively. T-2307 at 2.5 to 10 mg/kg of body weight/day exhibited activity against blood stage and liver stage parasites in rodent malaria models. In conclusion, T-2307 exhibited in vitro and in vivo antimalarial activity.
TEXT
Malaria, caused by protozoan parasites of the genus Plasmodium, is one of the world's leading killer infectious diseases. There are an estimated 200 to 300 million new cases of the disease each year worldwide, and 0.8 to 1 million deaths (5, 15). Many of these deaths occur in children and are the result of severe and cerebral malaria caused by Plasmodium falciparum, the most pathogenic of the four species that infect humans (5).
In the absence of a vaccine for malaria and in view of widespread resistance of the parasites to antimalarial drugs in current use, new agents are urgently needed to combat malaria (4).
We had previously reported that T-2307, a novel arylamidine, exhibits broad-spectrum in vitro and in vivo antifungal activity against clinically significant pathogens (7, 8, 16). The analogous arylamidine derivatives, such as pentamidine and DB75, exhibit antiprotozoan activities against Plasmodium, Trypanosoma, and Leishmania (2, 3, 14).
Accordingly, T-2307, similar to pentamidine and DB75, is expected to exhibit antiprotozoan activity. In the present study, we investigated the in vitro and in vivo antimalarial activity of T-2307.
The in vitro antimalarial activity of T-2307 against P. falciparum was examined. Parasite cultures were maintained in human erythrocytes suspended at 5% hematocrit in RPMI 1640 containing 0.5% AlbuMAX I solution and 5.95 g of HEPES, 2 g of NaHCO3, 0.5 g of l-glutamine, and 50 mg of hypoxanthine per liter. After the parasites had been synchronized to the ring stage by sorbitol lysis (6), T-2307 and reference agents were added to the synchronized parasite culture (ring stage, >90%, and parasitemia, 0.5%) in a 96-well plate. The plate was incubated for one intraerythrocytic life cycle (FCR-3, 40 h, and K-1, 48 h) at 37°C under a gas mixture of 5% O2 and 5% CO2. In order to assess parasite growth, lysis buffer containing 0.02% SYBR green I was added to the parasite culture, and after incubation for 1 h, fluorescence was measured at excitation and emission wavelengths of 485 and 535 nm, respectively (12). The 50% inhibitory con-centrations (IC50s) of T-2307 and the reference agents against the chloroquine-sensitive P. falciparum FCR-3 strain and the chloro-quine-resistant P. falciparum K-1 strain are shown in Table 1. The IC50s of T-2307 against FCR-3 and K-1 strains were 0.47 and 0.17 μM, respectively, indicating that T-2307 exhibited no cross-resistance against chloroquine.
Table 1.
IC50s of T-2307 and reference agents against P. falciparum FCR-3 and K-1 in vitroa
| Compound | IC50 (μM)b |
|
|---|---|---|
| FCR-3 | K-1 | |
| T-2307 | 0.47 ± 0.01 | 0.17 ± 0.00 |
| Pentamidine | 0.18 ± 0.00 | 0.083 ± 0.003 |
| Chloroquine | 0.051 ± 0.002 | 1.4 ± 0.0 |
T-2307, chloroquine, and pentamidine were added to the 0.5% parasitemia culture (>90% ring stage). Then, the culture was incubated for one intraerythrocytic life cycle (FCR-3, 40 h; K-1, 48 h) at 37°C under a gas mixture of 5% O2 and 5% CO2. Parasite growth was assessed by SYBR green I-based fluorescence assay, and IC50s were calculated using WinNonlin software.
The results are expressed as means ± standard errors of the means of duplicate experiments.
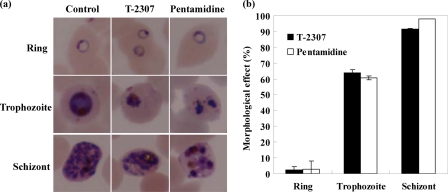
The stage-specific activities of T-2307 and pentamidine against P. falciparum K-1 strain were assessed by evaluating a morphological effect. The synchronized ring, trophozoite, and schizont stages were exposed to approximately the 5 times the IC50 of T-2307 and pentamidine against the K-1 strain (800 nM and 400 nM, respectively) for 12 h.
The morphology of the parasites treated with these agents was compared with that of untreated parasites by using microscopy. T-2307 and pentamidine caused altered morphologies, such as condensation in trophozoite stage parasites and abnormal cell division in schizont stage parasites (Fig. 1a). On the other hand, these compounds had no effect on the morphology in ring stage parasites (Fig. 1a).
Fig 1.
Stage-specific activity of T-2307 and pentamidine against P. falciparum K-1 in vitro. Giemsa-stained thin blood smears were examined with microscopy to assess the morphology of 50 parasites at the ring, trophozoite, and schizont stages that had been exposed to 800 nM T-2307 and 400 nM pentamidine for 12 h. (a) Giemsa-stained microscope image of the parasites treated with T-2307 and pentamidine at each stage (×1,000 magnification). (b) Morphological effect of T-2307 and pentamidine at each stage. Morphological effect (%) = [(D − C)/(100 − C)] × 100, where D is the rate of morphologically abnormal parasites (%) in agent-treated culture and C is the rate of morphologically abnormal parasites (%) in control culture. The results are expressed as the means ± standard errors of the means of duplicate experiments.
Next, morphological effect (%) was calculated by assessing the morphology of 50 parasites at each stage. The definition of the morphological effect is given in detail in the Fig. 1b legend. These results indicated that both T-2307 and pentamidine exhibited potent activity against mature stages, especially the schizont stage (Fig. 1b).
The in vivo antimalarial activities of T-2307 and pentamidine were evaluated in mice infected with Plasmodium vinckei PV strain. It has been reported that P. vinckei infection was a good murine model of falciparum malaria for testing the in vivo activity of diamidine derivatives (1). BALB/c mice (male, 5 to 6 weeks of age) were intravenously injected with 0.2 ml of the parasitized erythrocytes (1 × 104 cells/mouse), and thereafter, T-2307 or a reference agent was subcutaneously administered once a day for 8 days beginning 2 h postinfection. Animal experiments in this study were carried out in compliance with the Guide for Animal Experimentation at Obihiro University of Agriculture and Veterinary Medicine.
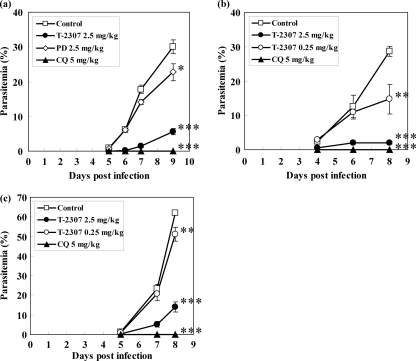
As shown in Fig. 2a, the parasitemia in the mice administered T-2307 at 2.5 mg/kg of body weight/day decreased significantly compared to that in the control group. At the same dose, T-2307 exhibited antimalarial activity superior to that of pentamidine, while chloroquine administration at 5 mg/kg/day resulted in a decrease in parasitemia to an undetectable level.
Fig 2.
In vivo antimalarial activity of T-2307 against blood stage parasites of P. vinckei PV (a), P. berghei ANKA (b), and P. chabaudi AJ (c). Mice were intravenously injected with parasitized erythrocytes (1 × 104 cells/mouse). T-2307, chloroquine, and pentamidine were subcutaneously administered once a day for 8 days beginning 2 h postinfection. Detection of parasitemia was performed by preparing Giemsa-stained blood smears. Statistical analysis was performed by parametric Dunnett testing against the results for the control group (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The results are expressed as the means ± standard errors of the means of 4 or 5 experiments. CQ, chloroquine; PD, pentamidine.
Similar results were obtained when the in vivo activity of T-2307 was evaluated in the mice infected with Plasmodium berghei ANKA strain or Plasmodium chabaudi AJ strain. In both models, parasitemia in the mice administered T-2307 at 0.25 and 2.5 mg/kg/day decreased significantly compared to that in the control group, with a greater decrease at 2.5 mg/kg/day (Fig. 2b and c), while chloroquine at 5 mg/kg/day decreased parasitemia to a nearly undetectable level.
It has been observed that pentamidine is almost inactive against infection with P. berghei but is effective against P. vinckei infection (1). In contrast, T-2307 showed antimalarial activity in vivo against not only P. vinckei but also P. berghei and P. chabaudi. This suggests that there is a difference in the spectrum of activity between T-2307 and pentamidine in these rodent malaria models.
The in vivo antimalarial activity of T-2307 against the liver stage was assessed by evaluating a prepatent period for the blood stage parasites in the mice after the sporozoite inoculation (11). Sporozoites were isolated from the salivary glands of P. berghei-infected Anopheles stephensi mosquitoes. BALB/c mice (male, 6 weeks of age) had been intravenously injected with 0.2 ml of the sporozoite suspension (1 × 103 cells/mouse). T-2307 at 2.5 mg/kg was subcutaneously administered 4 times a day for 4 days, and primaquine at 50 mg/kg was intraperitoneally administered once a day for 4 days, both beginning 2 days before infection. Parasitemia was observed between 5 and 7 days after infection. The prepatent period was defined as the number of days between sporozoite infection and detection of 0.1% parasitemia of the blood stage parasites. The prepatent period of mice administered T-2307 was found to be approximately 1 day longer than that of the control group, indicating that T-2307 decreased the liver stage parasite burden by approximately 10-fold compared with that of the control group (17), while no parasites were detected in mice treated with 50 mg/kg of primaquine (Table 2).
Table 2.
In vivo antimalarial activity of T-2307 against liver stage parasites of P. berghei ANKAa
| Compound | No. infected/no. injectedb | Prepatent period (days)c |
|---|---|---|
| Control | 5/5 | 6.0 ± 0.3 |
| T-2307 | 4/4 | 7.0 ± 0.0d |
| Primaquine | 0/4 | >7e |
Mice were intravenously injected with a sporozoite suspension (1 × 103 cells/mouse). T-2307 at 2.5 mg/kg was subcutaneously administered 4 times a day for 4 days and primaquine at 50 mg/kg was intraperitoneally administered once a day for 4 days beginning 2 days before infection. Parasitemia was determined between 5 and 7 days after infection.
No. infected, number of mice that developed >0.1% parasitemia at the blood stage until 7 days after infection; no. injected, number of mice injected with sporozoites.
Prepatent period, number of days between sporozoite infection and detection of 0.1% parasitemia at the blood stage. The results are expressed as the means ± standard errors of the means of 4 of 5 experiments. The prepatent period of the T-2307-treated group and that of the control group were compared by paired t test using SAS analytical software, release 8.2 (SAS Institute Japan Ltd., Tokyo, Japan).
P < 0.05.
The prepatent period could not be calculated because parasite was not detected. Therefore, statistical analysis could not be performed.
It has been shown that pentamidine targets the hemoglobin degradation pathway and that DB75 targets the nucleus (9, 13). Although several hypotheses have been proposed, the precise mechanism of diamidine compounds against the malaria parasites remains unclear. We had previously reported that T-2307 disrupts mitochondrial function in yeast (10). However, the mechanism of action of T-2307 against the malaria parasites also remains unclear at present. On the basis of the difference in the spectrum of activity of T-2307 and pentamidine against the rodent malaria models, the mechanism of action of T-2307 against malaria parasites may be different from that of pentamidine. Further studies will be required to elucidate the mechanism of action of T-2307.
In the present study, we showed that T-2307 exhibited in vitro and in vivo antimalarial activities against P. falciparum and the rodent malaria parasites, respectively.
Further research is now required to confirm the characteristics of efficacy and safety of T-2307 and its derivative compounds as new antimalaria agents.
ACKNOWLEDGMENTS
We are grateful to S. Kano (National Center for Global Health and Medicine) for providing the P. falciparum strains. We are thankful to the Hokkaido Kushiro Red Cross Blood Center for supplying human red blood cells. We are grateful to K Tanabe (Osaka University) for providing the P. chabaudi AJ strain. We also thank the Malaria Research and Reference Reagent Resource Center (MR4/ATCC) for providing the P. vinckei PV strain.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Angulo-Barturen I, et al. 2008. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One 3:e2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakunova SM, et al. 2009. Structure-activity study of pentamidine analogues as antiprotozoal agents. J. Med. Chem. 52:2016–2035 [DOI] [PubMed] [Google Scholar]
- 3. Bell CA, et al. 1990. Structure-activity relationships of analogs of pentamidine against Plasmodium falciparum and Leishmania mexicana amazonensis. Antimicrob. Agents Chemother. 34:1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gelb HM. 2007. Drug discovery for malaria: a very challenging and timely endeavor. Curr. Opin. Chem. Biol. 11:440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenwood BM, et al. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stage in culture. J. Parasitol. 65:418–420 [PubMed] [Google Scholar]
- 7. Mitsuyama J, et al. 2008. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 52:1318–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishikawa H, et al. 2010. Uptake of T-2307, a novel arylamidine, in Candida albicans. J. Antimicrob. Chemother. 65:1681–1687 [DOI] [PubMed] [Google Scholar]
- 9. Purfield AE, Tidwell RR, Meshnick SR. 2009. The diamidine DB75 targets the nucleus of Plasmodium falciparum. Malar. J. 8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata T, et al. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr F1-943.2007. [Google Scholar]
- 11. Singh AP, et al. 2010. Lipophilic bisphosphonates are potent inhibitors of Plasmodium liver stage growth. Antimicrob. Agents Chemother. 54:2987–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stead AM, et al. 2001. Diamidine compounds: selective uptake and targeting in Plasmodium falciparum. Mol. Pharmacol. 59:1298–1306 [DOI] [PubMed] [Google Scholar]
- 14. Werbovetz K. 2006. Diamidine as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 7:147–157 [PubMed] [Google Scholar]
- 15. World Health Organization 2010. World Malaria Report 2010. World Health Organization, Geneva, Switzerland [Google Scholar]
- 16. Yamada E, Nishikawa H, Nomura N, Mitsuyama J. 2010. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob. Agents Chemother. 54:3630–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yano K, et al. 2008. Disruption of the Plasmodium berghei 2-Cys peroxiredoxin TPx-1 gene hinders the sporozoite development in the vector mosquito. Mol. Biochem. Parasitol. 159:142–145 [DOI] [PubMed] [Google Scholar]