Abstract
The aim of this study was to determine the potential application of N-chlorotaurine (NCT), N,N-dichloro-2,2-dimethyltaurine (NVC-422), and N-monochloro-2,2-dimethyltaurine (NVC-612) as catheter lock solutions for the prevention of catheter blockage and catheter-related bloodstream infections by testing their anticoagulant and broad-spectrum antimicrobial activities in human blood. NCT, NVC-422, NVC-612, and control compounds were serially diluted in fresh human blood to evaluate the effects on prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, and direct thrombin inhibition. Quantitative killing assays against pathogens, including methicillin-resistant Staphylococcus aureus, Escherichia coli, and Candida albicans, were performed in the presence of heparin and human blood. NCT and NVC-612 (1.38 mM each) and 1.02 mM NVC-422 prolonged prothrombin time (Quick value, 17 to 30%), activated partial thromboplastin time 3- to 4-fold to 76 to 125 s, and thrombin time 2- to 4-fold to 34 to 68 s. Fibrinogen decreased from 258 to 283 mg/dl (range of controls) to <40 mg/dl. No direct thrombin inhibition was observed by NVC-422 or NVC-612. Heparin did not influence the bactericidal activity of NCT. The microbicidal activities of NCT, NVC-422, and NVC-612 were maintained in diluted human blood. NCT, NVC-612, and NVC-422 have broad-spectrum antimicrobial activity in blood and anticoagulant activity targeting both intrinsic and extrinsic pathways of the coagulation system. These properties support their application as catheter lock solutions.
INTRODUCTION
Central venous catheters (CVCs) are devices that are commonly used in critically ill patients for the administration of parenteral nutrition, medications, and fluids and to monitor the patient's hemodynamic status. Complications such as infection, blockage, thrombosis, and hemorrhage have been associated with the use of CVCs (14, 28). The International Nosocomial Infection Control Consortium recently estimated that in intensive care units, 7.6 CVC-associated bloodstream infections occur per 1,000 CVC-days (19). The use of anticoagulants such as heparin or citrate reduces the risk of catheter blockage (14). However, due to their lack of intrinsic antimicrobial activity, heparin and citrate are ineffective in preventing catheter-associated bloodstream infections. In the last decade, several studies have found a benefit for antimicrobial lock solutions such as 30% citrate, ethanol, chlorhexidine, and antibiotics for their efficacy (1, 2, 5, 12). However, antibiotics may have the drawback of resistance development (12).
N-Chlorotaurine (ClHN-CH2-CH2-SO3H) is an endogenous mild oxidant belonging to the class of active chlorine compounds (chloramines) with broad-spectrum microbicidal activity against Gram-positive and Gram-negative bacteria, viruses, fungi (yeasts and molds), protozoa, and worm larvae (reviewed in reference 9). It can be synthesized chemically as a sodium salt (ClHN-CH2-CH2-SO3Na, abbreviated NCT), which is very well water soluble and can be stored at 4°C for 1 year and at 20°C for 3 weeks with a loss of activity of approximately 10% (8). In clinical trials, NCT at a concentration of 1% (55 mM) has been shown to be very well tolerated and effective at different body sites, such as the eye, the outer ear, skin ulcerations, the urinary tract, and other body cavities (9, 17, 18, 24). Recently, dimethylated analogs of NCT which are also water soluble but which can be stored at room temperature (20°C) for 1 year without a significant loss of activity have been synthesized. Namely, N,N-dichloro-2,2-dimethyltaurine[NVC-422; Cl2N-CH2-(CH3)2-CH2-SO3Na] and N-monochloro-2,2-dimethyltaurine [NVC-612; ClHN-CH2-(CH3)2-CH2-SO3Na] have similar broad-spectrum microbicidal activity as NCT (26, 27). The clinical benefit of NVC-422 as an antibacterial agent was recently demonstrated in a phase 2 clinical trial for the treatment of impetigo (11).
There is an unmet medical need for safe and effective CVC lock solutions for the prevention of both catheter blockage and infection. To address this need, we evaluated three novel broad-spectrum antimicrobial agents, NCT, NVC-422, and NVC-612, against both intrinsic and extrinsic pathways of the coagulation system. Since catheters may fill with blood while inserted in vivo, the bactericidal activity in the presence of blood was tested. Furthermore, we monitored the influence of heparin on the microbicidal activity as well as on the anticoagulant activity.
MATERIALS AND METHODS
Antimicrobial agents.
The pure crystalline sodium salt of NCT was prepared according to published procedures (8), as were its dimethylated analogs, NVC-612 and NVC-422 (13, 26, 27). Sodium thiosulfate, taurine, and buffers were purchased from Merck (Darmstadt, Germany); 2-amino-2-methylpropanesulfonic acid (dimethyltaurine [DMT]) was obtained from NovaBay Pharmaceuticals Inc. (Emeryville, CA). Heparin was purchased as Heparin Immuno 5,000 IU/ml from Ebewe Pharma GmbH (Unterach, Austria) and 200-fold diluted in 0.9% saline to a stock solution containing 25 IU/ml.
Anticoagulant activities of NCT, NVC-612, and NVC-422.
Initially, in the first step, whole blood was taken from a single volunteer (M.N.) and enriched with trisodium citrate (0.3 ml of a 0.106 M solution plus 2.7 ml whole blood; S-Monovette; Sarstedt, Nümbrecht, Germany). Portions of 1.8 ml citrate blood were mixed with 0.2 ml NCT in 0.9% saline. The following final (after dilution in blood) NCT concentrations were tested separately: 1% (55 mM), 0.5%, 0.25%, 0.1%, 0.05%, 0.025%, and 0.01%. A saline control was performed in parallel. Tests were chosen to test all essential pathways of coagulation. The extrinsic system, naturally initiated by tissue damage and activation of coagulation factors VII and X, was tested by prothrombin time using the Thromborel S test. Prothrombin time is presented as a percentage, calculated by the dilution of standard plasma necessary to achieve the coagulation time of the sample. Therefore, a lower percentage indicates a prolonged coagulation time of the sample. Since prothrombin time tests (stated as percentage of an average clotting time of standard human plasma; reference interval, 70 to 130%; test limits, <10% and >130%) slightly depend on the manufacturer, the international normalized ratio (INR; reference interval, 0.8 to 1.2), which includes a correction factor for every registered test, was also calculated. The test is hardly influenced by heparin. The intrinsic system, naturally initiated by contact activation of factor XII by the endothelium, was tested by activated partial thromboplastin time using the Pathromtin* SL test, stated as clotting time, in seconds (reference interval, 26 to 37 s; test limit, >400 s). The test is strongly influenced by heparin. The final path of coagulation, the polymerization of fibrin from fibrinogen, naturally initiated by thrombin, was measured by thrombin time using the BC thrombin reagent test, stated as clotting time, in seconds (reference, <21 s; test limit, >400 s). To detect an influence of NCT on fibrinogen itself, this parameter was tested using the Multifibren* U test, stating the concentration in mg/dl (reference interval, 180 to 350 mg/dl; test limit, <40 mg/dl). The test measures the coagulation time of citrated plasma with a high excess of thrombin, where the coagulation time largely depends on the fibrinogen content of the specimen. The result is evaluated using a reference curve, and the concentration is calculated in mg/dl. All tests were from Dade Behring GmbH, Marburg, Germany.
Second, a blood sample was taken as described above from 11 healthy volunteers after they provided informed consent, according to the guidelines of the Declaration of Helsinki. Since the therapeutically applied final concentration of 1% (55 mM) NCT largely led to an excess of the test limits in the pilot test (see Results and Table 1) and numeric results were desirable, lower concentrations were used in these tests. Portions of 1.8 ml citrate blood were mixed with 0.2 ml of 0.9% saline containing the following ingredients: blank control (saline), 40 μl of 25 IU/ml heparin, 0.1% NCT, 0.25% NCT, or 0.1% NCT plus 40 μl 25 IU/ml heparin. Tests were performed as described above. In addition, antithrombin III was determined using the Berichrom* antithrombin III test, and D dimer, which consists of cleavage products of polymerized fibrin molecules and is an indicator of fibrinolysis, was determined using the D-Dimer Plus test (Dade Behring GmbH, Marburg, Germany). Antithrombin III was tested as an important inhibitor of coagulation and D dimer to confirm indirectly the absence of fibrin formation by the absence of fibrin decay products.
Table 1.
Influence of NCT at different concentrations on coagulation parameters in whole human blooda
| Concn of NCTb (mM) | PT (%) | aPTT (s) | TT (s) | Fibrinogen concn (mg/dl) |
|---|---|---|---|---|
| 55 (1) | < limit | > limit | > limit | Not detectable |
| 27.5 (0.5) | < limit | > limit | > limit | Not detectable |
| 13.8 (0.25) | < limit | > limit | 101.8 | Not detectable |
| 5.5 (0.1) | < limit | 371.4 | 92.2 | Not detectable |
| 2.75 (0.05) | 33.4 | 71.3 | 48.8 | Not detectable |
| 1.38 (0.025) | 70.6 | 37.7 | 26.6 | 199.4 |
| 0.55 (0.01) | 91.4 | 30.6 | 19.5 | 281.4 |
| 0 NCT, salinec | 89.3 | 29.9 | 19.5 | 289.5 |
| 0 NCTd | 100.1 | 29.1 | 19.6 | 388.3 |
Results are from a single pilot experiment. PT, prothrombin time; aPTT, activated partial thromboplastin time; TT, thrombin time. > limit/< limit, value exceeded/went below the test limit.
NCT was dissolved in saline and 10-fold diluted in whole human citrate blood (0.2 ml plus 1.8 ml). Data in parentheses are percentages.
Blood (1.8 ml) plus 0.2 ml physiological saline solution.
Citrate blood without any additives.
As a third step, NVC-612 and NVC-422 were used. A blood sample was taken from 4 volunteers, and 1.8-ml portions were mixed with 0.25% NCT (final concentration, 0.025%, 1.375 mM), 0.34% NVC-612 (final concentration, 1.38 mM for 83.5% potency), 0.25% NVC-422 (final concentration, 1.02 mM). Controls were performed with blank saline, 1.38 mM dimethyltaurine or taurine, or 0.5 IU heparin (final concentration for each).
The direct thrombin activities of NVC-422, NCT, and DMT (used as a negative control) were tested using a SensoLyte 520 thrombin activity assay kit (AnaSpec, Inc., Fremont, CA). A known inhibitor, N-α-(2-naphthylsulfonylglycyl)-4-amidinophenylalanine piperidine (N-α-NAPAP) (10), was applied as a positive control at a concentration of 0.6 μM.
Quantitative killing of microorganisms by NCT with and without heparin in buffer solution.
Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 11229, Streptococcus pyogenes d68, Staphylococcus epidermidis ATCC 12228, Pseudomonas aeruginosa ATCC 27853, Proteus mirabilis ATCC 14153, Candida albicans CBS 5982, and methicillin-resistant Staphylococcus aureus (MRSA; clinical isolate), all deep-frozen for storage, were grown on Mueller-Hinton agar plates and subsequently in tryptic soy broth overnight. They were washed twice and 10-fold diluted in saline before use. NCT at a final concentration of 1% (55 mM) was dissolved in 0.1 M phosphate buffer (pH 7.1) containing either 125 IU/ml heparin or no heparin. Controls were performed in buffer without additives and in buffer containing 125 IU/ml heparin. All of the solutions were prewarmed immediately before the tests and incubated at 37°C. Bacterial suspensions (40 μl) were added to 4 ml of NCT, NCT-heparin, and control solutions. After 1, 3, 5, 8, and 10 min (30, 60, 80, and 120 min for C. albicans), aliquots of 100 μl were removed and diluted 10-fold or 100-fold in 0.6% sodium thiosulfate solution to inactivate NCT. Aliquots (50 μl) of these dilutions were spread in duplicate on tryptic soy agar plates with an automatic spiral plater (model WASP 2; Don Whitley Scientific, Shipley, United Kingdom), allowing a detection limit of 100 CFU/ml, taking into account both plates and the dilution. The plates were incubated at 37°C, and the numbers of CFU of microorganisms were counted after 24 and 48 h.
Quantitative killing of microorganisms by NCT, NVC-612, and NVC-422 in blood.
Blood was drawn from volunteers and enriched with 125 IU/ml heparin to avoid immediate coagulation. Different volumes of a 0.9% saline solution of NCT, NVC-612, and NVC-422 were added so that the test samples contained 10%, 25%, 50%, and 75% blood. In addition, 100% blood was tested, in which the oxidants were directly dissolved in heparinized blood. The final test article concentrations after mixing in blood were 55 mM for both NCT and NVC-612 and 27.5 mM for NVC-422 in all samples. Controls contained heparinized 100% blood and 10%, 25%, 50%, and 75% blood in saline, without additives. To investigate the dependency on the concentration of the oxidants at a defined blood concentration, additional tests were performed with 5.5, 13.8, 27.5, and 55 mM NCT in parallel in 10% blood. All of the solutions were incubated at 37°C. S. aureus and E. coli grown in tryptic soy broth overnight were washed twice and 10-fold diluted in saline. An aliquot (40 μl) was suspended in the test solutions containing the different concentrations of blood. After 1, 3, 5, 10, 15, 30, 45, and 60 min, aliquots of 100 μl were removed and diluted 10-fold or 100-fold in 0.6% sodium thiosulfate. Quantitative cultures from the aliquots were performed as detailed above.
Statistics.
Tests applied were unpaired Student's t test and one-way analysis of variance (ANOVA) with Bonferroni's or Dunnett's multiple-comparison test. P values of <0.05 were considered significant.
RESULTS
Anticoagulant activities of NCT, NVC-422, and NVC-612.
In a first pilot experiment, the influence of different concentrations of NCT on the coagulation system was tested in one blood sample. At the lowest concentration of 0.55 mM (0.01%), NCT had minimal effects on the activated partial thromboplastin time and fibrinogen tests and no influence on prothrombin time and thrombin time. NCT had a dose-dependent impact on all parameters, including prothrombin time and thrombin time (Table 1). Furthermore, the values of prothrombin time and fibrinogen were lower in blood containing 0.09% saline than blood without additives. Therefore, in the following, all values of coagulation tests were statistically compared to those for the control, containing a final saline concentration of 0.09%.
On the basis of these results, 0.55 mM (0.01%) NCT and 1.38 mM (0.025%) NCT were tested in blood samples from 11 donors (Table 2). Prothrombin time, activated partial thromboplastin time, and thrombin time were prolonged, and fibrinogen decreased in samples containing 1.38 mM NCT (P < 0.01 for both concentrations and all parameters compared to the plain saline control). Consistent with Table 1, 0.55 mM NCT had only a minimal and statistically not significant effect on the coagulation parameters (Table 2). Heparin (0.5 IU/ml) had the expected marked impact on activated partial thromboplastin time and thrombin time but a minimal effect on prothrombin time. Heparin's effect was further enhanced by 0.55 mM NCT (P < 0.01 for prothrombin time). Vice versa, heparin did not inhibit but rather enhanced the anticoagulant effect of NCT (P < 0.01 for prothrombin time, activated partial thromboplastin time, and thrombin time). Antithrombin III and D dimer were not affected by NCT or by heparin at the applied concentrations.
Table 2.
Influence of NCT and heparin on coagulation parameters in whole human blooda
| Anticoagulant | PT (%) | INR | aPTT (s) | TT (s) | Fib (mg/dl) | AT III (%) | D dimer (μg/liter) |
|---|---|---|---|---|---|---|---|
| Control | 84.1 (10.5) | 1.1 (0.1) | 33.5 (3.9) | 19.5 (1.6) | 246.5 (54.1) | 87.5 (7.7) | 185.8 (72.1) |
| Heparin | 70.8 (11.3) | 1.2 (0.1) | 247.4b (87.2) | ≥400b (NC) | 253.1 (67.3) | 88.1 (11.0) | 193.5 (74.2) |
| 0.55 mM NCT | 74.8 (11.7) | 1.2 (0.1) | 37.6 (4.4) | 23.5 (2.9) | 192.1 (51.6) | 86.2 (8.1) | 179.9 (72.6) |
| 1.38 mM NCT | 25.3b,c (11.1) | 3.2b,c (1.3) | 114.0d (53.6) | 45.8b,c (7.2) | ≤40b,c (NC) | 83.7 (8.3) | 165.5 (68.1) |
| 0.55 mM NCT + heparin | 62.5b (10.5) | 1.3 (0.1) | 295.5b (80.2) | ≥400b (NC) | 192.5 (50.7) | 84.5 (9.0) | 184.6 (73.7) |
NCT was dissolved in saline and 10-fold diluted in whole human citrate blood (0.2 ml plus 1.8 ml) with or without heparin (0.5 IU/ml) to 0.55 mM (0.01%) or 1.38 mM (0.025%). Control consisted of 0.2 ml 0.9% NaCl plus 1.8 ml whole citrate blood. NC, not calculable; PT, prothrombin time; INR, international normalized ratio; aPTT, activated partial thromboplastin time; TT, thrombin time; Fib, fibrinogen; AT III, antithrombin III; D dimer, cleavage products of polymerized fibrin. Values are means (standard deviations) of 3 to 4 independent experiments.
P < 0.01 versus control (one-way ANOVA and Bonferroni's multiple-comparison test).
P < 0.01 versus all other test rows (one-way ANOVA and Bonferroni's multiple-comparison test).
P < 0.05 versus control (one-way ANOVA and Bonferroni's multiple-comparison test).
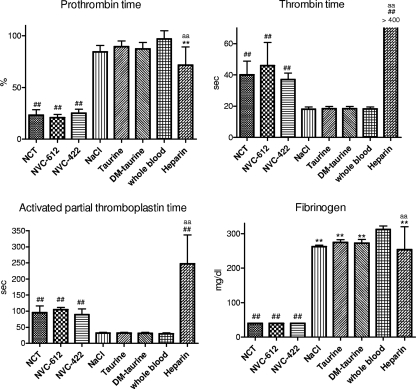
In blood samples from four donors, 1.38 mM NVC-612 and 1.02 mM NVC-422 had similar anticoagulant activity as 1.38 mM NCT (Fig. 1). In contrast, taurine and 2,2-dimethyltaurine, which exert no oxidative activity, did not show any effect (Fig. 1). Direct thrombin activity was not influenced by 0.4 to 40 mM NVC-612, NVC-422, or dimethyltaurine, while 0.6 μM N-α-NAPAP inhibited it by 84% (data not shown).
Fig 1.
Influence of NCT, NVC-612, NVC-422, and heparin, as well as the controls taurine, dimethyltaurine, and saline, on coagulation parameters after 10-fold dilution in whole citrate human blood (0.2 ml plus 1.8 ml) to a final concentration of 1.38 mM (1.02 mM for NVC-422) compared to whole citrate blood without any additives. Values are means ± SDs of 3 to 4 independent experiments. **, P < 0.01 versus whole blood; ##, P < 0.01 against all controls (blood without additives, saline, taurine, dimethyltaurine); aa, P < 0.01 versus NCT, NVC-612, and NVC-422. One-way ANOVA and Bonferroni's multiple-comparison test.
Quantitative killing of microorganisms by NCT, NVC-422, and NVC-612 in human blood.
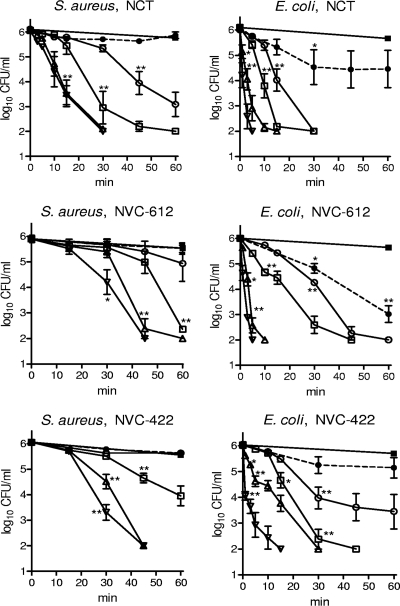
At 55 mM, NCT and NVC-612 as well as NVC-422 at 27.5 mM (with the same oxidation capacity as 55 mM NCT and NVC-612) had bactericidal activities in 10%, 25%, 50%, and 75% heparinized blood (Fig. 2). In general, E. coli was slightly more susceptible than S. aureus. The activities of all three test substances decreased with increasing blood concentration. In the presence of 10 to 50% blood, a significant reduction of both bacterial species was achieved within 60 min in all cases. In the presence of 75% blood, there was still significant bactericidal activity (except for NVC-612 and NVC-422 against S. aureus), while whole blood (100%) greatly diminished (E. coli) or negated (S. aureus) the bactericidal activity. Generally, there was no significant difference between the results for all three test compounds.
Fig 2.
Bactericidal activity of 55 mM NCT, NVC-612, and 27.5 mM NVC-422 against S. aureus and E. coli in the presence of 10% (▽), 25% (△), 50% (□), 75% (○), and 100% (●) whole blood at 37°C. ■, control without oxidant in 100% blood. Values are means ± SEMs of three independent experiments each; detection limit, 100 CFU/ml. *, threshold values for P < 0.05; **, P < 0.01 versus control without test substances. One-way ANOVA and Dunnett's multiple-comparison test.
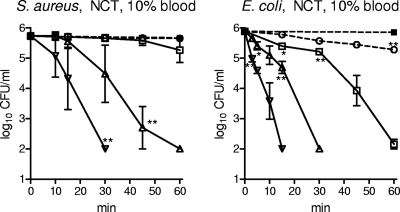
The dependency on the concentration of the oxidants at a defined blood concentration was clearly disclosed with 5.5 to 55 mM NCT in 10% blood against S. aureus and E. coli (Fig. 3). While 55 mM and 27.5 mM demonstrated good activity, 13.8 mM killed only E. coli within 60 min. A longer incubation time of 5 h disclosed killing of S. aureus to the detection limit by 13.8 mM NCT, too. The CFU count of E. coli was reduced by 2.55 to 3.09 log10 units (range of n = 3) after 5 h in 5.5 mM NCT, while S. aureus was not killed any more by this concentration.
Fig 3.
Bactericidal activity of 55 mM (▽), 27.5 mM (△), 13.8 mM (□), and 5.5 mM (○ with dashed line) NCT against S. aureus and E. coli in the presence of 10% blood at 37°C. ■ with dashed line, control without oxidant in 10% blood. Values are means ± SEMs of three independent experiments each; detection limit, 100 CFU/ml. *, threshold values for P < 0.05; **, P < 0.01 versus control without test substances. One-way ANOVA and Dunnett's multiple-comparison test.
Quantitative killing of microorganisms by NCT with and without heparin in buffer solution.
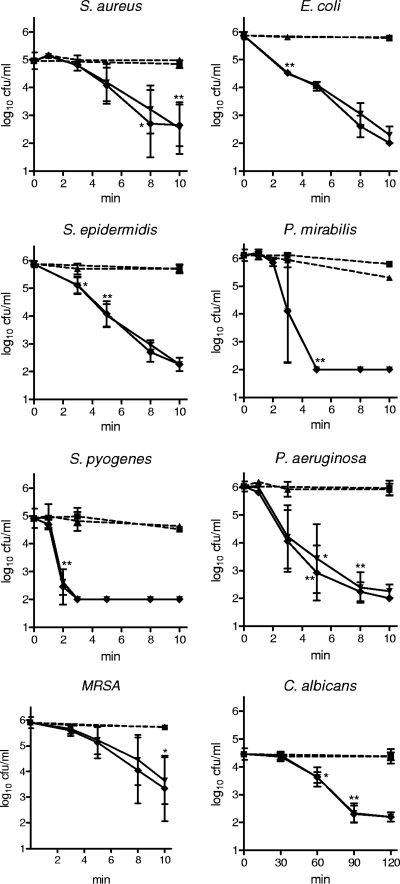
At body temperature (37°C) and pH 7.1, 55 mM (1%) NCT reduced viable counts of microorganisms by 3 log10 units within 5 min (S. pyogenes, P. mirabilis), 8 min (E. coli, S. epidermidis, P. aeruginosa), 15 min (S. aureus, MRSA), and 120 min (C. albicans) (Fig. 4). For 55 mM NVC-612, the times for the same reduction were 5 min (E. coli, P. mirabilis, S. pyogenes), 8 min (P. aeruginosa), 15 min (S. aureus), and 45 min (C. albicans), and for 55 mM NVC-422 the times were 1 min (S. pyogenes, P. mirabilis), 3 min (P. aeruginosa), 5 min (E. coli), 10 min (S. aureus), and 30 min (C. albicans). Addition of 125 IU/ml heparin had no influence on the bactericidal effect, and heparin by itself did not kill microorganisms within the tested incubation times (Fig. 4).
Fig 4.
Microbicidal activity of 55 mM (1%) NCT against bacteria and Candida in the presence (♦) and absence (▼) of 125 IU/ml heparin in 0.1 M phosphate buffer at pH 7.1 and 37°C; ■, phosphate buffer without additives; ▲, phosphate buffer with 125 IU/ml heparin. Values are means ± SDs of three independent experiments each; detection limit, 100 CFU/ml. P > 0.05 between NCT and NCT plus heparin for all incubation times and all strains. *, threshold values for P < 0.05; **, P < 0.01 versus control without test substances. One-way ANOVA and Dunnett's multiple-comparison test.
DISCUSSION
In the present study, we demonstrate that the N-chloramines NCT, NVC-422, and NVC-612 have anticoagulant activity and retain their broad-spectrum antimicrobial activities in the presence of up to 75% human blood. These two critical properties make NCT, NVC-422, and NVC-612 potential CVC lock solutions for the prevention of CVC-related bloodstream infections and catheter blockage. Since tolerability has been shown for 1% (55 mM) aqueous NCT solution in several clinical trials and at different body sites, for instance, the skin, outer ear canal, and the eye (9, 17, 18, 24), and also for 1.5% (61 mM) NVC-422 gel on the skin (11), a concentration of about 1% NCT, NVC-422, and NVC-612 in aqueous or buffered solution is estimated to be preferable for the development of these compounds as catheter lock solutions. This is confirmed by all presently available data or considerations on safety (see below). Addition of heparin is conceivable, but the benefit remains to be investigated.
The anticoagulant activity was dose dependent, and the activity of a concentration of 55 mM NCT, which is generally applied therapeutically (9), by far exceeded the test limits in the pilot tests. To gain numeric results, which enabled investigation of the combined effect with heparin and a reasonable comparison with previous literature on active chlorine compounds, we applied lower concentrations for the more comprehensive experiments. NCT (1.38 mM), NVC-422 (1.02 mM), and NVC-612 (1.38 mM) still had anticoagulant activity in all main coagulation tests in human blood (Fig. 1). These results confirm those of a previous study, in which it was found that NaOCl, chloramine T, and NCT (prepared from hypochlorous acid and taurine and not from the sodium salt, as in our study) at concentrations of 2 to 3 mM oxidize and inactivate plasminogen activator inhibitor-1, fibrinogen, factor V, factor VIII, and factor X and inhibit platelet aggregation at 1 mM (22, 23). The latter effect has recently been confirmed for NCT, N,N-dichlorotaurine, and N-chloro-N-methyltaurine (16). Moreover, the inhibition of primary and secondary aggregation of thrombocytes and of the release of the contents of their dense granules by chloramines has been demonstrated (15). Because of these results, human granulocytes have been considered to participate in physiologic thrombolysis (21). The absence of any influence on the D-dimer test fits with the observed anticoagulant effect and absence of formation of fibrin. Thus, no cleavage products of fibrin occur. The significance of our study compared to the previous studies is that NCT in its clinically applied form (prepared from the crystalline sodium salt [8]) and its novel analogs NVC-422 and NVC-612 were tested in a setting of experiments designed for their use as catheter lock solutions.
Besides a limited additive anticoagulant effect, there was no interaction with heparin, which is widely used to avoid clotting of central venous catheters. Since it is presently unknown whether or not chloramines can replace heparin for that purpose, it is conceivable that a combination of both compounds could provide an antimicrobial and an anticoagulant effect. Heparin consists of sulfonated glycosaminoglycans (glucosamine and glucuronic acid) and contains amidic groups, which do not react with NCT, suggesting compatibility between heparin and NCT (8).
Efficacy criteria are that the lock solution has to be antimicrobial even in the presence of blood on the tip of the catheter or trace amounts leaked back into the catheter (20). Active chlorine compounds are known to be consumed by organic substances, mainly by sulfhydryl, thio, and aromatic compounds (6, 8). Despite this, NVC-422, NVC-612, and NCT at a concentration of 55 mM, which appears to be very suitable for most clinical applications (9, 11, 17, 18, 24), continued to kill bacteria even in 50 to 75% blood. Only 100% blood largely prevented bacterial killing. Differences between the three tested chloramines are small and are probably irrelevant for practical use. Since catheters leading to blood vessels are irrigated with saline after use and the instillation of a lock solution further dilutes possible blood residues, only low concentrations of organic matter remain to consume any of the chloramines. Therefore, it is likely that they will have sufficient antimicrobial efficacy as lock solutions in vivo. This is confirmed by the finding that NCT had microbicidal activity down to 13.8 to 5.5 mM in 10% blood. On the other hand, these results (Fig. 3) clearly show that 55 mM should be preferred regarding efficacy. Its tolerability seems probable, as discussed in the following.
Regarding the safety of catheter lock solutions, no systemic toxic side effects should occur in case of leakage into the bloodstream (3, 12). Other chloramines, namely, N,N-dichlorotaurine and N-chlorophenylalanine, had antithrombotic activity and obviously were well tolerated when applied intravenously into mice at concentrations of 3.4 to 6.8 mg/kg of body weight (0.25 to 0.37 mM) and 13.6 mg/kg (1 mM), respectively (15). Their lethal doses causing death of 50% of mice were 48 and 105 mg/kg, respectively (15). Another active chlorine compound consisting of a chlorodioxide, tetrachlorodecaoxide (WF10) (7), is already in use as an intravenous infusion in humans for immune stimulation and treatment of postradiation chronic inflammatory cystitis and proctitis (25). A comprehensive battery of good laboratory practice safety pharmacology and toxicology studies has been conducted to support the use of NVC-422 in a number of clinical indications under several investigational new drug dossiers (4). Concentrations up to 80 mg/kg were tested intravenously in animals. Even in the case that 10 ml of a 1% NVC-422 or 1% NCT solution (containing 100 mg of drug) was accidentally applied intravenously in humans, this would amount to 1.5 mg/kg for a 70-kg person, which is more than 50 times below the 50% lethal dose of NVC-422 and more than 30 times below that of N,N-dichlorotaurine in mice (4, 15). All these data and considerations may predict a high degree of safety of small amounts of NCT, NVC-422, and NVC-612 entering the circulation.
The results of this study confirm that millimolar concentrations of NCT, NVC-612, and NVC-422 have broad-spectrum microbicidal activity in blood and anticoagulant properties, which suggest their further investigation as catheter lock solutions.
ACKNOWLEDGMENTS
This study was supported by the Austrian Science Fund, grant no. L313-B13, and by NovaBay Pharmaceuticals, Inc.
We thank John A. Soderquist, University of Puerto Rico, for his valuable comments and critical review of the manuscript. We are grateful to Andrea Windisch and Margit Lanthaler for excellent technical assistance.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Casey AL, Elliott TS. 2010. Prevention of central venous catheter-related infection: update. Br. J. Nurs. 19:78–87 [DOI] [PubMed] [Google Scholar]
- 2. Casey AL, Leonard AM, Peter N, Tom SE. 2008. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet 8:763–776 [DOI] [PubMed] [Google Scholar]
- 3. Chittick P, Sherertz RJ. 2010. Recognition and prevention of nosocomial vascular device and related bloodstream infections in the intensive care unit. Crit. Care Med. 38:S363–S372 [DOI] [PubMed] [Google Scholar]
- 4. Darouiche D, et al. 2011. NVC-422. Drugs Future 36:651–656 [Google Scholar]
- 5. del Pozo JL. 2009. Role of antibiotic lock therapy for the treatment of catheter-related bloodstream infections. Int. J. Artif. Organs 32:678–688 [DOI] [PubMed] [Google Scholar]
- 6. Dychdala GR. 2001. Chlorine and chlorine compounds, p 135–158 In Block SS. (ed), Disinfection, sterilization and preservation. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 7. Giese T, et al. 2004. Differential effects on innate versus adaptive immune responses by WF10. Cell. Immunol. 229:149–158 [DOI] [PubMed] [Google Scholar]
- 8. Gottardi W, Nagl M. 2002. Chemical properties of N-chlorotaurine sodium, a key compound in the human defence system. Arch. Pharm. (Weinheim) 335:411–421 [DOI] [PubMed] [Google Scholar]
- 9. Gottardi W, Nagl M. 2010. N-Chlorotaurine, a natural antiseptic with outstanding tolerability. J. Antimicrob. Chemother. 65:399–409 [DOI] [PubMed] [Google Scholar]
- 10. Henriques ES, Fonseca N, Ramos MJ. 2004. On the modeling of snake venom serine proteinase interactions with benzamidine-based thrombin inhibitors. Protein Sci. 13:2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iovino SM, et al. 2011. NVC-422 topical gel for the treatment of impetigo. Int. J. Clin. Exp. Pathol. 4:587–595 [PMC free article] [PubMed] [Google Scholar]
- 12. Kulawik D. 2010. Antimicrobial lock solutions: an approach to reduce catheter-related bacteremia. Nephrol. Nurs. J. 37:553–555 [PubMed] [Google Scholar]
- 13. Low E, et al. 2009. N,N-Dichloroaminosulfonic acids as novel topical antimicrobial agents. Bioorg. Med. Chem. Lett. 19:196–198 [DOI] [PubMed] [Google Scholar]
- 14. Mitchell MD, Anderson BJ, Williams K, Umscheid CA. 2009. Heparin flushing and other interventions to maintain patency of central venous catheters: a systematic review. J. Adv. Nurs. 65:2007–2021 [DOI] [PubMed] [Google Scholar]
- 15. Murina MA, Fesenko OD, Sergienko VI, Chudina NA, Roshchupkin DI. 2002. Antithrombotic activity of N,N-dichlorotaurine on mouse model of thrombosis in vivo. Bull. Exp. Biol. Med. 134:36–38 [DOI] [PubMed] [Google Scholar]
- 16. Murina MA, Roshchupkin DI, Chudina NA, Petrova AO, Sergienko VI. 2009. Antiaggregant effect of taurine chloramines in the presence of serum albumin. Bull. Exp. Biol. Med. 147:704–707 [DOI] [PubMed] [Google Scholar]
- 17. Nagl M, Nguyen VA, Gottardi W, Ulmer H, Höpfl R. 2003. Tolerability and efficacy of N-chlorotaurine compared to chloramine T for treatment of chronic leg ulcers with purulent coating. Br. J. Dermatol. 149:590–597 [DOI] [PubMed] [Google Scholar]
- 18. Neher A, et al. 2004. Acute otitis externa: efficacy and tolerability of N-chlorotaurine, a novel endogenous antiseptic agent. Laryngoscope 114:850–854 [DOI] [PubMed] [Google Scholar]
- 19. Rosenthal VD, et al. 2010. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am. J. Infect. Control 38:95–104 [DOI] [PubMed] [Google Scholar]
- 20. Steczko J, Ash SR, Nivens DE, Brewer L, Winger RK. 2009. Microbial inactivation properties of a new antimicrobial/antithrombotic catheter lock solution (citrate/methylene blue/parabens). Nephrol. Dial. Transplant. 24:1937–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stief TW. 2007. Singlet oxygen potentiates thrombolysis. Clin. Appl. Thromb. Hemost. 13:259–278 [DOI] [PubMed] [Google Scholar]
- 22. Stief TW, Aab A, Heimburger N. 1988. Oxidative inactivation of purified human alpha-2-antiplasmin, antithrombin III, and C1-inhibitor. Thromb. Res. 49:581–589 [DOI] [PubMed] [Google Scholar]
- 23. Stief TW, Kurz J, Doss MO, Fareed J. 2000. Singlet oxygen inactivates fibrinogen, factor V, factor VIII, factor X, and platelet aggregation of human blood. Thromb. Res. 97:473–480 [DOI] [PubMed] [Google Scholar]
- 24. Teuchner B, et al. 2005. Tolerability and efficacy of N-chlorotaurine in epidemic keratoconjunctivitis—a double-blind randomized phase 2 clinical trial. J. Ocular Pharmacol. Ther. 21:157–165 [DOI] [PubMed] [Google Scholar]
- 25. Veerasarn V, Boonnuch W, Kakanaporn C. 2006. A phase II study to evaluate WF10 in patients with late hemorrhagic radiation cystitis and proctitis. Gynecol. Oncol. 100:179–184 [DOI] [PubMed] [Google Scholar]
- 26. Wang L, et al. 2011. Chemical characterization and biological properties of NVC-422, a novel, stable N-chlorotaurine analog. Antimicrob. Agents Chemother. 55:2688–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Khosrovi B, Najafi R. 2008. N-Chloro-2,2-dimethyltaurines: a new class of remarkably stable N-chlorotaurines. Tetrahedron Lett. 49:2193–2195 [Google Scholar]
- 28. Weber DJ, Rutala WA. 2011. Central line-associated bloodstream infections: prevention and management. Infect. Dis. Clin. North Am. 25:77–102 [DOI] [PubMed] [Google Scholar]