Abstract
Primaquine is currently the only drug available for radical cure of Plasmodium vivax and P. ovale liver infection stages, but limited safety data exist for children <10 years of age. Detailed daily assessments of side effects in glucose-6-phosphate dehydrogenase (G6PD)-normal children treated with 14 days of primaquine plus chloroquine (3 days; n = 252) or artesunate (7 days; n = 141) (0.5 mg/kg of body weight) showed that both treatments are well tolerated, do not lead to reductions in hemoglobin levels, and can thus safely be used in children 1 to 10 years of age.
TEXT
Primaquine (PQ), one of the oldest synthetic antimalarial drugs, remains to date the only licensed product that can eliminate the hepatic dormant stages—the hypnozoites—of the two Plasmodium species (P. vivax and P. ovale) capable of producing relapses. The latest World Health Organization (WHO) recommendations for the prevention of hypnozoite-derived relapses state that the drug should be used contemporaneously with an effective blood schizontocidal for 14 days at a dosage of 0.25 mg/kg of body weight (in a single daily dose) (11). In areas where tolerance to primaquine has been observed, such as in Oceania and Southeast Asia, this dose should be doubled to 0.5 mg/kg. While strongly endorsing this recommendation, the WHO explicitly states that it is based on limited evidence.
Despite over 60 years of continuous use, primaquine still carries a reputation of being an “unsafe” drug. Side effects can be summarized into three main categories, the most important of which is the array of potentially life-threatening hemolytic side effects that it can cause among glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals (12). This enzymatic deficiency, of which ∼140 different variants exist (3), is an absolute contraindication for the use of primaquine when the enzyme's activity is below the threshold of 5%, but the drug can be used in milder cases with the provision of spreading the treatment on a weekly basis over a 2-month-long schedule of 0.75 mg/kg (11). The second important side effect is an increase in the level of methemoglobin, which is mild and generally well tolerated (5) unless the patient has an inborn deficiency of the methemoglobin reductase metabolic pathway. Finally, primaquine can cause dose-dependent abdominal discomfort when taken on an empty stomach (4) and, as a consequence, is best taken with food (11). Apart from the aforementioned side effects, primaquine is usually safe and well tolerated in patients without inborn deficiencies (1, 2).
There are, however, virtually no published data available on the safety and tolerability of primaquine in children, and the WHO therefore maintains that primaquine is contraindicated in children less than 4 years of age (11), even though children in that age group suffer the brunt of P. vivax disease in areas of high endemicity such as the southwest Pacific (6–8). A recent study that included the coadministration of a single dose of 0.75 mg/kg primaquine with artesunate plus sulfadoxine-pyrimethamine (SP) to children 1 to 12 years of age as part of a mass-administration trial resulted in significantly reduced hemoglobin (Hb) levels 7 days after treatment (10). The Hb reduction was largest in children with G6PD deficiency but was also present in G6PD heterozygote- and homozygote-normal children, raising concerns that PQ may cause moderate anemia when coadministered with artemisinins and that excluding individuals based on G6PD status alone may not be sufficient to prevent PQ-induced hemolysis.
There is thus an urgent need for a more extensive evaluation of PQ's safety and tolerability in young children, in particular if coadministered with an artemisinin. Here, we report the results from two different pediatric cohorts treated with different primaquine-containing antimalarial schedules as part of a wider epidemiological study, for the evaluation of their tolerability and safety.
This study was performed in two cohorts and was conducted in the Maprik region of the East Sepik province in Papua New Guinea (PNG). The study region is an area of hyperendemicity for both P. falciparum and P. vivax, with P. vivax the predominant source of infection and disease in the first 3 years of life and a progressive replacement by P. falciparum as the main cause of disease, extending even to adulthood (6, 8, 9). Since 2011, PNG has adopted artemether-lumefantrine for the treatment of uncomplicated malaria, irrespective of the species. If an infection by P. vivax or P. ovale is confirmed, an additional 14-day course of primaquine at a dose of 0.25 mg/kg/day is recommended but rarely implemented.
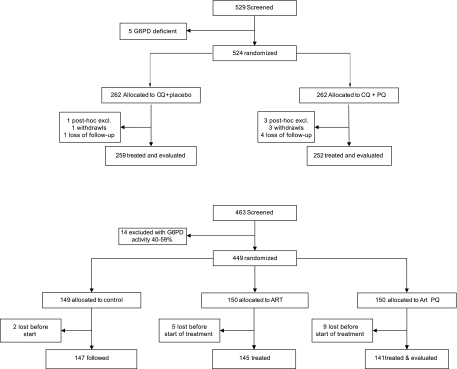
Two different pediatric cohorts of children 5 to 10 and 1 to 5 years of age, arranged to assess the epidemiology of malaria according to age, were treated with antimalarials at the beginning of a 9-month-long follow-up. All children living the study villages whose parents agreed to their participation were tested for G6PD deficiency using a G6PD assay kit (Dojindo Laboratories, Japan) and OSMMR-D G6PD assay (R&D Diagnostics, Greece) according to the manufacturers' instructions, with all G6PD-deficient children excluded prior to enrollment and drug treatment. The first community-based cohort included 529 children 5 to 10 years of age recruited in Albinama; 524 (99.1%) were G6PD normal and randomized to receive either chloroquine (standard 25 mg/kg/total dose, divided over 3 days) and high-dose primaquine (0.5 mg/kg/day for 14 days [group 1; n = 262]) or chloroquine and placebo (3 or 14 days, respectively [group 2; n = 262]) (Fig. 1). Three children in the placebo arm and 10 in the primaquine arm were excluded post hoc due to protocol violations. The study was double blinded, with randomization performed using preallocated, block-randomized treatment codes. The second cohort was recruited among children aged 1 to 5 years of age, in Ilaita, an area neighboring Albinama. A total of 463 children were screened for G6PD deficiency, and 449 (97.0%) were randomized to receive 7 days of artesunate treatment (4 mg/kg/day) and 14 days of high-dose primaquine treatment (0.5 mg/kg/day, starting on day 1 of artesunate treatment; Fig. 1), only artesunate monotherapy for 7 days, or no antimalarial treatment. A total of 9 children allocated to the artesunate-plus-primaquine arm were lost in the 3 months between randomization and the start of treatment.
Fig 1.
Trial design and drugs administered in the two different study cohorts. (Upper panel) Albinama cohort (5 to 10 years of age). (Lower panel) Ilaita cohort (1 to 5 years of age).
All primaquine doses were administered under supervision; those given to children 5 to 10 years of age were given with a snack, while parents or guardians of children 1 to 5 years of age were advised to make sure children had a meal or were breastfed prior to drug administration. Children in both arms of the cohort of those 5 to 10 years of age and the artesunate-plus-primaquine arm in the cohort of those 1 to 5 years of age were followed up on a daily basis. The occurrences of different signs and symptoms and adverse events and the general tolerability of the study drugs were recorded daily using standardized questionnaires by specifically trained study nurses. Illnesses detected at recruitment were treated according to PNG national guidelines. The proportions of children with reported signs or symptoms were compared using Fisher's exact or χ2 tests, and (paired) t tests were used to compare differences in hemoglobin levels at baseline and day 8 (5 to 10 years of age) and day 14 (1 to 5 years of age).
Table 1 summarizes the presence of different signs and symptoms at baseline and the cumulative incidence of adverse events throughout the 14-day follow-up period. Among the children 5 to 10 years of age, no significant differences were observed at baseline between the primaquine and placebo groups for any of the signs and symptoms (Table 1; P > 0.41). Symptoms were rare during the 14 days of follow-up (frequency < 7%), and no differences in the occurrence of new symptoms and their cumulative incidence during the 14 days of follow-up were observed between the groups (P > 0.24).
Table 1.
Prevalence at baseline and cumulative occurrence of different signs and symptoms according to treatment group and cohorta
| Sign, symptom, or parameter | Children age 5–10 yr |
Children age 1–5 yr |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline |
P valueb | Days 1–14 (cumulative) |
P valueb | n/141 (%) at baseline for group 3 (ART + PQ) | n/141 (%) at days 1–14 for group 3 (ART + PQ) | P valuec | |||
| n/252 (%) for group 1 (CQ + PQ) | n/259 (%) for group 2 (CQ + placebo) | n/252 (%) for group 1 (CQ + PQ) | n/259 (%) for group 2 (CQ + placebo) | ||||||
| Signs and symptoms | |||||||||
| Feverd | 29 (11.5) | 24 (9.3) | 0.41 | 11 (4.4) | 10 (3.9) | 0.83 | 26 (18.4) | 11 (7.8) | 0.16 |
| Yellow sclera | 0 | 0 | 3 (2.1) | 0.043 | |||||
| Dizziness | 0 | 1 (0.4) | 1.00 | 0 | 2 (0.8) | 0.50 | 1 (0.7) | 0.36 | |
| Headache | 5 (2.0) | 6 (2.3) | 0.80 | 8 (3.2) | 10 (3.9) | 0.81 | 2 (1.4) | 4 (2.8) | 0.85 |
| Earache | 2 (0.8) | 0 | 0.24 | 0 | |||||
| Tiredness | 1.00 | 5 (2.0) | 7 (2.7) | 0.77 | 5 (3.6) | 0.35 | |||
| Itchiness | 3 (1.2) | 1 (0.4) | 0.37 | 1 (0.7) | 1.00 | ||||
| Skin rash | 1 (0.4) | 1 (0.4) | 1.00 | 2 (1.3) | 1.00 | ||||
| Chest tightness | 1 (0.4) | 0 | 0.49 | 0 | |||||
| Cough | 13 (5.2) | 13 (5.0) | 0.94 | 19 (7.5) | 16 (6.2) | 0.60 | 16 (11.4) | 17 (12.1) | 0.15 |
| Shortness of breath | 1 (0.4) | 3 (1.2) | 0.62 | 1 (0.4) | 1 (0.4) | 1.00 | 2 (1.4) | 2 (1.4) | 0.30 |
| Nausea | 1 (0.4) | 0 | 0.49 | 8 (3.2) | 5 (1.9) | 0.42 | 4 (2.8) | 4 (2.8) | 0.85 |
| Vomiting | 0 | 1 (0.4) | 1.00 | 6 (2.4) | 3 (1.2) | 0.33 | 0 | 2 (1.4) | 0.72 |
| Stomachache | 0 | 0 | 1 (0.4) | 0 | 0.49 | 2 (1.4) | 6 (4.3) | 0.010 | |
| Diarrhea | 0 | 0 | 1 (0.4) | 2 (0.8) | 1.00 | 4 (2.8) | 2 (1.4) | 0.30 | |
| Myalgia | 1 (0.4) | 0 | 0.49 | 2 (1.4) | 0.30 | ||||
| Any sign or symptom | 30 (11.9) | 25 (9.7) | 0.41 | 29 (11.5) | 26 (10.0) | 0.67 | 30 (21.3) | 27 (19.2) | 0.05 |
| Laboratory measures | |||||||||
| Mean hemoglobin (gl/dl)e | 10.8 | 10.9 | 0.71 | 10.3 | 10.5 | 0.007 | 9.31 | 9.81 | |
| ΔHb | −0.56 | −0.34 | 0.24 | 0.48 | |||||
| CI95 | −0.29 to −0.81 | −0.59 to −0.08 | 0.30 to 0.66 | ||||||
ART, artesunate; CQ, chloroquine; PQ, primaquine; CI95, 95% confidence interval. Only limited clinical assessment was conducted at baseline for the cohort of children 1 to 5 years of age.
P value for comparison of groups 1 and 2.
P value for comparison of groups 1 and 3 for days 1 to 14 (cumulative).
Axillary temperature >37.5°C or reported fever.
Hemoglobin levels assessed at baseline and day 8 (5 to 10 years of age) and day 14 (1 to 5 years of age) of follow-up.
Among the younger children, the prevalence of adverse events at baseline was higher (21%) due to a higher prevalence of malarial fevers (1 to 5 years of age, 14.9%; 5 to 10 years of age, 5.3% [P < 0.001]). Similarly, the occurrence of new symptoms and their cumulative incidence during the 14 days of follow-up were higher (Table 1). However, most of the symptoms were related to febrile illness and/or cough. Although a somewhat higher rate of stomachaches was observed (P = 0.010), the rates of nausea and vomiting were comparable to those observed in the older children who received a snack with each of the primaquine doses. No treatments had to be discontinued due to poor tolerability or repeated vomiting in either cohort.
Among the older children, a marginally larger (0.56 versus 0.33 g/dl; P = 0.24) but clinically and statistically insignificant drop in mean hemoglobin (Hb) levels (measured by Hemacue, Angholm, Sweden) was observed at day 8 in the primaquine group versus the placebo group. Similarly, equal numbers of children in both groups experienced clinically relevant drops of >2 gl/dl (22/247 versus 22/257; P = 0.89). Among the children 1 to 5 years of age treated with artesunate plus primaquine, Hb levels did not change in the first 3 days of treatment (9.31 versus 9.35 g/dl; P = 0.77) and then increased by 0.48 g/dl after 14 days of treatment (9.31 versus 9.81 g/dl; P < 0.001). Only 1 child experienced a drop in Hb of 2 g/dl, with no clinical evidence of hemolysis. Both primaquine schemes were therefore well tolerated and safe.
This report of these two cohorts provides the first published detailed evidence of acceptable safety and tolerability of the 14-day high-dose (0.5 mg/kg) primaquine schedule in G6PD-normal children 1 to 10 years of age. In the placebo-controlled study of children 5 to 10 years of age, the side effects were observed infrequently and were thought to be associated with the primaquine treatment only in one child. The moderately higher rate of adverse events in the younger cohort was almost entirely due to the higher levels of preexisting illness observed at baseline. The well-known gastrointestinal side effects of primaquine were rare even in children 1 to 5 years of age when the drug was not coadministered with food. As some children may not have had a meal at home prior to treatment, the tolerability could have been even better if food had been given as part of the primaquine treatment itself. The low level of side effects noticed and the lack of any notable reduction in Hb levels after 7 days of concurrent high-dose primaquine (0.5 mg/kg) and artesunate daily treatment indicate that the combination of these two drugs is safe and that primaquine can be safely given to G6PD-normal Melanesian children 1 to 5 years of age. Given the good tolerability in both cohorts, it might be possible to investigate shorter courses (<14 days) of higher doses of primaquine (>0.5 mg/kg) that might improve compliance with primaquine treatment (2). While further pediatric safety studies need to be conducted in other populations, the WHO recommendations for primaquine use should be regularly reviewed to assess the adequacy of primaquine treatment in G6PD-normal patients >1 year of age.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Baird JK, Hoffman SL. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336–1345 [DOI] [PubMed] [Google Scholar]
- 2. Baird JK, Rieckmann KH. 2003. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 19:115–120 [DOI] [PubMed] [Google Scholar]
- 3. Cappellini MD, Fiorelli G. 2008. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371:64–74 [DOI] [PubMed] [Google Scholar]
- 4. Clayman CB, et al. 1952. Toxicity of primaquine in Caucasians. JAMA 149:1563–1568 [DOI] [PubMed] [Google Scholar]
- 5. Fryauff DJ, et al. 1995. Randomised placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet 346:1190–1193 [DOI] [PubMed] [Google Scholar]
- 6. Genton B, et al. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 5:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karyana M, et al. 2008. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar. J. 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin E, et al. 2010. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One 5:e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mueller I, et al. 2009. High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar. J. 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shekalaghe SA, et al. 2010. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrob. Agents Chemother. 54:1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf [Google Scholar]
- 12. Youngster I, et al. 2010. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 33:713–726 [DOI] [PubMed] [Google Scholar]