Abstract
The microdomain of the integral membrane protein (MIMP) has been shown to adhere to mucin and to antagonize the adhesion of enteropathogenic Escherichia coli (EPEC) to epithelial cells; however, the mechanism has not been fully elucidated. In this study, we further identified the receptor of MIMP on NCM460 cells and investigated the mechanism (the p38 mitogen-activated protein kinase [MAPK] pathway) following the interaction of MIMP and its corresponding receptor, mannose receptor. We first identified the target receptor of MIMP on the surfaces of NCM460 cells using immunoprecipitation-mass spectrometry technology. We also verified the mannose receptor and examined the degradation and activation of the p38 MAPK signaling pathway. The results indicated that MIMP adhered to NCM460 cells by binding to the mannose receptor and inhibited the phosphorylation of p38 MAPK stimulated after EPEC infection via inhibition of the Toll-like receptor 5 pathway. These findings indicated that MIMPs relieve the injury of NCM460 cells after enteropathogenic E. coli infection through the mannose receptor and inhibition of the p38 MAPK signaling pathway, both of which may therefore be potential therapeutic targets for intestinal diseases, such as inflammatory bowel disease.
INTRODUCTION
There is a widespread assumption that bacterial species that closely adhere to the mucosa are more likely to play a contributory role in major intestinal infection and inflammatory bowel diseases (IBD) (40, 43). During such diseases, the homeostasis of the gut mucosa is altered, leading to gut barrier dysfunction (2, 47). IBD is characterized by increased numbers of Bacteroides spp., adherent/invasive Escherichia coli, enterococci, and Clostridium perfringens and reduced numbers of Bifidobacterium and Lactobacillus species in the gut (16, 22, 53). Maintaining normal epithelial barrier function is important in resisting the invasion of pathogens. Over the past few decades, some studies have reported that treatment with probiotics, including lactic acid bacteria, has been proven to be effective in patients with colonic inflammation (26, 35, 38, 42). Bacteria belonging to the genus Lactobacillus that reside in the mammalian gut play an important role in maintaining homeostasis of the gut flora by adhering to and colonizing the intestinal mucosa, where they compete with pathogenic bacteria (8, 34, 36). However, the underlying molecular mechanisms of probiotics have not been fully elucidated (13).
Surface layer proteins (SLPs), including numerous identical protein/glycoprotein subunits (3), are ubiquitous cell envelope structures of lactobacilli that can spontaneously form regular layers either in a solution or on a solid support under certain conditions (46, 51). It is reported that SLPs have been investigated in regard to the interaction of lactobacilli and the intestinal epithelium (6, 10, 15). SLPs play an important part in the binding process of lactobacilli to the intestinal epithelial cells (IECs) (5, 10, 57). However, only a few studies (10, 57) have investigated the binding process of lactobacillus SLPs, which may be because lactobacillus SLPs are difficult to isolate, purify, and synthesize (59). Therefore, the roles of SLPs in the process of adhesion to the intestine remain poorly understood (24).
Our previous studies have demonstrated that Lactobacillus plantarum was able to prevent enteroinvasive E. coli (EIEC)-induced damage and then confer therapeutic effects on colorectal intestinal epithelial (Caco-2) cells (41) by competitive inhibitive effects of EIEC, immunologic regulation of the reaction of dendritic cells and T cell differentiation (32), and protection of tight junction (TJ) protein structure and function both in vivo and in vitro (31, 33). Furthermore, a small functional protein domain, the microdomain of the integral membrane protein (MIMP), was identified, and its adhesion activity was verified in a normal human colon mucosal epithelial cell line, NCM460. Our recent study (28) also described the SLP functional domain, indicating that the integral membrane protein (IMP), which was responsible for adhesion to human IECs, could mediate the adherence of L. plantarum strain CGMCC 1258 to IECs. Moreover, MIMP (IMP515 to -575), the small active-domain adhesive protein within IMP, was further purified and successfully characterized using bioinformatics and molecular techniques. Competitive-inhibition assays were performed in this study to further confirm the ability of MIMP to interfere with enteropathogenic E. coli (EPEC) adherence to NCM460 (27). Our previous data (27) also confirmed that MIMP reduced intestinal permeability and restored the expression and distribution of TJ proteins in both NCM460 cell monolayers and interleukin-10−/− (IL-10−/−) mice. Moreover, MIMP stimulated the expression of anti-inflammatory cytokines in colonic mucosa and attenuated colitis in IL-10−/− mice (27). Therefore, our above-mentioned findings indicated that MIMP was the main L. plantarum component that conferred protective effects on IECs, establishing a foundation from which the anti-infective role of MIMP could be further defined. To further analyze the protective effects of MIMP on IECs, we established transiently MIMP-expressing NCM460 cells (NCM460/MIMP) that possessed EPEC anti-infective properties related to the activation of protein kinase C-η and occludin phosphorylation (25).
The aim of our present study was to identify the receptor of MIMP on NCM460 cells and to further investigate the mechanism (the p38 mitogen-activated protein kinase [MAPK] pathway) following the interaction of MIMP and its corresponding receptor in order to explain the molecular mechanism of MIMP in protecting against barrier dysfunction of NCM460 cells.
MATERIALS AND METHODS
MIMP, antibodies, and major reagents.
Recombinant MIMP was expressed in E. coli as described in our previous studies (27, 28). Anti-MIMP antibodies were prepared by immunizing an adult male New Zealand White rabbit with MIMP. Briefly, 0.3 ml (2 g/liter) of the purified protein emulsified in complete Freund's adjuvant was injected intraperitoneally at multiple points on the back and groin (200 μg at each point) for the first immunization, and half the dose in incomplete Freund's adjuvant was used for the booster immunization after 2 weeks and repeated two more times. Fourteen days after the last booster, serum was collected and purified by affinity chromatography on a 1-ml MIMP-Sepharose 4B column and stored at −20°C until use. Primary antibodies, including those against mannose receptor (MR), occludin, phosphorylated p38 MAPK, p38 MAPK, and actin, were purchased from Santa Cruz Biotechnology (California). Secondary antibodies, including horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and HRP-conjugated anti-human IgG antibodies, were purchased from Sigma (Missouri). Dulbecco's modified Eagle's medium (DMEM), Iscove's modified Dulbecco's medium, fetal bovine serum (FBS), and TRIzol reagent were obtained from Gibco (California). EDTA solution, d-mannose, brefeldin A, and saponin were from Sigma-Aldrich (Steinheim, Germany). Anisomycin from Streptomyces griseolus (176880-MG) and p38 MAPK activator were purchased from Merck (KGaA, Darmstadt, Germany).
Bacterial strains, culture conditions, and the infection model.
The intestinal epithelial monolayers were treated with EPEC for 24 h in the presence or absence of MIMP, as described previously (27, 28). The EPEC strain ATCC 43887 (O111:NM; Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China) was grown in static DMEM at 37°C for 24 h to allow intimate adherence and pedestal formation. Quantification of bacterial density was measured at 600 nm (Beckman DU-50 spectrophotometer), along with the CFU. Furthermore, mannose was also added to prevent type 1 fimbria-mediated binding in a concentration-dependent manner (>80% inhibition in the presence of 3% mannose). NCM460 cells were purchased from Incell Corporation (San Antonio, TX) and cultured in M3 medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 95% humidified atmosphere with 5% CO2. Cells were passaged at preconfluent densities using 0.05% trypsin and 0.5 mm EDTA (Invitrogen, Carlsbad, CA) (29). NCM460 cells were passaged for 24 h before further treatment.
Quantitative real-time PCR.
Occludin gene expression was determined by quantitative real-time PCR. Total RNA was isolated from NCM460 cells in each group using TRIzol reagent, as previously described (21), followed by DNase I treatment. The quantity and quality of RNA was verified by the ratios of absorbance values at 260 and 280 nm and by visualization of the bands on agarose gels. For each sample, 600 ng of mRNA was used in the reverse transcription reaction (iScript kit; Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's specifications. Further analysis of the mRNA levels of each group was performed by real-time PCR with a light-cycling system (LightCycler; Roche Diagnostics GmbH, Mannheim, Germany). All values were expressed as a fold increase or decrease relative to the expression of actin. The sequences of the primers were as follows: occludin, F, 5′-GCAGCTACTGGACTCTACG-3′, and R, 5′-ATGGGACTGTCAACTCTTTC-3′; β-actin, F, 5′-CTCCATCCTGGCCTCGCTGT-3′, and R, 5′-GCTGTCACCTTCACCGTTCC-3′. All values are presented as the mean and standard deviation (SD).
Western blotting.
For Western blotting, proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Massachusetts) using a semidry electroblotter (Bio-Rad) for 120 min at 100 V. The membrane was then incubated with the appropriate primary antibody for 2 h at room temperature, washed three times (20 min each time) with Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBS-T buffer), and then incubated for 1 h with the appropriate HRP-conjugated secondary antibody in TBS-T buffer for 4 h at 4°C. The membrane was washed three times (60 min each time) with TBS-T buffer and developed by the enhanced chemiluminescence method (ECL kit; Pierce, Illinois) according to the manufacturer's instructions.
Fluorescent staining.
For the fluorescent staining of cells, NCM460 cell monolayers were cultured, treated with EPEC or MIMP according to the different groups, and then fixed in acetone-methanol (1:1) at 0°C for 5 min. Fluorescent staining of occludin protein was performed using a previously described method (41). Briefly, samples prepared as described above were permeabilized in 0.2% Triton X-100 and incubated with a primary antibody and corresponding fluorescein isothiocyanate (FITC)-conjugated specific secondary antibody in 3% nonfat milk. The fluorescence was then visualized by confocal laser scanning microscopy (CLSM) (MRC 1024; Bio-Rad).
Immunoprecipitation.
Ni-nitrilotriacetic acid (NTA) agarose beads were resuspended by alternately inverting and gently tapping the polypropylene column (Invitrogen). The 6×His-tagged recombinant MIMP protein was incubated with gentle agitation at room temperature in the prepared purification column for 1 h. The resin was then precipitated by gravity or low-speed centrifugation (800 × g), and the supernatant was carefully aspirated. The extracted membrane protein from the NCM460 cells was mixed with the resin-MIMP protein complex and incubated at 4°C overnight, followed by three washes with wash buffer (8 M urea, 20 mM sodium phosphate, pH 6.0, 500 mM NaCl). The supernatant was stored at 4°C for SDS-PAGE analysis. A negative control was included that lacked MIMP protein.
SDS-PAGE and MS.
Immunoprecipitation samples were mixed with loading buffer containing SDS and beta-mercaptoethanol, boiled for 3 min, centrifuged, and loaded onto 4 to 20% precast Novex Tris-glycine gels in a Miniprotean II apparatus (Bio-Rad). The gels were minimally stained with Coomassie brilliant blue R-250. The molecular weight of the protein was determined by comparing its electrophoretic mobility with those of marker proteins. By comparison with the IgG bands, the unique band in the samples was excised from the SDS-PAGE gel and analyzed by liquid chromatography coupled with tandem mass spectrometry (LC–MS-MS). The MS data were initially analyzed with SEQUEST software (Thermo Fisher Scientific, San Jose, CA) and then with Trans-Proteomics Pipeline (TPP) software (Center for Systems Biology, Institute for Systems Biology, Seattle, WA) and FASTA software to further identify the protein.
Statistical analysis.
Statistical analyses were performed using the SPSS 10.0 system (SPSS Inc., Illinois). The SD between multiple groups was assumed to satisfy a normal distribution. Data were analyzed by one-way analysis of variance (ANOVA), assuming homogeneity of variance. The Dunnett t test was used for multiple comparisons between experimental and control groups. The Student-Newman-Keuls (SNK) t test was employed to examine differences between two sets of data or variables between two groups. A P value of <0.05 was considered to be statistically significant.
RESULTS
MIMP relieved the decreased occludin protein levels in EPEC-infected NCM460 cells.
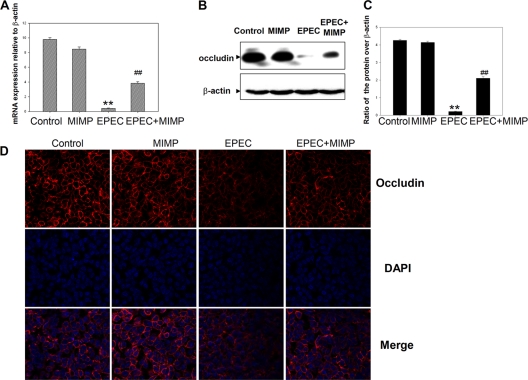
In comparison with the uninfected control cells, a significant decrease in occludin gene expression in NCM460 cells infected with EPEC was observed. Treatment with MIMP relieved the decrease in the occludin gene expression level induced by EPEC, similarly to uninfected control cells (Fig. 1A). The results of Western blot analysis showed that the occludin protein levels were decreased in NCM460 cells infected with EPEC (Fig. 1B) compared with the control cells. However, in NCM460 cells treated with MIMP, the occludin protein levels reached a higher level than in EPEC-infected cells. Relatively low levels of fluorescence of the occludin protein were evident in the NCM460 cells infected with EPEC (Fig. 1C). In NCM460 cell monolayers treated with MIMP before EPEC infection, the intensity and distribution of fluorescence appeared similar to those in the control cells. Both the mRNA and protein expression data confirmed that MIMP prevented the decrease in occludin protein levels induced by EPEC in NCM460 cells.
Fig 1.
Protective effect of MIMP on NCM460 cells infected with EPEC. (A) Occludin gene expression in each group. The data represent the mean values and SD. (B) Expression of the occludin protein in each group was examined by Western blotting. (C) Quantitative analysis of the occludin protein levels in the different groups shown in panel B. The data from each time point represent the mean value and SD obtained from individual NCM460 monolayers. (D) Expression of the occludin protein in each group was examined by fluorescent staining. Columns from left to right: control cells, control cells plus MIMP, control cells plus EPEC, and control cells plus EPEC plus MIMP. **, P < 0.001 compared with the control group; ##, P < 0.001 compared with the EPEC group.
Identification of the extracted SLP responsible for adhesion by MIMP.
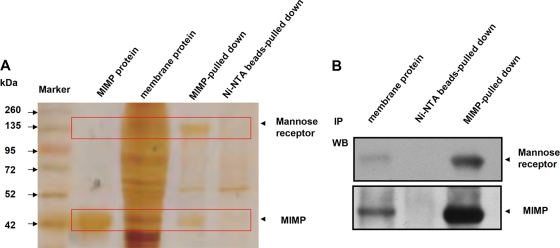
The membrane proteins were isolated from the NCM460 cells incubated with MIMP, and protein fractions were isolated using the MIMP antibody and beads. The proteins in each group were separated and detected by SDS-PAGE. There were several bands of interest with different immunoprecipitates (IPs) pulled down by the MIMP antibody compared with the proteins pulled down by IgG (Fig. 2A). A band with a molecular mass of 160 to 190 kDa, the most strongly reacting single band, was excised from the gel and subjected to LC–MS-MS, followed by analysis using the SEQUEST, Trans-Proteomics Pipeline, and FASTA software, as described in Materials and Methods. According to the ProteinProphet algorithm (29), the putative protein was identified as mannose receptor (see Figure S1 in the supplemental material). Further analysis of this band by Western blotting identified the putative target protein using a mannose receptor antibody (Fig. 2B).
Fig 2.
Identification of the extracted SLP responsible for adhesion by MIMP in NCM460 cells infected with EPEC. (A) Lanes (from left to right): molecular marker (kDa), MIMP protein, total membrane protein, and the IPs stained after SDS-PAGE analysis. (B) Proteins excised from the gel were detected by Western blot (WB) analysis as mannose receptor (top) and MIMP (bottom). Lanes (from left to right): extracted SLP, IPs pulled down by the Ni-NTA beads, and IPs pulled down by the MIMP–Ni-NTA beads.
Transduction signal pathway following MIMP adhesion.
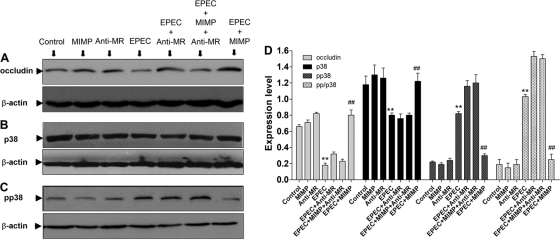
As shown by the Western blot results for the EPEC-infected cells compared with those for the control cells, EPEC decreased the occludin protein levels (Fig. 3A). The occludin protein level was similar to that of control cells when the EPEC-infected cells were treated with MIMP (Fig. 3A). However, when NCM460 cells were treated with EPEC, MIMP, and anti-MR, the occludin protein level was similar to that in cells treated with EPEC alone (Fig. 3A). The anti-MR antibody abolished the protective effect of MIMP on the TJ structure in NCM460 cells. The expression of p38 MAPK and phosphorylated p38 MAPK was assessed to determine whether the signal pathway was activated. As shown in Fig. 3B, infection with EPEC led to a slight decrease in p38 MAPK, and the addition of MIMP could reverse this effect (Fig. 3B). The anti-MR antibody abolished the effect of MIMP (Fig. 3B). The phosphorylation of p38 MAPK increased significantly after infection with EPEC (Fig. 3C), and the addition of MIMP abolished this effect (Fig. 3C). However, after the anti-MR was added, the effect of MIMP was also abolished (Fig. 3C). In Fig. 3D, we investigated the ratio of phosphorylated p38 to total p38 (pp/p38) for each group and found that pp/p38 was enhanced following the addition of EPEC, which could be relieved by MIMP. The effect of MIMP could also be abolished by anti-MR (P < 0.001) (Fig. 3D). Figure 3A to D indicate that MIMP abrogates EPEC-induced occludin degradation and p38 activation through a mechanism that requires the MR.
Fig 3.
Influence of occludin, p38 MAPK, and phosphorylated p38 (pp38) MAPK expression on blocking of the MR. (A to C) Western blotting of occludin protein (A), p38 MAPK (B), and pp38 MAPK (C). (D) Quantitative analysis of different groups with the p/pp38 ratio. The data at each time point represent the mean value and SD obtained from 3 individual NCM460 monolayers. Among columns with the same shading: **, P < 0.001 compared with the control group; ##, P < 0.001 compared with the EPEC group.
MIMP blocks the TLR5 pathway through the mannose receptor.
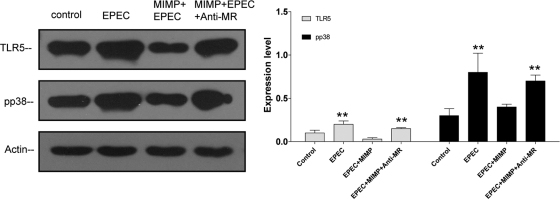
The expression levels of Toll-like receptor 5 (TLR5) and p38 were determined by Western blotting. The results showed that EPEC enhanced the expression level of phosphorylated p38, while addition of MIMP prevented the change of phosphorylated p38 and the expression of TLR5 was inhibited. However, after anti-MR was added, the effects of MIMP were abolished (Fig. 4).
Fig 4.
MIMP blocks the TLR5 pathway through the mannose receptor. (A) EPEC enhanced the expression level of phosphorylated p38, while addition of MIMP prevented the change in phosphorylated p38 and the expression of TLR5 was inhibited. However, after anti-MR was added, the effects of MIMP were abolished. (B) Quantitative analysis of the different groups. The data at each time point represent the mean value and SD obtained from 3 individual NCM460 monolayers. **, P < 0.001 compared with the control group.
The effect of blocking the transduction signal pathways, as detected by fluorescence staining.
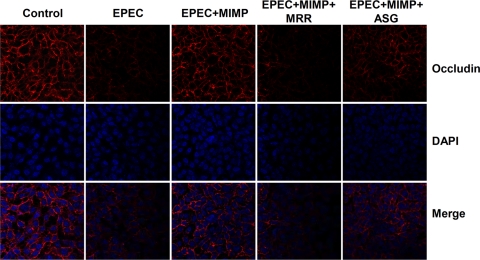
Anisomycin, a bacterial antibiotic isolated from S. griseolus, is an extremely potent activator of p38 MAPK. In our study, we also used anisomycin to activate the p38 MAPK pathway to investigate the protective effects of MIMP. The occludin protein was distributed throughout the cytoskeleton in the NCM460 cell monolayers not infected with EPEC (Fig. 5, column 1 from the left). As shown in Fig. 4B, the intensity of fluorescence of the NCM460 cells treated with EPEC was lower than that of the control cells. MIMP increased the intensity and distribution of fluorescence to a level similar to that in the control cells (Fig. 5, column 3). The anti-MR antibody inhibited the ability of MIMP to maintain the intensity and distribution of fluorescence at normal levels (Fig. 5, column 4). The intensity of fluorescence in cells treated with the p38 MAPK activator was lower than that in the control cells, indicating that anisomycin from S. griseolus could partially abolish the protective effect of MIMP by the activation of p38 MAPK (Fig. 5, column 5).
Fig 5.
Protective mechanism of MIMP in NCM460 cells infected with EPEC as detected by fluorescent staining. Columns from left to right: normal NCM460 cells, normal NCM460 cells plus EPEC, normal NCM460 cells plus EPEC plus MIMP, normal NCM460 cells plus EPEC plus MIMP plus MR antibody (MRR), and normal NCM460 cells plus EPEC plus MIMP plus anisomycin from S. griseolus (ASG). The images were collected in 1-μm increments beginning at the apical aspect of the monolayers and optically sectioning to the basolateral membrane. Original magnification, ×630.
DISCUSSION
It has been established that probiotics have important protective effects on the intestinal barrier function (17, 56, 58). However, the SLP potency of clinical probiotics is relatively low, and the use of large doses of probiotics may increase the risk of translocation of lactic acid bacteria, especially for clinical patients with poor gastrointestinal function (14, 23, 50). Furthermore, when antibiotics are used at the same time, the antibiotics also cause damage to the probiotics, which can reduce their therapeutic effects. In recent years, studies on the adhesion mechanism of lactobacilli have shown that SLP plays an important role in their adhesion to human IECs through their protective biological function (30). It has been reported that SLP not only can mediate the adhesion of bacteria to target cells, but also can stimulate intracellular signaling transduction pathways and block bacterial adhesion by competitive inhibition of receptor activity with a similar structure (30). However, current research on SLP has focused mainly on the high-molecular-weight proteins directly, instead of the highly effective microdomain protein of SLP. As a result of larger protein fragments, an unclear domain, and an undefined mechanism, the disease resistance of SLP is still weak, and the clinical application of SLP is still limited. Our recent study showed that the SLP functional domain MIMP, which was responsible for adhesion to human IECs, could mediate the adherence of L. plantarum to IECs (28). Moreover, we further purified and successfully characterized the biological function of MIMP (27).
Occludin (but not ZO-1, claudin-1, or cell adhesion proteins) has been observed to be downregulated even in non-actively inflamed tissue in ulcerative colitis (1, 19, 52). Trinitrobenzene sulfonic acid (TNBS)/ethanol-induced colitis in rats caused disruption of normal immunofluorescent staining patterns of intestinal epithelial occludin (but not ZO-1 or cingulin), even though freeze fracture strand patterns seen in electron micrographs were normal (11). Another experimental colitis model, however, the IL-2 knockout mouse, showed increased occludin protein levels and increased barrier function in the colon (4). Strategies to prevent and/or reverse occludin downregulation may be an important therapeutic target. L. plantarum was able to antagonize the adhesion of EPEC, as it adheres to NCM460 colonic cells. In previous studies, we determined the region of MIMP responsible for maintaining intestinal epithelial barrier function and altered TJ structure, for inhibiting intestinal permeability, and for decreasing the production of proinflammatory cytokines (27). In our study, we used quantitative real-time PCR, Western blotting, and fluorescent staining to verify the protective effects of MIMP on NCM460 cells after infection with EPEC. In comparison with the uninfected control cells, a significant decrease in occludin gene expression in NCM460 cells infected with EPEC was observed (Fig. 1A, EPEC). Treatment with MIMP prevented the decrease in the gene expression level induced by EPEC, similarly to uninfected control cells (Fig. 1A, EPEC + MIMP compared with the EPEC group; P < 0.001). The results of Western blot analysis showed that the occludin protein levels had decreased in NCM460 cells infected with EPEC (Fig. 1B, EPEC) compared with the control cells. However, in NCM460 cells treated with MIMP, the occludin protein levels reached a higher level than in EPEC-infected cells. Relatively low levels of fluorescence of the occludin protein were evident in the NCM460 cell infected with EPEC (Fig. 1C, EPEC). In NCM460 cell monolayers treated with MIMP before EPEC infection, the intensity and distribution of fluorescence appeared similar to those in the control cells. Both the mRNA and protein expression data confirmed that MIMP prevented the decrease in occludin protein levels induced by EPEC in NCM460 cells.
To identify the MIMP receptor on the surfaces of the NCM460 cells infected with EPEC, we employed immunoprecipitation followed by mass spectrometry and used the data to analyze protein complex components and cellular protein networks. We purified a novel component of the MIMP complex, mannose receptor, and analyzed the composition of this protein to demonstrate core complex modules and several novel subcomplex interactions. Mannose receptor is a C-type lectin carbohydrate-binding protein. The structure of mannose receptor allows it to bind to high-mannose structures on the surfaces of potentially pathogenic viruses and bacteria so that they can be neutralized by phagocytic engulfment or be engulfed by the cell (12, 45). The function of this receptor is to recognize complex carbohydrates located on glycoproteins that are a part of many different biological processes, such as cell-cell recognition, serum glycoprotein turnover, and the neutralization of pathogens, mediating the endocytosis of glycoproteins by macrophages. Antigens are targeted to dendritic cells through dendritic-cell-specific receptors, and mannose receptor is one of these targets (49). By identifying the receptor for MIMP and the subsequent transduction signal pathways in this study, we found that MIMP abrogates EPEC-induced occludin degradation and p38 activation through a mechanism that requires the MR. Because there is strong evidence that p38 is upregulated in response to EPEC flagellin, we further investigated the effects of MIMP on TLR5 signal. The results showed that MIMP is capable of blocking the TLR5 pathway through the mannose receptor and then decreasing the expression level of phosphorylated p38.
p38 MAPK is a Ser/Thr kinase belonging to the family of MAPKs. The signaling pathways induced by MAPKs are responsive to stress stimuli, such as cytokines, UV irradiation, heat shock, and cell apoptosis. Activation of protein kinase C (PKC) by exposure of cultured human corneal epithelial cells to phorbol myristate acetate (PMA) (a PKC activator) has been shown to result in an increase in paracellular permeability (9). However, when the cells were treated with PMA in the presence of a specific inhibitor of MAPK kinase, all barrier characteristics were maintained, which indicated that PKC modulates TJ function via the activation of MAPK. This was further supported by the finding that occludin distribution was altered in these cells (54). Therefore, p38 MAPK is closely related to TJ proteins. The p38 MAPK pathway is important in macrophages and polymorphonuclear neutrophils (37, 44), and inflammatory stimuli, such as lipopolysaccharide and N-formyl-methionyl-leucyl-phenylalanine, also activate p38 MAPK (7, 39, 48, 60). p38 MAPK has been demonstrated to be involved in the upregulation of expression of several inflammatory genes, such as those encoding inducible nitric oxide synthase (iNOS), IL-8, and IL-6 (20, 55). As for the mechanism of MIMP stimulation of the p38 MAPK pathway, it has been reported that p38 is upregulated in response to EPEC flagellin (18). In our study, we further verified the p38 MAPK signal pathway following the interaction of MIMP and MR. We treated NCM460 cells with anisomycin from S. griseolus, the p38 MAPK activator, and found that it could partially abolish the protective effect of MIMP, which further verified that MIMP might exert its protective effects via the p38 MAPK signal pathway.
Our study indicated MIMP could interact with MR and then exert its protective effects on NCM460 cells. However, it has been reported that MR mainly interacts with mannose residues, such as the structures on the surfaces of potentially pathogenic viruses, bacteria, and fungi. Therefore, one limitation of our study is that the structures of MIMP and MR may not conjugate, and the mechanism of binding between MIMP and MR also needs to be further investigated. Taken together, our findings enhance our understanding of the therapeutic effects of MIMP on the cellular and molecular mechanisms involved in intestinal barrier dysfunction and intestinal inflammation during some intestinal diseases, such as IBD.
Conclusion.
MIMP relieves the injury to NCM460 cells after EPEC infection through the mannose receptor and the p38 MAPK signaling pathway, which may be potential therapeutic targets for intestinal barrier dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by research grants from the National Natural Science Foundation of China (no. 81070293 and 81100255).
There is no conflict of interest.
Authors' contributions: Zhihua Liu and Huanlong Qin conceived the study and participated in its design and coordination; acquired the funding; and wrote, revised, and approved the final manuscript. Zhihua Liu carried out all the experiments (except for those described below), data collection, statistical analyses, and paper preparation. Mary Pat Moyer provided NCM460 cells and revised the manuscript. Jianjun Yang, Peng Zhang, Yanlei Ma, and Chenzhang Shi carried out the quantitative real-time PCR, Western blotting, and fluorescence staining. All authors read and approved the final manuscript.
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://iai.asm.org.
REFERENCES
- 1. Alemka A, et al. 2010. Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect. Immun. 78:2812–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alverdy JC, Laughlin RS, Wu L. 2003. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit. Care Med. 31:598–607 [DOI] [PubMed] [Google Scholar]
- 3. Avall-Jaaskelainen S, et al. 2008. Identification and characterization of domains responsible for self-assembly and cell wall binding of the surface layer protein of Lactobacillus brevis ATCC 8287. BMC Microbiol. 8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barmeyer C, et al. 2004. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G244–G252 [DOI] [PubMed] [Google Scholar]
- 5. Bernet MF, Brassart D, Neeser JR, Servin AL. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buck BL, Altermann E, Svingerud T, Klaenhammer TR. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 71:8344–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Detmers PA, et al. 1998. Role of stress-activated mitogen-activated protein kinase (p38) in beta 2-integrin-dependent neutrophil adhesion and the adhesion-dependent oxidative burst. J. Immunol. 161:1921–1929 [PubMed] [Google Scholar]
- 8. Eaton KA, Honkala A, Auchtung TA, Britton RA. 2011. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect. Immun. 79:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franchimont N, Rydziel S, Canalis E. 1997. Interleukin 6 is autoregulated by transcriptional mechanisms in cultures of rat osteoblastic cells. J. Clin. Invest. 100:1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francius G, et al. 2009. Stretching polysaccharides on live cells using single molecule force spectroscopy. Nat. Protoc. 4:939–946 [DOI] [PubMed] [Google Scholar]
- 11. Fries W, et al. 1999. Experimental colitis increases small intestine permeability in the rat. Lab. Invest. 79:49–57 [PubMed] [Google Scholar]
- 12. Hattori T, et al. 2010. Genetic variants in mannose receptor gene (MRC1) confer susceptibility to increased risk of sarcoidosis. BMC Med. Genet. 11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunter CJ, et al. 2009. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect. Immun. 77:1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Ishibashi N, Yamazaki S. 2001. Probiotics and safety. Am. J. Clin. Nutr. 73:465S–470S [DOI] [PubMed] [Google Scholar]
- 15. Johnson-Henry KC, Hagen KE, Gordonpour M, Tompkins TA, Sherman PM. 2007. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 9:356–367 [DOI] [PubMed] [Google Scholar]
- 16. Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 17. Kerac M, et al. 2009. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet 374:136–144 [DOI] [PubMed] [Google Scholar]
- 18. Khan MA, et al. 2008. Flagellin-dependent and -independent inflammatory responses following infection by enteropathogenic Escherichia coli and Citrobacter rodentium. Infect. Immun. 76:1410–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. 2001. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 159:2001–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larsen CM, et al. 1998. Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J. Biol. Chem. 273:15294–15300 [DOI] [PubMed] [Google Scholar]
- 21. Lelievre V, Hu Z, Byun JY, Ioffe Y, Waschek JA. 2002. Fibroblast growth factor-2 converts PACAP growth action on embryonic hindbrain precursors from stimulation to inhibition. J. Neurosci. Res. 67:566–573 [DOI] [PubMed] [Google Scholar]
- 22. Lepage P, et al. 2008. Dysbiosis in inflammatory bowel disease: a role for bacteriophages? Gut 57:424–425 [DOI] [PubMed] [Google Scholar]
- 23. Liong MT. 2008. Safety of probiotics: translocation and infection. Nutr. Rev. 66:192–202 [DOI] [PubMed] [Google Scholar]
- 24. Liu Z, Ma Y, Qin H. 2011. Potential prevention and treatment of intestinal barrier dysfunction using active components of lactobacillus. Ann. Surg. 254:832–833 [DOI] [PubMed] [Google Scholar]
- 25. Liu Z, et al. 2011. Expression of the Lactobacillus plantarum surface layer MIMP protein protected NCM460 epithelial cells from enteroinvasive Escherichia coli infection. Cell Physiol. Biochem. 27:99–108 [DOI] [PubMed] [Google Scholar]
- 26. Liu Z, et al. 2011. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—a double-blind study. Aliment Pharmacol. Ther. 33:50–63 [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, et al. 2012. Functional characterization of MIMP for its adhesion to the intestinal epithelium. Front. Biosci. 17:2106–2127 [DOI] [PubMed] [Google Scholar]
- 28. Liu Z, Shen T, Moyer MP, Qin H. 2012. Identification of the Lactobacillus SLP domain that binds gastric mucin. Front. Biosci. 17:2128–2143 [DOI] [PubMed] [Google Scholar]
- 29. Liu Z, Shen T, Zhang P, Ma Y, Qin H. 2011. Lactobacillus plantarum surface layer adhesive protein protects intestinal epithelial cells against tight junction injury induced by enteropathogenic Escherichia coli. Mol. Biol. Rep. 38:3471–3480 [DOI] [PubMed] [Google Scholar]
- 30. Liu Z, et al. 2012. Molecular regulation of the intestinal epithelial barrier: implication in human diseases. Front. Biosci. 17:2903–2909 [DOI] [PubMed] [Google Scholar]
- 31. Liu Z, et al. 2011. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the intestinal permeability. Mol. Biol. Rep. 38:1353–1361 [DOI] [PubMed] [Google Scholar]
- 32. Liu ZH, et al. 2011. Identification of DC-SIGN as the receptor during the interaction of Lactobacillus plantarum CGMCC 1258 and dendritic cells. World J. Microbiol. Biotechnol. 27:603–611 [Google Scholar]
- 33. Liu ZH, et al. 2010. Protective effects of Lactobacillus plantarum against epithelial barrier dysfunction of human colon cell line NCM460. World J. Gastroenterol. 16:5759–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madsen K, et al. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580–591 [DOI] [PubMed] [Google Scholar]
- 35. Makharia GK, et al. 2008. A randomized, double-blind, placebo-controlled trial of a probiotic preparation, Vsl#3, for the treatment of mild to moderate active ulcerative colitis. Gastroenterology 134:A99 [Google Scholar]
- 36. Matsuzaki T, Takagi A, Ikemura H, Matsuguchi T, Yokokura T. 2007. Intestinal microflora: probiotics and autoimmunity. J. Nutr. 137:798S–802S [DOI] [PubMed] [Google Scholar]
- 37. McLeish KR, Klein JB, Coxon PY, Head KZ, Ward RA. 1998. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. J. Leukoc. Biol. 64:835–844 [PubMed] [Google Scholar]
- 38. Miele E, et al. 2009. Effect of a probiotic preparation (VSL# 3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 104:437–443 [DOI] [PubMed] [Google Scholar]
- 39. Nick JA, et al. 1997. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J. Clin. Invest. 99:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nuding S, Fellermann K, Wehkamp J, Stange EF. 2007. Reduced mucosal antimicrobial activity in Crohn's disease of the colon. Gut 56:1240–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qin H, Zhang Z, Hang X, Jiang Y. 2009. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon ATR. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635–639 [DOI] [PubMed] [Google Scholar]
- 43. Rhodes JM. 2007. The role of Escherichia coli in inflammatory bowel disease. Gut 56:610–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rose DM, et al. 1997. Fc gamma receptor cross-linking activates p42, p38, and JNK/SAPK mitogen-activated protein kinases in murine macrophages: role for p42MAPK in Fc gamma receptor-stimulated TNF-alpha synthesis. J. Immunol. 158:3433–3438 [PubMed] [Google Scholar]
- 45. Royer PJ, et al. 2010. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J. Immunol. 185:1522–1531 [DOI] [PubMed] [Google Scholar]
- 46. Sara M, Sleytr UB. 2000. S-layer proteins. J. Bacteriol. 182:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sartor RB. 2004. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126:1620–1633 [DOI] [PubMed] [Google Scholar]
- 48. Sham HP, et al. 2011. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-kappaB and p38 mitogen-activated protein kinase activation. Infect. Immun. 79:3552–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shimizu K, Fujii S. 2008. An adjuvant role of in situ dendritic cells (DCs) in linking innate and adaptive immunity. Front. Biosci. 13:6193–6201 [DOI] [PubMed] [Google Scholar]
- 50. Simakachorn N, et al. 2011. Tolerance, safety, and effect on the faecal microbiota of an enteral formula supplemented with pre- and probiotics in critically ill children. J. Pediatr. Gastroenterol. Nutr. 53:174–181 [DOI] [PubMed] [Google Scholar]
- 51. Spurbeck RR, Arvidson CG. 2010. Lactobacillus jensenii surface-associated proteins inhibit Neisseria gonorrhoeae adherence to epithelial cells. Infect. Immun. 78:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. 2010. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect. Immun. 78:4958–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Swidsinski A, et al. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54 [DOI] [PubMed] [Google Scholar]
- 54. Ungureanu-Longrois D, et al. 1995. Induction of nitric oxide synthase activity by cytokines in ventricular myocytes is necessary but not sufficient to decrease contractile responsiveness to beta-adrenergic agonists. Circ. Res. 77:494–502 [DOI] [PubMed] [Google Scholar]
- 55. Vanden Berghe W, et al. 1998. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 273:3285–3290 [DOI] [PubMed] [Google Scholar]
- 56. van Zanten SV. 2010. ACP Journal Club: probiotics improve symptoms in adults with the irritable bowel syndrome. Ann. Intern. Med. 153:JC3–JC7 [DOI] [PubMed] [Google Scholar]
- 57. Wang B, et al. 2008. Identification of a surface protein from Lactobacillus reuteri JCM1081 that adheres to porcine gastric mucin and human enterocyte-like HT-29 cells. Curr. Microbiol. 57:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weizman Z. 2010. Probiotics therapy in acute childhood diarrhoea. Lancet 376:233. [DOI] [PubMed] [Google Scholar]
- 59. Wells JM, Mercenier A. 2008. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6:349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoon YM, et al. 2010. Bacteroides fragilis enterotoxin induces human beta-defensin-2 expression in intestinal epithelial cells via a mitogen-activated protein kinase/I kappaB kinase/NF-kappaB-dependent pathway. Infect. Immun. 78:2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.