Abstract
Antibodies to pneumococcal capsular polysaccharide (PPS) are required for PPS-based vaccine-mediated protection against Streptococcus pneumoniae. Previous work established that 1E2, a mouse IgG1 to PPS3 that does not induce serotype 3 (ST3) S. pneumoniae killing by phagocytes in vitro, protects mice from death after intranasal infection with ST3, but its efficacy was abrogated in FcγR (F common gamma receptor)-deficient mice. In this study, we determined whether 1E2 efficacy against pulmonary ST3 infection requires FcγRIII. 1E2 did not protect FcγRIII-deficient (FcγRIII−/−) mice. Studies of the mechanism of 1E2-mediated effects showed that it resulted in a marked reduction in lung inflammation in ST3-infected wild-type (Wt [C57BL/6]) mice that was abrogated in FcγRIII−/− mice. 1E2 had no effect on early bacterial clearance in the lungs of ST3-infected Wt, FcγRIIB−/−, or FcγRIII−/− mice, but it reduced levels of bacteremia and serum macrophage inflammatory protein-2) (MIP-2), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) in Wt and FcγRIIB−/− mice, strains in which it is protective. As previous work showed that neutrophils were dispensable for 1E2 efficacy, we investigated whether macrophages are required for 1E2 efficacy against intranasal infection with ST3 and found that its efficacy was abrogated in Wt mice depleted of macrophages intranasally. In vitro studies revealed that1E2 promoted ST3 internalization by naïve alveolar macrophages but did not induce early intracellular killing. Macrophages from 1E2-treated ST3-infected mice studied ex vivo exhibited more apoptosis than those from FcγRIII−/− mice. These findings suggest that 1E2 mediates protection against ST3 in mice by affecting the inflammatory response, perhaps in part via macrophage apoptosis, rather than by inducing early bacterial clearance.
INTRODUCTION
There are ample data that the induction of pneumococcal capsular polysaccharide (PPS) serotype (ST)-specific opsonic antibody (Ab) correlates with vaccine protection against invasive pneumococcal disease (41). However, ST-specific antibodies that mediate protection against pneumococcus in mouse models, but do not promote opsonophagocytic killing in vitro (9, 10, 18, 49), have been identified. Such antibodies include PPS3-specific human and mouse monoclonal antibodies (MAbs) that protect mice against lethal pneumonia and sepsis with ST3 (49). Understanding the mechanism(s) by which antibodies to PPS3 mediate protection is important. ST3 pneumonia is associated with a higher risk of death than other STs (29, 55), severe ST3 disease attributed to serotype replacement has been reported in children (4, 11), and to date, although data from the newly introduced 13-valent pneumococcal capsular polysaccharide-protein conjugate vaccine are not yet available, investigational ST3 conjugate vaccines failed to prevent ST3 mucosal disease (36).
The ability of antibodies of the IgG isotype to mediate phagocytosis depends on the availability of FcγR (F common gamma receptors). The murine FcγR family consists of three activating receptors, FcγRI, FcγRIII, and FcγRIV, which when cross-linked induce cellular activation, and an inhibitory receptor, FcγRIIB, which inhibits the activating signal (33). Activating receptors promote and the inhibitory receptor inhibits FcγR-dependent antigen internalization and phagocytosis (45). Murine FcγRI binds IgG2a; FcγRIIB binds IgG1, IgG2a, and IgG2b; FcγRIII binds IgG1, IgG2a, and IgG2b; and FcγRIV binds IgG2a and IgG2b (33). In a previous study, Tian et al. reported that PPS3-specific mouse IgG1 MAbs that do (7A9 and 5F6) and do not (1E2) promote phagocyte-mediated opsonophagocytic killing of ST3 in vitro were each able to protect wild-type C57BL/6 (Wt) mice against lethal intranasal infection with ST3 (49). The MAbs that promoted in vitro killing (7A9 and 5F6) required FcγRIIB and neutrophils to mediate protection, whereas the one that did not (1E2) required the FcR common gamma chain (FcγR) but not FcγRIIB or neutrophils (49). Given that 1E2 requires an activating FcγR to mediate protection and FcγIII is the activating FcγR to which mouse IgG1 binds (2), we determined whether the efficacy of 1E2 against ST3 pneumonia depends on FcγRIII.
MATERIALS AND METHODS
Streptococcus pneumoniae and PPS.
The ST3 S. pneumoniae WU2 strain was grown in tryptic soy broth (TSB) to mid-log phase as described previously (49). WU2 has been used extensively to study the host response to and survival after infection with ST3 in mice (6, 26, 30, 37, 48, 49, 54). Purified PPS3, isolated from strain 6303 and obtained from the American Type Culture Collection, was used for enzyme-linked immunosorbent assay (ELISA)-based analyses of MAb binding.
Mice.
Wt C57BL/6 male mice (National Cancer Institute) (6 to 8 weeks old) were used. FcγRIII-deficient (FcγRIII−/−) male and female mice (22) obtained from Jackson Laboratories and FcγRIIB-deficient (FcγRIIB−/−) male and female mice (46) obtained from Taconic Laboratories and bred in the Animal Institute of the Albert Einstein College of Medicine (AECOM) were used. All mouse experiments were conducted according to the rules, regulations, and ethical standards for animal use of the Animal Care and Use Committee of AECOM.
MAbs and F(ab′)2 Fragments of MAbs.
Mouse IgG1 MAbs to PPS3, 1E2, 7A9, and 5F6 were used in this study. Their production, PPS specificity, and efficacy were described previously (49). Each MAb has a different PPS3 specificity and protects Wt mice against intranasal (49) and intraperitoneal (19) infection with ST3 (strain WU2) in Wt mice. None of the MAbs required complement to mediate protection in Wt mice (49). An IgG1 MAb that binds PPS8, 31B12 (56), was used as an isotype control.
MAb F(ab′)2 fragments were produced and purified with a mouse IgG1 F(ab′)2 preparation kit as described by the manufacturer (Pierce). The purity of the F(ab′)2s was analyzed by nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining: neither an Fc fragment nor an IgG1 protein band was detected for either MAb (data not shown). Protein concentrations of F(ab′)2 fragments were determined by protein assay as described by the manufacturer (Bio-Rad). F(ab′)2 fragments were sterile filtered, and aliquots were stored at −80°C. The antigen binding capability of F(ab′)2 fragments to PPS3 was determined by ELISA as previously described (49). F(ab′)2 fragments of both MAbs bound to PPS3 (data not shown).
Pneumococcal infection.
Mice were infected intranasally with ST3 (WU2) as previously described (49). For MAb protection experiments, 10 μg of purified MAb was diluted in phosphate-buffered saline (PBS) and 100 μl given intraperitoneally to mice 2 h before intranasal infection with 108 CFU ST3 as previously described (49). Inocula were confirmed by CFU on Trypticase soy agar with 5% sheep's blood plates (BD), plated before and after infection. For all studies that did not evaluate survival, groups of 4 to 14 mice were infected intranasally with 2 × 107 CFU of ST3 2 h after intraperitoneal administration of 10 μg of the PPS3 and control MAbs. Mice were killed 24 h after infection.
Lung and blood bacterial burden.
Mice were anesthetized and bled from the retro-orbital plexus by the use of heparinized hematocrit capillary tubes (Fisher Scientific) and killed by cervical dislocation, and the lungs were aseptically removed and homogenized in Hanks balanced salt solution. Samples were serially diluted in TSB and plated onto blood agar plates. The plates were incubated for 18 h at 37°C in 5% CO2, and then the number of CFU was counted.
Measurement of cytokine levels in the lungs and blood.
Cytokine levels were measured as described previously (10, 40). Sera were separated from clotted blood by centrifugation at 3,000 × g for 30 min. ELISA Duoset kits for macrophage inflammatory protein-2 (MIP-2), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (R&D Systems) were used for cytokine determinations according to the manufacturer's protocols. These mediators were chosen because they are increased by FcγRIII-mediated phagocytosis (21), in models of pneumococcal pneumonia and sepsis (13, 14, 24, 27, 54) and pneumococcal phagocytosis in S. pneumoniae-infected inhibitory FcγR (FcγRIIB)-deficient mice (12).
Histopathology.
Mice were infected as described above and killed 24 h after infection. Lungs were inflated with buffered formalin for 48 h, removed, and paraffin embedded. Thin sections were stained with hematoxylin and eosin (H&E) and evaluated by a veterinary pathologist.
Alveolar macrophage depletion.
To deplete alveolar macrophages, Wt mice were given a dose of 1 mg liposome-encapsulated clodronate intranasally as described for other studies (25, 42, 53) 2 days before infection. Liposomes without clodronate were used as a control. Clodronate was a gift of Roche Diagnostics GmbH and encapsulated in liposomes as previously described (51). Macrophage depletion was confirmed by performing bronchial alveolar lavage (see details below) on several “test” mice, staining the samples with F4/80-fluorescein isothiocyanate and determining percent expression of F4/80 on a FACScan gating on F4/80+ cells. This analysis revealed <20% macrophages in clodronate-liposome-treated compared to control mice.
Bacterial uptake and killing assay.
These experiments were performed as described previously (58) with some modifications. ST3 was incubated with 1 μg/ml of the PPS3 or control MAbs for 30 min at 37°C in veronal buffer (VB) (Lonza). Alveolar macrophages were isolated from mice by bronchial lavage using 10 ml per mouse through an intratracheal catheter with PBS supplemented with 0.6 mM EDTA. Lavage fluids were pooled and centrifuged at 300 × g for 7 min; fluids contained 97% monocytes/macrophages by morphology on trypan blue-stained cells counted in a hemocytometer. PPS3/MAb mixtures were then incubated for 1 h at 37°C with 10% mouse serum and naïve Wt or FcγRIII−/− alveolar macrophages with a macrophage-to-ST3 (E:T) ratio of 1:10, after which the mixtures were washed with VB to remove unbound bacteria. Then, cold water at pH 10.5 was added to part of the mixture (aliquot A), processed with a vortexer for 1 min, and incubated for 30 min at room temperature to lyse the macrophages. The nonlysed mixture was then treated with 100 μg/ml of gentamicin for 15 min at 37°C to kill extracellular bacteria and divided in half (aliquots B and C). After washing, aliquot B was lysed with water and aliquot C was incubated for an additional hour and then lysed with water. Experiments were also performed with Wt macrophages in which aliquot C was incubated for 2 h and with FcγRIII−/− macrophages with 1-h incubations as described above. Each lysed aliquot was diluted in VB, plated on sheep blood agar plates, and incubated at 37°C in 5% CO2 overnight. The total number of bound (extra- and intracellular) bacteria was defined as aliquot A. The total number of bacteria internalized was defined as aliquot B, and the number of bacteria that were killed by the macrophages was determined by subtracting CFU of aliquot C from aliquot B.
Cellular apoptosis.
Apoptosis of alveolar macrophages obtained from MAb-treated, infected Wt and FcγRIII−/− mice was determined using annexin V and 7-aminoactinomycin D (7-AAD) staining and an annexin V kit (BD) as described previously (27) according to the manufacturer's instructions. F4/80 fluorescein isothiocyanate was added to the samples with annexin V allophycocyanin and 7-AAD according to the manufacturer's instructions. Samples were run on a FACScan and analyzed with FlowJo software by gating on cell size using forward/side scatter and then F4/80 positivity. F4/80-positive (F4/80+) cells that stained as positive for annexin V and negative for 7-AAD were considered apoptotic, and 7-ADD+ cells were considered necrotic.
Statistical analysis.
Mouse survival was evaluated statistically by Kaplan-Meier plotting and the log rank test as described previously (49). The levels of blood and lung CFU and cytokines were analyzed using one-way analysis of variance (ANOVA) with Dunn's multiple-comparison test. Comparisons of the effects of the MAbs in the macrophage uptake and apoptosis assays were performed using one-way ANOVA with Bonferroni's multiple-comparison test. All statistical analyses were performed using Prism (GraphPad Software). A P value of less than 0.05 was considered statistically significant.
RESULTS
Effect of MAb on survival in mice.
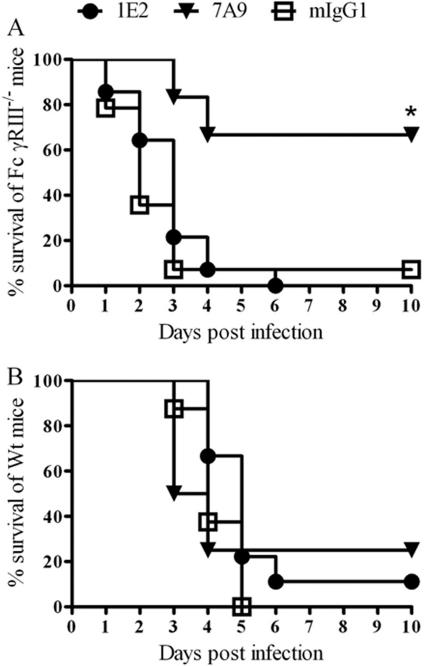
A previous study established that MAbs 1E2 and 7A9 (and 5F6) protect Wt (C57BL/6) mice against lethal intranasal infection with ST3 and that 1E2 efficacy required the common Fcγ chain (49). Hence, this study was undertaken to determine whether the efficacy of 1E2 requires the presence of FcγRIII. As shown in Fig. 1A, ST3-infected FcγRIII−/− mice were protected from death by 7A9 but not 1E2 (P < 0.01, comparing 7A9 to control MAb or 1E2). F(ab′)2 fragments of 7A9 and 1E2 did not protect ST3-infected Wt (Fig. 1B), FcγRIIB−/−, or FcγRIII−/− (data not shown) mice. Hence, 1E2 and 7A9 require an Fc region to mediate protection in the infection model used in this study.
Fig 1.
The dependence of PPS3-specific MAbs on FcγRIII and their Fc regions to protect mice against intranasal infection with 108 CFU ST3. (A) Survival of ST3-infected FcγRIII−/− mice after intraperitoneal immunization with PPS3 MAbs, 1E2 and 7A9, or a control MAb (mIgG1) 2 h prior to infection. *, P < 0.01, comparing 7A9- to 1E2- and mIgG1-treated mice. Kaplan-Meier log-rank test, n = 12 to 14 mice per group. (B) Survival of ST3-infected Wt mice after passive immunization with F(ab′)2 fragments of 1E2 and 7A9 2 h prior to infection. F(ab′)2 fragments did not mediate protection; there was no significant difference in survival between the groups. Kaplan-Meier log-rank test, n = 8 mice per group.
Bacterial burden in blood and lungs. (i) Lung CFU.
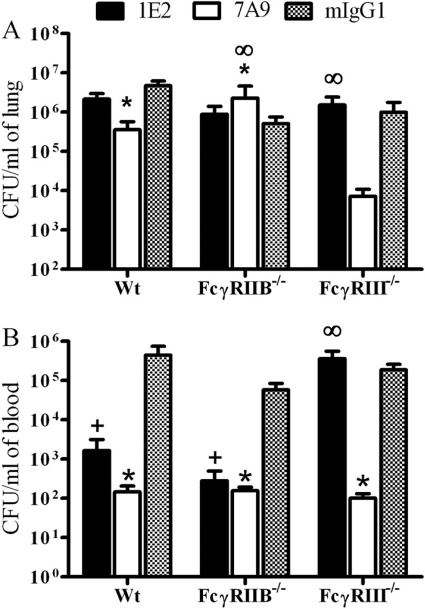
At 24 h after infection, 7A9-treated Wt and FcγRIII−/− mice had, respectively, fewer lung CFU than control mIgG1-treated and 1E2-FcγRIII−/− mice (P < 0.01 and P < 0.01, respectively) (Fig. 2A). Among ST3-infected FcγRIIB−/− mice, 7A9-treated mice had more lung CFU than control mIgG1-treated mice (P < 0.01) (Fig. 2A). For 1E2-treated mice, lung CFU in Wt mice and each FcγR-deficient strain were not statistically different from CFU in mIgG1-treated mice. Hence, lung CFU correlated with protection in 7A9- but not 1E2-treated mice 24 h after infection.
Fig 2.
Lung (A) and blood (B) of 1E2-, 7A9-, and mIgG1-treated Wt, FcγRIIB−/−, and FcγRIII−/− mice 24 h after intranasal infection with 2 × 107 CFU ST3. Lung CFU were lower among 7A9- than mIgG1-treated Wt and FcγRIII−/− mice. Blood CFU were lower in 1E2- than mIgG1-treated Wt and FcγRIIB−/− mice and in 7A9- than mIgG1-treated Wt, FcγRIIB−/−, and FcγRIII−/− mice. P < 0.05, comparing (+) 1E2- to mIgG1-treated mice, (*) 7A9- to mIgG1-treated mice, and (∞) 1E2- to 7A9-treated mice. One-way ANOVA with Dunn's multiple-comparison test. n = 8 to 14 mice per group. Error bars represent standard errors of the means.
(ii) Blood CFU.
At 24 h after infection, 7A9-treated Wt, FcγRIIB−/−, and FcγRIII−/− mice had fewer blood CFU than mIgG1-treated mice (P < 0.01, P < 0.01, and P < 0.01, respectively) (Fig. 2B). For 1E2-treated mice, Wt and FcγRIIB−/− mice had fewer blood CFU than mIgG1-treated mice (P < 0.01 and P < 0.01, respectively), but 1E2-treated FcγRIII−/− mice had more CFU than 7A9-treated FcγRIII−/− mice (P < 0.01). Hence, blood CFU correlated with protection for 1E2 and 7A9, except for 7A9-treated FcγRIIB−/− mice 24 h after infection.
Cytokine levels in the lungs and sera. (i) Lung cytokines.
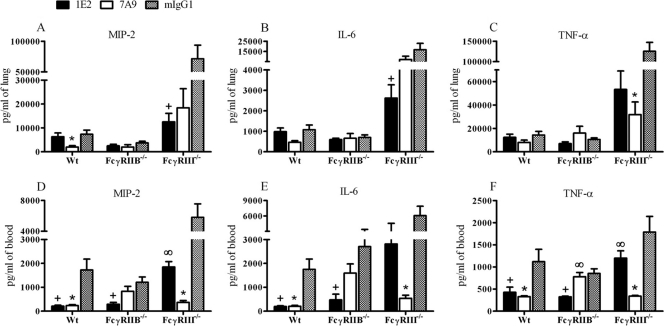
At 24 h after infection, 7A9-treated Wt mice had lower levels of lung MIP-2 and 7A9-treated FcγRIII−/− mice had lower levels of TNF-α (P < 0.03 and P < 0.01, respectively) (Fig. 3A and C) than mIgG1-treated mice. Hence, for 7A9, lower levels of these mediators in the lungs 24 h after infection correlated with lung CFU and protection. At 24 h after infection, 1E2-treated FcγRIII−/− mice had lower lung levels of MIP-2 and IL-6 than mIgG1-treated mice (P < 0.01 and P < 0.05, respectively) (Fig. 3A and B).
Fig 3.
Cytokines in lung and serum from 1E2-, 7A9-, and mIgG1-treated Wt, FcγRIIB−/−, and FcγRIII−/− mice 24 h after intranasal infection with 2 × 107 CFU ST3. (A) MIP-2 in lung, (B) IL-6 in lung, (C) TNF-α in lung, (D) MIP-2 in serum, (E) IL-6 in serum, (F) TNF-α in serum. P < 0.05 comparing (+) 1E2- to mIgG1-treated mice, (*) 7A9- to mIgG1-treated mice, and (∞) 1E2- to 7A9-treated mice. One-way ANOVA with Dunn's multiple-comparison test. n = 8 to 14 mice per group. Error bars represent standard errors of the means.
(ii) Serum cytokines.
At 24 h after infection, 7A9-treated Wt and FcγRIII−/− mice had lower levels of MIP-2 (P < 0.01 and P < 0.01, respectively), IL-6 (P < 0.01 and P < 0.01, respectively), and TNF-α (P < 0.01 and P < 0.01, respectively) than mIgG1-treated mice (Fig. 3D, E, and F). At 24 h after infection, 1E2-treated Wt and FcγRIIB−/− mice had lower levels of MIP-2 (P < 0.01 and P < 0.01, respectively), IL-6 (P < 0.01 and P < 0.01, respectively), and TNF-α (P < 0.01 and P < 0.01, respectively) than mIgG1-treated Wt and 7A9-treated FcγRIIB−/− mice (Fig. 3D, E, and F), but 1E2-treated FcγRIII−/− mice had higher levels of MIP-2 and TNF-α (P < 0.01 and P < 0.01, respectively) than control mIgG1- and 7A9-treated FcγRIII−/− mice. Hence, for 7A9 and 1E2, lower levels of serum cytokines 24 h after infection correlated with protection, except for 7A9-treated FcγRIIB−/− mice.
Inflammatory response in the lungs.
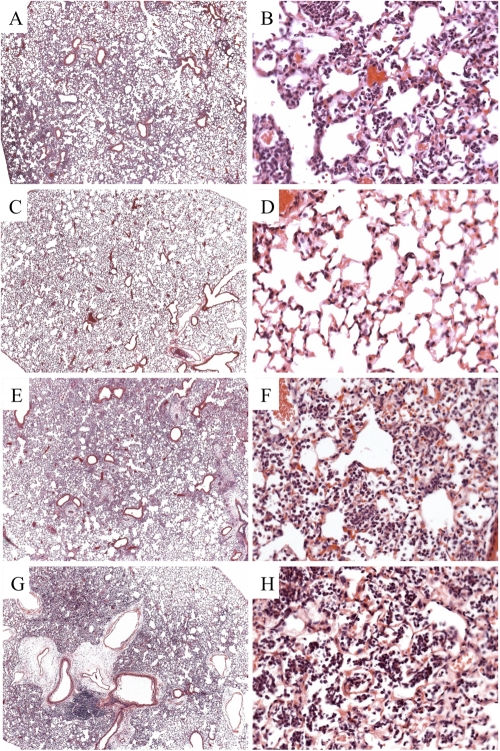
Histopathological studies were performed to evaluate inflammation in lung sections from Wt and FcγRIII−/− mice 24 h after infection. ST3-infected mIgG1-treated Wt mice exhibited neutrophilic and necrotizing pneumonia, with areas of fragmented, degenerating neutrophils and diffuse cellular infiltration radiating from blood vessels (Fig. 4A and B). 1E2-treated Wt mice had no evidence of pneumonia and very few infiltrating cells (Fig. 4C and D). In contrast, 1E2-treated (Fig. 4G and H) and mIgG1-treated (Fig. 4E and F) FcγRIII−/− mice each had pneumonia with similar patterns of wide-spread inflammation with cellular infiltration in alveolar spaces that resembled the inflammation in mIgG1-treated Wt mice. Hence, 1E2 mediated a reduction in inflammation in Wt mice that was abrogated in FcγRIII−/− mice.
Fig 4.
Histological sections of Wt and FcγRIII−/− mice treated with MAbs 24 h after intranasal infection with 2 × 107 CFU ST3. H&E sections of control mIgG1-treated Wt mice (A and B) had increased cellular infiltration compared to 1E2-treated Wt mice (C and D). In contrast, mIgG1-treated (E and F) and 1E2-treated (G and H) FcγRIII−/− mice had similar patterns of cellular infiltration. Panels A, C, E, and G are ×4 magnification; panels B, D, F, and H are ×40 magnification.
1E2 requires alveolar macrophages to mediate protection.
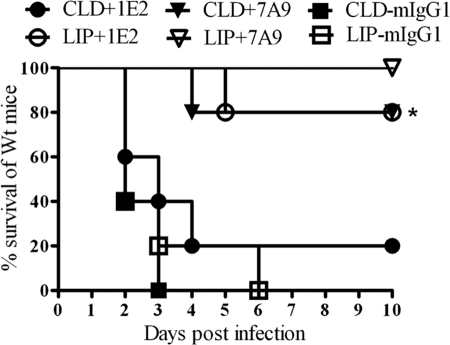
The survival of ST3-infected control PBS-liposome-treated Wt mice that received 1E2 was significantly longer than that of clodronate-liposome-treated mice (P < 0.01) (Fig. 5). There was no statistical difference in the survival rates of PBS-liposome- and clodronate-liposome-treated mice that received 7A9. Hence, in our model, alveolar macrophages were required for 1E2- but not 7A9-mediated protection.
Fig 5.
Survival of ST3-infected alveolar macrophage-depleted Wt mice after intraperitoneal administration of MAbs and intranasal infection with 108 CFU ST3. Alveolar macrophage depletion with chlodronate liposomes had no significant effect on protection by 7A9, but it abrogated protection by 1E2. *, P < 0.001, Kaplan-Meier log rank test. n = 5 mice per group. CLD, chlodronate liposomes; LIP, liposome control.
1E2 Promotes internalization but not intracellular killing of ST3 by macrophages.
To investigate whether 7A9 and 1E2 promote macrophage interaction with ST3, we assessed three processes: binding, internalization, and killing. Results are shown in Table 1. Compared to control mIgG1, 1E2 and 7A9 each induced more binding of ST3 to (naïve) alveolar macrophages (P < 0.03 and P < 0.01, respectively) and more ST3 internalization (P < 0.01 and P < 0.01, respectively). In the killing experiments there were more viable bacteria in 1E2- than 7A9- or mIgG1-treated cells (P < 0.01). We would have liked to perform additional studies with 7A9, but unfortunately, the cell line was lost. Instead, we used another protective IgG1 MAb, 5F6, that like 7A9 mediated killing in the opsonophagocytosis assay (OPA) and required FcγRIIB and neutrophils to mediate protection against ST3 (49). Results with 5F6 in Wt cells were similar to those with 7A9: compared to mIgG1-treated control cells, 1E2 and 5F6 each induced more ST3 binding (P < 0.03 and P < 0.05, respectively) and internalization (P < 0.05 and P < 0.03, respectively), and there were more viable ST3 in 1E2- than in 5F6- or mIgG1-treated cells (P < 0.01) (data not shown). To determine if there was a delay in 1E2-mediated killing, we extended the final incubation from 1 to 2 h and found there were no viable CFU inside 1E2-, 5F6-, or mIgG1-treated cells (data not shown). We also performed the binding, internalization, and killing experiments with naïve alveolar macrophages from FcγRIII−/− mice (Table 1). ST3 binding to FcγRIII−/− macrophages was markedly less than to Wt macrophages, and the amounts of binding to FcγRIII−/− cells did not differ between the MAb-treated groups. 7A9 and 1E2 each induced less ST3 internalization by FcγRIII−/− than Wt macrophages, with the numbers of live intracellular CFU being similar. Although 7A9 internalized more ST3, this was not statistically significant. Hence, the ability of 7A9 and 1E2 to bind to and induce ST3 uptake in FcγRIII−/− macrophages was impaired and the increased viability of ST3 in 1E2-ST3-treated Wt macrophages was abrogated.
Table 1.
PPS3-specific MAb-mediated binding, uptake, and intracellular killing of ST3 by naïve alveolar macrophages from Wt and FcγRIII−/− micea
| Strain | No. of ST3 bound to macrophages |
No. of ST3 internalized(% of bound ST3 ± SD) |
No. of ST3 alive inside(% of internalized ST3 ± SD) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1E2 | 7A9 or 5F6b | mIgG1 | 1E2 | 7A9 or 5F6b | mIgG1 | 1E2 | 7A9 or 5F6b | mIgG1 | |
| Wt | 60,000 ± 3,536+ | 77,000 ± 4,950* | 4,000 ± 1,414 | 5,400 ± 502+ (9.0 ± 0.3)+ | 7,700 ± 206* (9.0 ± 0.3)* | 80 ± 58 (2.0 ± 0.8) | 7,074 ± 960+∞ (131.0 ± 5.7)+∞ | 154 ± 39 (2.3 ± 0.5) | 35 ± 11 (42.5 ± 17.1) |
| FcγRIII−/− | 18,000 ± 3,857 | 33,000 ± 9,232 | 15,000 ± 8,550 | 1,188 ± 437 (6.6 ± 3.7) | 2,607 ± 3,893 (7.9 ± 6.7) | 210 ± 34 (1.4 ± 0.6) | 520 ± 426 (43.8 ± 36.2) | 521 ± 698 (20.0 ± 26.5) | 10 ± 11 (4.3 ± 5.1) |
Numbers shown are means with standard deviations; P < 0.05, comparing + 1E2- to mIgG1-treated cells, * 7A9- or 5F6- to mIgG1-treated cells, and ∞ 1E2- to 7A9- or 5F6-treated cells. Data represent results of one-way ANOVA with Bonferroni's multiple-comparison test. n = 4 separate experiments with data combined.
5F6, an IgG1 that, like 7A9, promotes in vitro killing (49) was used in experiments with FcγRIII−/− mice.
1E2 promotes alveolar macrophage apoptosis.
Annexin V staining was performed to examine alveolar macrophage apoptosis 24 h after ST3 infection. The results are shown in Table 2. Among Wt mice, 1E2-treated mice had significantly more apoptotic (annexin V+/7-AAD− F4/80+) alveolar macrophages than 7A9-treated (P < 0.01)- and mIgG1 (P < 0.01)-treated mice; mIgG1-treated mice had more apoptotic alveolar macrophages than 7A9-treated Wt mice (P < 0.01). 1E2- and 7A9-treated mice each had fewer 7-ADD+ alveolar macrophages than mIgG1-treated mice (P < 0.01 and P < 0.01, respectively). The level of 7-AAD+ macrophages among mIgG1-treated Wt mice could represent either necrosis or late apoptosis (52, 57). Similar studies were performed in FcγRIII−/− mice 24 h after ST3 infection. These mice had fewer F4/80+ cells than Wt mice. There were no differences in the numbers of apoptotic alveolar macrophages among 1E2-, 7A9-, or mIgG1-treated FcγRIII−/− mice (Table 2), but 1E2- and 7A9-treated mice each had fewer 7-ADD+ macrophages than mIgG1-treated mice (P < 0.01 and P < 0.01, respectively). Hence, the 1E2-mediated increase in apoptotic macrophages observed in Wt mice was abrogated in FcγRIII−/− mice.
Table 2.
Analysis of apoptosis of alveolar macrophages from MAb-treated Wt and FcγRIII−/− mice 24 h after intranasal infection with 2 × 107 CFU ST3a
| Strain | No. of F4/80+ macrophages (× 105) |
No. of annexin V+/7-AAD−/F4/80+ macrophages (× 105)(% ± SD) |
No. of 7-AAD+/F4/80+ macrophages (× 105)(% ± SD) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1E2 | 7A9 or 5F6b | mIgG1 | 1E2 | 7A9 or 5F6b | mIgG1 | 1E2 | 7A9 or 5F6b | mIgG1 | |
| Wt | 96 ± 64 | 111 ± 36 | 120 ± 20 | 16 ± 2+∞ (13 ± 1)+∞ | 3 ± 1* (3 ± 1)* | 6 ± 2 (6 ± 2) | 5 ± 2+ (4 ± 1)+ | 3 ± 1* (3 ± 1)* | 13 ± 2 (14 ± 2) |
| FcγRIII−/− | 28 ± 7 | 24 ± 16 | 30 ± 20 | 6 ± 2 (24 ± 3) | 2 ± 1 (18 ± 6) | 7 ± 2 (19 ± 2) | 3 ± 1+ (12 ± 2)+ | 2 ± 1* (11 ± 1)* | 4 ± 2 (16 ± 1) |
Numbers shown are means with standard deviations of total F4/80+ macrophages. The percentages with standard deviations of apoptotic (annexin V+/7-ADD−) and necrotic (7-ADD+) F4/80+ macrophages are shown in parentheses. P < 0.05, comparing + 1E2- to mIgG1-treated mice, * 7A9- or 5F6- to mIgG1-treated mice, and ∞ 1E2- to 7A9-treated mice. Data represent results of one-way ANOVA with Dunn's multiple-comparison test. n = 4 to 5 mice/group.
5F6, an IgG1 that, like 7A9, promotes in vitro killing (49) was used in experiments with FcγRIII−/− mice.
DISCUSSION
There is ample evidence that PPS-specific antibodies that promote phagocyte killing of pneumococccus in vitro (referred to as “opsonophagocytic” or “opsonic” antibodies) are critical mediators of protection against invasive pneumococcal disease (17, 23, 39). However, there is less information on protective PPS-specific antibodies that do not enhance pneumococcal killing in the opsonophagocytic killing assay that has been used to evaluate pneumococcal conjugate vaccine responses (41). In this article, we show that the efficacy of MAb 1E2, a PPS3-specific IgG1 that does not promote ST3 killing in vitro and protects FcγRIIB−/− but not FcγR−/− mice against lethal intranasal infection with ST3 WU2 (49), depends on FcγRIII and alveolar macrophages. We also show that the efficacy of MAb 7A9, a PPS3-specific IgG1 that promotes ST3 killing in vitro and requires FcγRIIB−/− and neutrophils but not FcγR−/− to mediate protection (49), does not depend on FcγRIII or macrophages. Given that F(ab)′2s of 7A9 and 1E2 were not protective, the efficacy of these IgG1 MAbs requires an Fc region and FcγRs. Hence, the different requirements for neutrophils (7A9), macrophages (1E2), and an activating (1E2) or the inhibitory (7A9) FcγR exhibited by these MAbs parallel their difference in PPS3 specificity (49).
Analogous to its inability to mediate phagocyte killing in vitro (49), 1E2 did not mediate early bacterial clearance in ST3-infected Wt or FcγRIIB−/−mice, even though it is protective in these strains (49). In contrast, 7A9 was able to mediate a reduction in lung CFU in the strains in which it is protective, Wt and FcγRIII−/− mice. Given that FcγRIII is not expected to induce efficient neutrophil-mediated phagocytosis or bacterial killing (2, 20), the ability of 7A9 to mediate bacterial clearance in FcγRIII−/− mice corresponds to its ability to induce neutrophil-mediated killing in vitro and its requirement for neutrophils to mediate protection against intranasal infection (49). On the other hand, 1E2 and 7A9 each mediated a marked (>2 log) reduction in blood CFU in the mouse strains in which they are protective. However, blood CFU were also reduced in 7A9-treated FcγRIIB−/− mice, a strain in which 7A9 is not protective (49). The latter is likely to stem from the ability of 7A9 to induce phagocyte-mediated killing (49), as described by Clatworthy and Smith for FcγRIIB−/− mice that were able to phagocytose and control bacteremia but died of cytokine storm in a ST1 intraperitoneal infection model (12). Given that serum MIP-2, IL-6, and TNF-α were not reduced in 7A9-treated FcγRIIB−/− mice, the reduction in blood CFU in these mice is most consistent with phagocytosis-induced cytokine activation via FcγRIII in the absence of the anti-inflammatory effect of the inhibitory FcγR, FcγRIIB. Hence, in the setting of phagocytosis, neither bacterial killing in vitro nor bacterial clearance in vivo is predictive of protection against disease/death in vivo in our model.
The data herein reveal complex associations between the lung bacterial burden, lung cytokines, and survival. The absence of a decrease in cytokines in the lungs of 1E2-treated ST3-infected Wt or FcγRIIB−/− mice (mouse strains in which 1E2 is protective [49]) corresponds to the bacterial burden at the time these studies were performed, which was not reduced. In contrast, the reduction in lung CFU in 7A9-treated Wt and FcγRIII−/− mice, mouse strains in which 7A9 is protective, was accompanied by a decrease in lung cytokines, albeit only the reduction in TNF-α was statistically significant. The latter could reflect FcγRIIB-mediated inhibition of phagocytosis-induced cytokine release, as described by Clatworthy and Smith (12), but it could also correspond to the reduction in the lung bacterial burden in 7A9-treated Wt and FcγRIII−/− mice. Nonetheless, 1E2-treated FcγRIII−/− mice also exhibited a reduction in lung MIP-2 and IL-6 without a corresponding decrease in lung CFU, perhaps reflecting a lack of FcγRIII-mediated cytokine production and/or FcγRIIB-mediated inhibition of cytokine production in the setting of FcγRIII deficiency. Consistent with the latter, successful host defense requires both FcγR-mediated activation and inhibition to achieve a balance between detrimental and beneficial phagocytosis-mediated inflammation and bacterial clearance (12, 38). FcγRIII-mediated phagocytosis can induce IL-6 and TNF-α (21) and FcγRIIB mediates anti-inflammatory activity via inhibition of FcγRI/III (32) and clearance of immune complexes (50), but unbalanced/overexpression increases pneumococcal lethality in mice, despite phagocytosis (7). We are cognizant that cytokine levels in our model could have differed at other times and other cytokines might have exhibited relationships. In addition, we realize that more work is needed to understand the many factors influencing cytokine levels in our model.
Lung sections from 1E2-treated ST3-infected mice exhibited little to no lung inflammation, whereas 1E2-treated FcγRIII−/− mice had marked neutrophilic inflammation resembling the inflammatory response of mIgG1-treated Wt mice. Hence, the anti-inflammatory effect of 1E2 depends on FcγRIII, although it does not depend on early bacterial clearance, as the bacterial burdens in 1E2- and mIgG1-treated mice were comparable 24 h after infection, the time at which the histopathological analysis was performed. The precise mechanism by which 1E2 reduces lung inflammation remains under investigation. However, given that 1E2-mediated protection required alveolar macrophages in our model, our findings are analogous to those of Knapp et al. that alveolar macrophages enhance mouse resistance to ST3 (6303) by reducing lung inflammation, rather than mediating bacterial clearance (25). In the latter study, the anti-inflammatory effect of macrophages was attributed to clearance of apoptotic neutrophils. Although we did not examine neutrophils directly, the neutrophilic infiltrates observed in lung sections from control (mIgG1-treated) Wt and 1E2-treated FcγRIII−/− mice were not observed in 1E2-treated Wt mice. We also found that 1E2-treated mice had more apoptotic alveolar macrophages than either 7A9- or mIgG1-treated ST3-infected mice 24 h after infection, an effect that was abrogated in FcγRIII−/− mice, and that 1E2-, 5F6-, and mIgG1-treated FcγRIII−/− mice each had a similar amount of apoptotic macrophages. In view of the anti-inflammatory effect of 1E2 in Wt mice, our findings are consistent with data from Marriott et al. showing that apoptotic alveolar macrophages reduced lung inflammation and bacterial bloodstream invasion in a ST2 pulmonary infection model (28).
7A9 and 5F6, but not 1E2, were previously shown to promote ST3 killing by neutrophils and J774-like macrophages in vitro (49). Based on these findings, 7A9 and 5F6 were characterized as opsonic and 1E2 as nonopsonic. However, data presented herein challenge these characterizations, as 1E2 mediated an amount of ST3 uptake by naïve alveolar macrophages similar to that of 7A9 or 5F6 in vitro. Nonetheless, and corresponding to the lack of early bacterial clearance in the lungs of 1E2-treated mice, 1E2-opsonized ST3 induced significantly less intracellular bacterial killing in vitro after 1 h of incubation. Although ample data establish that mouse survival in pneumococcal infection requires bacterial clearance and control of bloodstream invasion (8, 13, 27, 44, 54), this study did not directly demonstrate how 1E2 mediates bacterial clearance. Given that pneumococcal pathogenesis stems from bacterial ligands binding to host receptors (3, 34) 1E2-induced macrophage internalization of ST3 could temporarily limit ST3 access to host receptors. However, 1E2-mediated protection is associated with alveolar macrophage apoptosis in vivo and our in vitro results suggest that macrophage internalization of 1E2 could result in delayed macrophage killing. 1E2-opsonized ST3 was viable in macrophages for 1 h in vitro, but there were no viable intracellular bacteria in 1E2-, 5F6-, or control mIgG1-treated cells 2 h after incubation. Consistent with our finding that 1E2- and 7A9-induced ST3 uptake was reduced but not abrogated in FcγRIII−/− macrophages, FcγRIIB and FcγRIII each promote (human) macrophage-associated apoptotic killing of S. pneumoniae (1) and opsonized S. pneumoniae induced delayed macrophage apoptosis-associated bacterial killing via FcγRIII and FcγRII (1). Hence, the kinetics of mouse and human macrophage apoptosis could differ. Nonetheless, Dockrell et al. showed that ST1 uptake resulted in alveolar macrophage apoptosis-mediated bacterial clearance in mice (15, 16) and killing in human macrophages (15). Further, Ali et al. described a delay in apoptosis-associated killing of S. pneumoniae by human macrophages (1), possibly reflecting recently described relationships between phagolysosomal events, apoptosis, and late-stage pneumococcal killing (5). Interestingly, Bewley et al. found that inhibition of macrophage apoptosis-associated killing of ST1 was associated with increased neutrophil recruitment to the lungs (5), providing a mechanistic hypothesis for our data that 1E2 efficacy requires macrophages and that its anti-inflammatory effect requires FcγRIII and is associated with alveolar macrophage apoptosis.
In summary, our data show that 1E2-mediated protection is a function of macrophage FcγRIII availability and reveal a critical role for FcγRIII in 1E2-mediated macrophage uptake and internalization of ST3 in vitro and alveolar macrophage apoptosis and immunomodulation in vivo. Fc-independent antibody-mediated immunomodulation was described in a Streptococcus mutans model (40). However, to our knowledge, despite several studies linking FcγRIII to inhibition of the inflammatory response (31, 35, 47), current thought holds that Fc-dependent immunomodulation depends on FcγRIIB (12, 43, 45, 50). Our data support but do not prove that 1E2 induces ST3 killing via late macrophage apoptosis, and the precise mechanism by which 1E2 mediates bacterial clearance in vivo remains to be determined. Nonetheless, our model provides a novel platform to dissect mechanisms of antibody-dependent macrophage apoptosis-associated pneumococcal killing and antibody-mediated regulation of the inflammatory response to pneumococcal infection. Finally, our data suggest that antibodies which do not promote phagocyte-mediated bacterial killing in vitro (41), but promote bacterial uptake by macrophages, warrant further study as potential modulators of the inflammatory response to pneumococcal pneumonia.
ACKNOWLEDGMENTS
We thank Rani Sellers of the Histopathology Core Facility for expert histopathological analysis. We also thank Lydia Tesfa of the Flow Cytometry Core Facility for assistance with the FACScan. The Flow Cytometry Core Facility is a shared facility supported by the Einstein Cancer Center (P30CAO13330).
This work was supported by the National Institutes of Health (R01-AI045459 and R01-AI044374 to L.P.) and an HIV, AIDS and Opportunistic Infection Institutional AIDS Training Grant (T32-AI007501 to S.W.).
No author has a conflict of interest with the data presented in this article.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Ali F, et al. 2003. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J. Infect. Dis. 188:1119–1131 [DOI] [PubMed] [Google Scholar]
- 2. Anderson CL, Shen L, Eicher DM, Wewers MD, Gill JK. 1990. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J. Exp. Med. 171:1333–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beisswenger C, Lysenko ES, Weiser JN. 2009. Early bacterial colonization induces Toll-like receptor-dependent transforming growth factor beta signaling in the epithelium. Infect. Immun. 77:2212–2220 doi:10.1128/IAI.01224-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bender JM, et al. 2008. Pneumococcal necrotizing pneumonia in Utah: does serotype matter? Clin. Infect. Dis. 46:1346–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bewley MA, et al. 2011. A cardinal role for cathepsin d in co-ordinating the host-mediated apoptosis of macrophages and killing of pneumococci. PLoS Pathog. 7:e1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Briles DE, Claflin JL, Schroer K, Forman C. 1981. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature 294:88–90 [DOI] [PubMed] [Google Scholar]
- 7. Brownlie RJ, et al. 2008. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J. Exp. Med. 205:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchwald UK, Lees A, Steinitz M, Pirofski L. 2005. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infect. Immun. 73:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burns T, Abadi M, Pirofski L. 2005. Modulation of the lung inflammatory response to serotype 8 pneumococcus infection by a human monoclonal IgM to serotype 8 capsular polysaccharide. Infect. Immun. 73:4530–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burns T, Zhong Z, Steinitz M, Pirofski LA. 2003. Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775–6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byington CL, et al. 2010. Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J. Clin. Microbiol. 48:520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clatworthy MR, Smith KG. 2004. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 199:717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coleman JR, Papamichail D, Yano M, Garcia-Suarez MM, Pirofski LA. 2011. Designed reduction of Streptococcus pneumoniae pathogenicity via synthetic changes in virulence factor codon-pair bias. J. Infect. Dis. 203:1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dallaire F, et al. 2001. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J. Infect. Dis. 184:292–300 [DOI] [PubMed] [Google Scholar]
- 15. Dockrell DH, Lee M, Lynch DH, Read RC. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184:713–722 [DOI] [PubMed] [Google Scholar]
- 16. Dockrell DH, et al. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380–5388 [DOI] [PubMed] [Google Scholar]
- 17. Ekström N, Vakevainen M, Verho J, Kilpi T, Kayhty H. 2007. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 75:1794–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fabrizio K, Groner A, Boes M, Pirofski LA. 2007. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin. Vaccine Immunol. 14:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fabrizio K, Manix C, Tian H, van Rooijen N, Pirofski LA. 2010. The efficacy of pneumococcal capsular polysaccharide-specific antibodies to serotype 3 Streptococcus pneumoniae requires macrophages. Vaccine 28:7542–7550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fossati G, Moots RJ, Bucknall RC, Edwards SW. 2002. Differential role of neutrophil Fcgamma receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum. 46:1351–1361 [DOI] [PubMed] [Google Scholar]
- 21. Hart SP, Alexander KM, Dransfield I. 2004. Immune complexes bind preferentially to Fc gamma RIIA (CD32) on apoptotic neutrophils, leading to augmented phagocytosis by macrophages and release of proinflammatory cytokines. J. Immunol. 172:1882–1887 [DOI] [PubMed] [Google Scholar]
- 22. Hazenbos WL, et al. 1996. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 5:181–188 [DOI] [PubMed] [Google Scholar]
- 23. Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. 2007. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 25:2518–2527 [DOI] [PubMed] [Google Scholar]
- 24. Knapp S, et al. 2004. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J. Infect. Dis. 189:1506–1515 [DOI] [PubMed] [Google Scholar]
- 25. Knapp S, et al. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171–179 [DOI] [PubMed] [Google Scholar]
- 26. Li J, Szalai AJ, Hollingshead SK, Nahm MH, Briles DE. 2009. Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect. Immun. 77:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marks M, et al. 2007. Influence of neutropenia on the course of serotype 8 pneumococcal pneumonia in mice. Infect. Immun. 75:1586–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marriott HM, et al. 2006. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J. Immunol. 177:6480–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. 2004. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect. Dis. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizrachi-Nebenzahl Y, et al. 2003. Differential activation of the immune system by virulent Streptococcus pneumoniae strains determines recovery or death of the host. Clin. Exp. Immunol. 134:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montalvão F, et al. 2010. Apoptotic lymphocytes treated with IgG from Trypanosoma cruzi infection increase TNF-alpha secretion and reduce parasite replication in macrophages. Eur. J. Immunol. 40:417–425 [DOI] [PubMed] [Google Scholar]
- 32. Nimmerjahn F, Ravetch JV. 2007. The antiinflammatory activity of IgG: the intravenous IgG paradox. J. Exp. Med. 204:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nimmerjahn F, Ravetch JV. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47 [DOI] [PubMed] [Google Scholar]
- 34. Novak R, Tuomanen E. 1999. Pathogenesis of pneumococcal pneumonia. Semin. Respir. Infect. 14:209–217 [PubMed] [Google Scholar]
- 35. Park-Min KH, et al. 2007. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity 26:67–78 [DOI] [PubMed] [Google Scholar]
- 36. Poolman J, et al. 2009. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 27:3213–3222 [DOI] [PubMed] [Google Scholar]
- 37. Ren B, et al. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506–7512 [DOI] [PubMed] [Google Scholar]
- 38. Rhein LM, Perkins M, Gerard NP, Gerard C. 2008. FcgammaRIII is protective against Pseudomonas aeruginosa pneumonia. Am. J. Respir. Cell Mol. Biol. 38:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robbins JB, Schneerson R, Szu SC. 1995. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171:1387–1398 [DOI] [PubMed] [Google Scholar]
- 40. Robinette RA, Oli MW, McArthur WP, Brady LJ. 2009. Beneficial immunomodulation by Streptococcus mutans anti-P1 monoclonal antibodies is Fc independent and correlates with increased exposure of a relevant target epitope. J. Immunol. 183:4628–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Romero-Steiner S, et al. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roscic-Mrkic B, et al. 2001. Roles of macrophages in measles virus infection of genetically modified mice. J. Virol. 75:3343–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samuelsson A, Towers TL, Ravetch JV. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484–486 [DOI] [PubMed] [Google Scholar]
- 44. Seyoum B, Yano M, Pirofski LA. 2011. The innate immune response to Streptococcus pneumoniae in the lung depends on serotype and host response. Vaccine 29:8002–8011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith KG, Clatworthy MR. 2010. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol. 10:328–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379:346–349 [DOI] [PubMed] [Google Scholar]
- 47. Thomas BN, Buxbaum LU. 2008. FcgammaRIII mediates immunoglobulin G-induced interleukin-10 and is required for chronic Leishmania mexicana lesions. Infect. Immun. 76:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian H, Groner A, Boes M, Pirofski L. 2007. Pneumococcal capsular polysaccharide vaccine-mediated protection of immunodeficient mice against serotype 3 Streptococcus pneumoniae. Infect. Immun. 75:1643–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. 2009. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect. Immun. 77:1502–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Lent P, et al. 2003. The inhibitory receptor FcgammaRII reduces joint inflammation and destruction in experimental immune complex-mediated arthritides not only by inhibition of FcgammaRI/III but also by efficient clearance and endocytosis of immune complexes. Am. J. Pathol. 163:1839–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Rooijen N, Sanders A. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83–93 [DOI] [PubMed] [Google Scholar]
- 52. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39–51 [DOI] [PubMed] [Google Scholar]
- 53. Wang J, Barke RA, Charboneau R, Schwendener R, Roy S. 2008. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling. J. Immunol. 180:3594–3600 doi:180/5/3594[pii]. [DOI] [PubMed] [Google Scholar]
- 54. Weber SE, Tian H, Pirofski LA. 2011. CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J. Immunol. 186:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weinberger DM, et al. 2010. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin. Infect. Dis. 51:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yano M, Pirofski LA. 2011. Characterization of gene use and efficacy of mouse monoclonal antibodies to Streptococcus pneumoniae serotype 8. Clin. Vaccine Immunol. 18:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zelenin AV, et al. 1984. 7-Amino-actinomycin D as a specific fluorophore for DNA content analysis by laser flow cytometry. Cytometry 5:348–354 [DOI] [PubMed] [Google Scholar]
- 58. Zhou H, Kobzik L. 2007. Effect of concentrated ambient particles on macrophage phagocytosis and killing of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 36:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]