Abstract
Pertussis is a highly contagious, acute respiratory illness caused by the bacterial pathogen Bordetella pertussis. Despite nearly universal vaccine coverage, pertussis rates in the United States have been rising steadily over the last 20 years. Our failure to comprehend and counteract this important public health concern is due in large part to gaps in our knowledge of the disease and the mechanisms of vaccine-mediated protection. Important questions about pertussis pathogenesis and mechanisms of vaccine effectiveness remain unanswered due to the lack of an animal model that replicates the full spectrum of human disease. Because current animal models do not meet these needs, we set out to develop a nonhuman primate model of pertussis. We inoculated rhesus macaques and olive baboons with wild-type B. pertussis strains and evaluated animals for clinical disease. We found that only 25% of rhesus macaques developed pertussis. In contrast, 100% of inoculated baboons developed clinical pertussis. A strong anamnestic response was observed when convalescent baboons were infected 6 months following recovery from a primary infection. Our results demonstrate that the baboon provides an excellent model of clinical pertussis that will allow researchers to investigate pertussis pathogenesis and disease progression, evaluate currently licensed vaccines, and develop improved vaccines and therapeutics.
INTRODUCTION
Whooping cough is a highly contagious, acute respiratory illness caused by the bacterial pathogen Bordetella pertussis (for a review, see references 10 and 17). The introduction of pertussis vaccines in the 1940s and nationwide coverage in excess of 95% led to a dramatic decrease in the incidence of the disease. However, for unexplained reasons, pertussis rates in the United States have been rising steadily over the last 20 years for infants, children, and adolescents (1). With more than 21,000 reported cases in the United States in 2010, the highest number since the 1950s, pertussis is the most common of the vaccine-preventable diseases (2). This resurgence is mirrored throughout the industrial world, despite similar high rates of vaccination (12, 20, 31). Several hypotheses have been suggested for the increase in cases, but there is no consensus within the scientific community (9). Our failure to comprehend and counteract this important public health concern is due in large part to gaps in our knowledge of the disease and the mechanisms of vaccine-mediated protection. In order to fill these gaps, a good animal model of pertussis is required.
In humans, infection with B. pertussis results in a wide spectrum of clinical manifestations that depends on the age and immune status of the host and ranges from mild respiratory symptoms to a severe cough illness which may be accompanied by the hallmark inspiratory whoop and posttussive emesis (4). Clinical signs include high leukocytosis, hypoglycemia, and reduced pulmonary capacity. Because of the acute nature of pertussis infections and because B. pertussis is a strict human pathogen with no known animal or environmental reservoir, maintenance of the organism within the population is thought to require continuous transmission of the disease from infected to naïve hosts.
A variety of animal models have been used to study the pathogenesis of pertussis, including mice, rabbits, guinea pigs, and newborn piglets (for a review, see reference 8). While these models are useful for studying certain aspects of pertussis, none of them adequately reproduces the full spectrum of the disease observed in humans. Previously published studies reported that nonhuman primates develop all of the characteristic markers of human pertussis (5, 13, 16, 21, 22, 24). However, these studies were published more than 50 years ago, with limited experimental detail, making it difficult to critically evaluate or reproduce these models. These studies focused mainly on two monkey species: rhesus macaques (Macaca mulatta) and the closely related species Macaca cyclopsis. The rhesus macaque models showed mixed success (5, 19, 22). Most rhesus macaques inoculated with B. pertussis failed to develop clinical signs of disease. In these studies, the ages, weights, and histories of monkeys were not described, and very little experimental detail was provided. In contrast to published results obtained using rhesus macaques, two studies in which M. cyclopsis monkeys were inoculated with B. pertussis strain 18323 were successful. Between the two M. cyclopsis studies, 13 of 14 directly infected monkeys developed clinical pertussis (13, 16). Disease was characterized by cough illness and 3- to 5-fold increases in circulating white blood cell (WBC) counts. In one study, transmission to naïve animals cohoused with infected animals was demonstrated (16). Although these results indicate that a nonhuman primate model of disease and transmission is achievable, these studies cannot be replicated directly because M. cyclopsis monkeys are endangered in the wild and are no longer available for biomedical research. It is not clear why very different results were reported for M. cyclopsis and the closely related rhesus macaque.
In our studies, we reevaluated the rhesus macaque model and confirmed that it is not a reliable model of pertussis. We subsequently evaluated the baboon (Papio anubis) and found that it provides an excellent model in which all aspects of pertussis clinical disease are reproduced. This model was used to evaluate colonization, cough illness, and serological immune responses following primary and secondary infections. Importantly, we also show that convalescent baboons were protected from reinfection.
MATERIALS AND METHODS
Ethics statement.
All animals were housed and handled in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care, with good animal practice as defined by the Institutional Animal Care and Use Committee at the Center for Biologics Evaluation and Research (CBER), U.S. Food and Drug Administration (FDA). All animal studies were approved by this committee.
Bacterial strains and media.
B. pertussis strain Tohama I was obtained from the collection of strains maintained by the FDA. A recent clinical isolate of B. pertussis (strain D420) was provided by the Centers for Disease Control. Bordet-Gengou (BG) agar plates were prepared with Bordet-Gengou agar (Becton Dickinson, Sparks, MD) containing 1% proteose peptone (Becton Dickinson) and 15% defibrinated sheep blood. Regan-Lowe plates were prepared from Regan-Lowe charcoal agar base (Becton Dickinson) with 10% defibrinated sheep blood and 40 μg/ml cephalexin. Stainer-Scholte (SS) medium was used for broth cultures and was prepared as described previously (23). All SS cultures were supplemented with 0.5% Casamino Acids.
Preparation of inoculum and infection of baboons and rhesus macaques.
Baboons (Papio anubis) were obtained from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center. Rhesus macaques were obtained from the FDA CBER breeding colony. Fresh plates of B. pertussis grown on BG agar were resuspended in phosphate-buffered saline (PBS) to a density of 109 to 1010 CFU per ml. The density of the inoculum was determined by measurement of optical density and confirmed by serial dilution and plating to determine the number of CFU per ml of inoculum. Baboons and rhesus macaques were anesthetized with ketamine at 10 to 15 mg/kg of body weight, administered intramuscularly (i.m.). Each animal's pharynx was swabbed with 2% lidocaine solution, and animals were intubated using a 2- to 3-mm endotracheal tube to deliver 1 ml of the inoculum to the top of the trachea. A 24-gauge, 3.2-cm intravenous (i.v.) catheter (Abbocath) was used to deliver 0.5 ml of inoculum to the back of each naris. Animals were placed in a sitting position for 3 min, returned to their cages, and observed until they recovered from anesthesia.
Evaluation of animals.
Baboons and rhesus macaques were anesthetized with ketamine, and weight and temperature were measured. Peripheral blood was drawn. Whole blood was evaluated for the number of circulating white blood cells by a complete blood count (CBC), and serum was saved at −80°C. The back of each naris was flushed with a 24-guage, 3.2-cm i.v. catheter containing 1 ml PBS. The recovered nasopharyngeal washes from both nares were combined, and 100 μl was divided and plated onto two Regan-Lowe plates. The number of CFU per plate was recorded after incubation at 37°C.
Quantification of coughing.
Each primate unit was monitored by a video recording system. Data were collected for six baboons in these studies. Videotape was reviewed at four 30-minute periods each day (6:00 a.m., 10:00 a.m., 2:00 p.m., and 6:00 p.m.). The number of coughs per hour was determined for each 30-minute observation period. The average number of coughs per hour for each day was calculated as the mean for all four observation periods for all six animals.
Measurement of transcriptional activity by quantitative real-time PCR.
B. pertussis strain D420 was grown on BG agar plates at 37°C and 39°C for 3 days. Bacteria were resuspended in RLT buffer (Qiagen, Valencia, CA), and samples were lysed in a FastPrep instrument using matrix B (MP Biomedicals, Solon, OH). RNA in the supernatant was purified using an RNeasy mini kit (Qiagen) and converted to cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer's protocol. Custom TaqMan gene expression assays for ptxA, fhaB, cyaA, and BPr01 (16S rRNA) were manufactured by Applied Biosystems, based on regions with limited homology to other B. pertussis genes. Gene expression was analyzed using TaqMan Fast Universal 2× PCR master mix (no AmpErase UNG). Reactions were performed in triplicate and run on an Applied Biosystems 7900HT real-time PCR system.
Evaluation of protein levels by Western blot analysis.
B. pertussis strain D420 was grown on BG agar plates at 37°C and 39°C for 3 days. Bacteria were scraped from the plates and lysed directly in Laemmli buffer (Bio-Rad, Hercules, CA). Lysate protein concentrations were measured by the bicinchoninic acid assay (Pierce, Rockford, IL). Samples were then normalized for equal protein loading and run in NuPAGE 4 to 12% Bis-Tris gels (Invitrogen, Carlsbad, CA). Proteins were transferred to polyvinylidene difluoride membranes and blocked in Tris-buffered saline solution containing 0.1% Tween 20 (TBST) with 5% nonfat dry milk. Membranes were then incubated with the following specific primary antibodies diluted in TBST with 1% milk: 1B7 mouse monoclonal antibody for pertussis toxin (PT) subunit 1, 1C6 mouse monoclonal antibody for filamentous hemagglutinin, and rabbit polyclonal antibody for adenylate cyclase toxin. Blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted in TBST with 1% bovine serum albumin (BSA). Signals were detected on HyperECL film with an ECL Plus chemiluminescence kit (GE Healthcare).
Detection of serum antibodies to pertussis toxin.
Anti-PT serum IgG was measured as described previously (7). For each set of enzyme-linked immunosorbent assay (ELISA) plates, a standard curve was constructed for human reference antiserum (FDA lot 5), and the relative units for each animal sample were calculated by comparison to the linear portion of this standard curve.
Statistics.
Error bars indicate standard errors for replicate experiments. For the anti-PT ELISA, data were log transformed to equalize variance among the sample sets. For the data obtained following primary infections, statistical analysis was performed by comparing preinfection to postinfection data with the two-tailed paired t test. For the data obtained following secondary infections, statistical analysis was performed by comparing naïve versus convalescent animals with the two-tailed unpaired t test. P values of <0.05 were considered statistically significant.
RESULTS
Infection of rhesus macaques with B. pertussis.
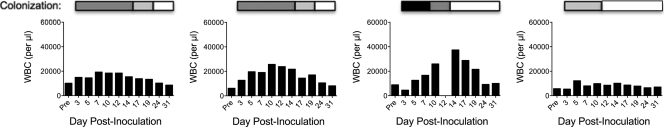
Previous studies suggested that very young M. cyclopsis monkeys appeared to provide a reliable model of clinical pertussis but that the closely related rhesus macaque model did not. Because the rhesus macaque studies did not list ages and weights of monkeys, we reasoned that the failure of past rhesus macaque studies may have been a consequence of using adult monkeys. Therefore, we began our studies by evaluating the ability to establish clinical pertussis in young rhesus macaques. We originally selected B. pertussis strain Tohama I for infections due to the extensive body of research conducted using this strain. Three rhesus macaques ranging in weight from 1.2 to 1.8 kg and between 43 and 64 weeks of age were inoculated with 109 to 1010 CFU B. pertussis strain Tohama I. All three monkeys were infected, as demonstrated by our ability to isolate B. pertussis from nasopharyngeal washes from day 3 until approximately day 24 postinoculation. Despite being infected, these monkeys did not exhibit overt signs of disease, and only mild increases in circulating white blood cell counts (≤2-fold) were observed. In order to address the concern that Tohama I may be attenuated because of lab adaptation, we obtained a recent clinical isolate of B. pertussis, designated strain D420, which was isolated from a human infant with severe respiratory distress. Four rhesus macaques ranging in weight from 1.7 to 1.9 kg and between 43 and 64 weeks of age were inoculated with 109 to 1010 CFU B. pertussis strain D420. All four monkeys were infected, as demonstrated by our ability to isolate B. pertussis from nasopharyngeal washes from day 3 until approximately day 15 postinoculation (Fig. 1). Two of the four monkeys developed a significant rise in white blood cells (4- and 6-fold) (Fig. 1). One of the two monkeys with an elevated white blood cell count developed a mild cough that persisted for several days.
Fig 1.
Leukocytosis and colonization of pertussis-infected rhesus macaques. Four weanling rhesus macaques were inoculated with B. pertussis strain D420 as described in Materials and Methods. Once prior to inoculation and on the indicated days postinoculation, blood was drawn from each rhesus macaque, and the number of circulating white blood cells per microliter of peripheral blood was determined as described in Materials and Methods and shown in the graph for each animal. Nasopharyngeal washes were performed as described in Materials and Methods, and the recovered washes were plated on two Regan-Lowe plates (50 μl per plate). The number of CFU per plate is indicated for each day by the shading of the bar over the WBC graph: white, 0 colonies; light gray, 1 to 100 colonies; dark gray, 101 to 1,000 colonies; and black, >1,000 colonies.
Growth of B. pertussis at 39°C.
It was striking that seven of seven rhesus macaques were persistently infected with B. pertussis but only one developed overt pertussis clinical signs. We considered the possibility that the elevated normal body temperature of rhesus macaques (38.7°C to 39.8°C) may not allow optimal B. pertussis growth. Using Stainer-Scholte liquid medium, we performed growth curve studies of D420 at 37°C and 39°C and found that B. pertussis exhibited a reduced growth rate at the elevated temperature (Fig. 2A). Moreover, when grown at 39°C on BG plates, B. pertussis failed to exhibit the characteristic hemolysis observed at 37°C (Fig. 2B). The hemolytic activity of B. pertussis is due to the RTX domain of adenylate cyclase toxin, an important virulence factor that is regulated at the level of transcription by the BvgAS two-component system (11). BvgAS, which regulates the expression of most pertussis virulence factors, exhibits low activity at 25°C and high activity at 37°C (14, 25). Because of this temperature-dependent modulation, we hypothesized that the lack of hemolysis at 39°C may have been due to inhibition of Bvg-mediated transcriptional activity at temperatures above 37°C. However, quantitative real-time PCR analysis of three key BvgAS-regulated genes (ptxA, fhaB, and cyaA) revealed that transcription of all three genes was the same in cells grown at 39°C as that in cells grown at 37°C (Fig. 2C). To analyze the protein expression of these three toxins, D420 was grown on Bordet-Gengou agar plates, and colonies were scraped into and lysed in sample buffer to collect both secreted and cell-associated proteins. Although transcription of the cyaA gene was unaffected at 39°C, Western blot analysis demonstrated that adenylate cyclase protein levels were significantly reduced in cells grown at 39°C relative to those in cells grown at 37°C (Fig. 2D).
Fig 2.
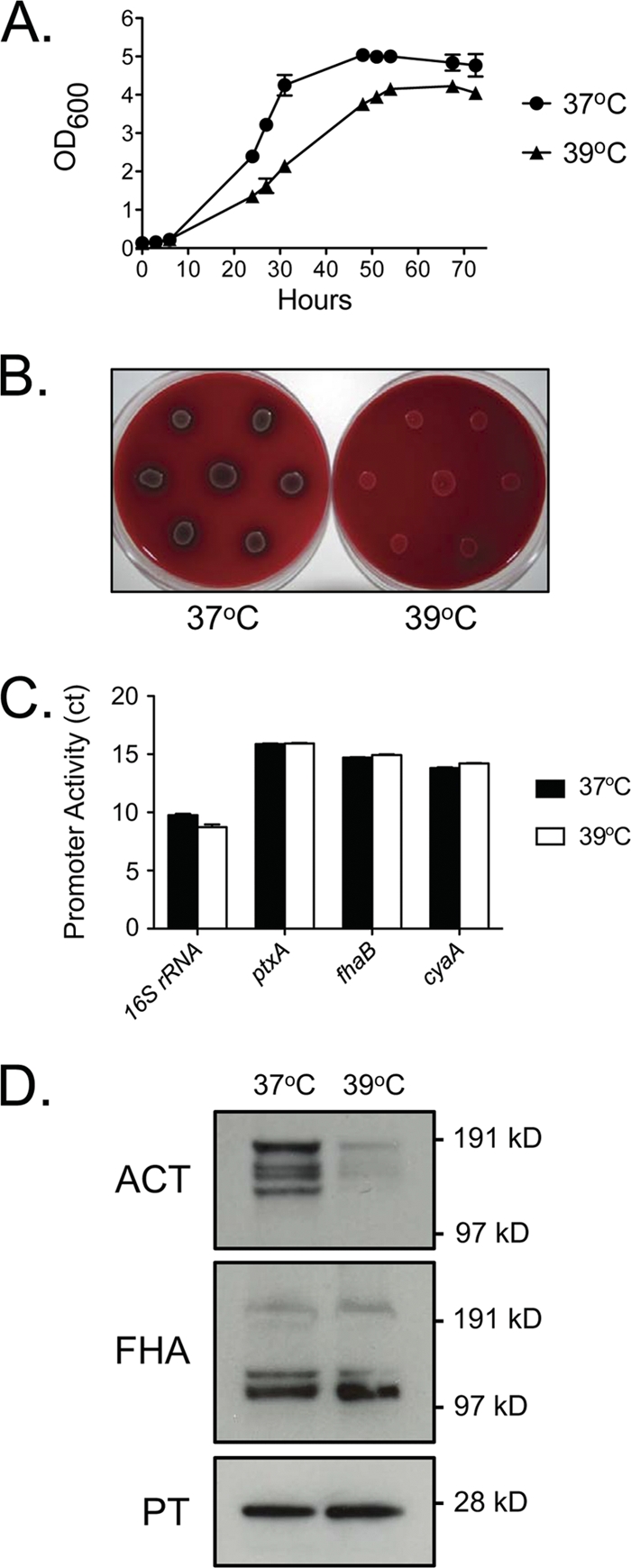
Characteristics of B. pertussis grown at 39°C. (A) Growth curve of B. pertussis grown in Stainer-Scholte medium at 37°C and 39°C (n = 3). (B) Hemolysis of B. pertussis strain D420 grown on BG agar plates at 37°C and 39°C. (C) Following growth of strain D420 on BG agar plates at 37°C and 39°C, the expression of the genes encoding pertussis toxin (ptxA), filamentous hemagglutinin (fhaB), and adenylate cyclase toxin (cyaA) were analyzed by real-time PCR as described in Materials and Methods. Data are presented as threshold cycle (CT) values (for 37°C, n = 4; for 39°C, n = 3). (D) Protein expression of adenylate cyclase toxin, filamentous hemagglutinin, and pertussis toxin was analyzed by Western blotting as described in Materials and Methods following growth of strain D420 on BG agar plates at 37°C and 39°C. Blots shown are representative of 4 separate experiments. The numbers on the right indicate the molecular size markers.
Infection of weanling baboons with B. pertussis.
Given the reduced growth rate of B. pertussis and the loss of expression of the key virulence factor adenylate cyclase at the elevated temperature of 39°C, we speculated that baboons, which have a normal core body temperature between 37°C and 39°C, may provide a more reliable model of clinical pertussis. In four independent studies, we inoculated a total of nine weanling baboons with 109 to 1010 CFU of B. pertussis strain D420. The baboons were between 2.0 and 3.2 kg and between 28 and 39 weeks of age at the time of infection. Nine of nine baboons developed classical symptoms of clinical pertussis. From the first examination of the infected animals on day 2 or 3 postinoculation, very large numbers of bacteria were recovered from the nasopharyngeal washes of all nine baboons (Table 1). In addition, all nine baboons exhibited very high WBC counts, with peak levels 5- to 10-fold above the preinfection baseline values (Fig. 3A). All nine baboons developed severe coughs that persisted for over 2 weeks (Fig. 3B). At peak illness, coughing fits were severe, consisting of 5 to 10 paroxysmal coughs. Most baboons exhibited a moderate increase in body temperature (approximately 1°C) between days 5 and 9 postinoculation (Fig. 3C).
Table 1.
Colonization of infected naïve weanling baboons
| Infected animal group | Colonization on indicated day postinoculationa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2/3 | 5 | 7 | 9/10 | 12 | 14 | 16/17 | 19 | 21 | 26 | 33 | |
| Naïve weanling | C | C | C | T | T | T | T | 14 | 0 | 0 | 0 |
| C | C | C | C | C | T | T | 0 | 0 | 0 | 0 | |
| C | C | C | C | T | T | T | 2 | ND | 0 | 0 | |
| C | C | C | C | T | T | 205 | 12 | ND | 0 | 0 | |
| C | C | C | C | T | 1,594 | 998 | 54 | ND | 0 | 0 | |
| C | C | C | C | 342 | 0 | 0 | 0 | ND | 0 | 0 | |
| C | C | C | C | C | T | T | 458 | ND | 54 | 0 | |
| C | C | C | C | 1,536 | 376 | 20 | 3 | ND | 0 | 0 | |
| C | C | C | T | T | 135 | 49 | 2 | ND | 0 | 0 | |
Nasopharyngeal washes were performed as described in Materials and Methods, and the recovered washes were plated in duplicate on Regan-Lowe plates with cephalexin. The number of CFU per 50 μl of nasopharyngeal wash was recorded for the indicated days postinoculation. T, colonies were distinguishable but were too numerous to count (>2,000); C, confluent growth; ND, not determined.
Fig 3.
Leukocytosis, cough illness, and fever in pertussis-infected baboons. (A) Once prior to inoculation and on the indicated days postinoculation, blood was drawn from each weanling baboon, and the number of circulating white blood cells per microliter of peripheral blood was determined (n = 9). (B) A subset of infected baboons (n = 6) were observed four times per day as described in Materials and Methods. The number of coughs per hour was determined for each animal at each observation period, and the overall mean was reported for each day postinoculation. (C) The core temperature of each baboon was measured three times prior to inoculation and at each examination postinoculation. One data point is presented for each baboon (n = 9) at each time point.
Bordetella pertussis infection causes a long-lived antibody response.
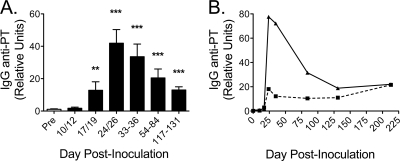
To analyze the antibody response to pertussis, we measured anti-PT IgG in serum. Preinfection samples were compared to samples taken postinfection, and relative units were calculated by comparison to a human reference standard (Fig. 4A). There was very little PT-reactive IgG in the preinfection samples. Titers were relatively unchanged on days 10 to 12 postinoculation before increasing significantly on days 17 to 19. Anti-PT levels peaked at days 24 to 26 in all seven animals, with a mean 40-fold enhancement relative to preinfection values. Anti-PT titers gradually declined at later time points but remained significantly enhanced through 4 months (Fig. 4A). The kinetics of the anti-PT response for the first two animals illustrates that the magnitude of the response varies greatly between infected animals and that the anti-PT antibody response is long-lived, having persisted through the latest serum collections at 7 months postinoculation (Fig. 4B).
Fig 4.
Serological response to pertussis toxin in B. pertussis-infected baboons. (A) Once prior to and on the indicated range of days after inoculation, sera were collected and anti-PT IgG was measured by ELISA as described in Materials and Methods (n = 7 for all time points except for days 117 to 131, for which n = 4). Data are presented as relative units (percent anti-PT IgG compared to a standard). (B) The complete anti-PT IgG kinetics for two of the seven baboons are shown. **, P < 0.01; ***, P < 0.001 versus preinfection.
Infection confers protective immunity.
Four baboons were held for 6 months following recovery from B. pertussis infection, and an additional two age-matched, naïve baboons were obtained. All six baboons were inoculated with 109 CFU of B. pertussis strain D420. The two naïve baboons developed symptoms of clinical pertussis that were indistinguishable from those observed in the pertussis-infected weanling baboons described above (Fig. 3 and Table 1). Very large numbers of bacteria were recovered from nasopharyngeal washes from both naïve baboons, and both exhibited very high WBC counts, with peak levels between 5- and 10-fold above the baseline values (Table 2 and Fig. 5A). Both naïve baboons developed severe coughs. In contrast, the four convalescent baboons did not develop disease upon reinfection. Very small numbers of B. pertussis CFU were isolated transiently from two of these baboons (Table 2). No increase in WBC counts and no coughing were observed in the four reinfected animals (Fig. 5B). An examination of anti-PT antibody titers demonstrated that in the convalescent animals, there was a rapid increase in titers that was evident as early as day 5 and peaked by day 7 (Fig. 5C). In the two naïve baboons, an anti-PT antibody response was not observed in the 2 weeks following inoculation, similar to the trend seen following primary infection of the naïve weanling baboons (Fig. 4 and 5C).
Table 2.
Colonization of infected naïve and reinfected adolescent baboons
| Infected animal group | Colonization on indicated day postinoculationa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2/3 | 5 | 7 | 9/10 | 12 | 14 | 16/17 | 19 | 21 | 26 | 33 | |
| Naïve adolescent | C | C | C | C | T | 310 | 0 | 0 | 0 | 0 | 0 |
| C | C | C | C | T | T | 150 | 0 | 0 | 0 | 0 | |
| Reinfected adolescent | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 14 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Nasopharyngeal washes were performed as described in Materials and Methods, and the recovered washes were plated in duplicate on Regan-Lowe plates with cephalexin. The number of CFU per 50 μl of nasopharyngeal wash was recorded for the indicated days postinoculation. T, colonies were distinguishable but were too numerous to count (>2,000); C, confluent growth.
Fig 5.
Reinfected convalescent baboons fail to develop leukocytosis and mount an anamnestic serological response. Convalescent baboons that had recovered from a previous pertussis infection (n = 4) and age-matched naïve baboons (n = 2) were inoculated with B. pertussis. The number of circulating white blood cells per microliter of peripheral blood was determined for the naïve baboons (A) and the convalescent baboons (B) once prior to inoculation and on the indicated days postinoculation. (C) Anti-PT IgG was measured by ELISA, as described in Materials and Methods, for naïve (gray bars) and convalescent (black bars) baboons. Data are presented as relative units on a logarithmic scale. **, P < 0.01; ***, P < 0.001 for convalescent versus naïve baboons.
DISCUSSION
The lack of an adequate animal model of pertussis has resulted in significant gaps in our knowledge of pertussis pathogenesis and the mechanisms of vaccine-mediated protection. This has hindered our ability to understand the ongoing rise in pertussis cases and means we do not have the tools for effective preclinical evaluation of therapeutics and next-generation vaccines. Reports in the literature show that M. cyclopsis provided a good model of pertussis. However, these studies were conducted 50 years ago, and this species of monkey is no longer available for biomedical research (13, 16). Other reports suggested that rhesus macaques were not particularly susceptible to pertussis, but little experimental detail was provided (5, 19, 22). Our studies with rhesus macaques confirmed that this species does not provide a reliable model of pertussis disease. The reasons that B. pertussis causes only mild infections in these animals are unknown and could involve physiological and/or genetic differences between rhesus macaques and pertussis-susceptible hosts. We believe that one important factor that contributes to the lack of disease in these animals is the relatively high body temperature of rhesus macaques compared to humans and baboons. In accordance with this hypothesis, we observed that B. pertussis exhibited a reduced growth rate in vitro at 39°C relative to that at 37°C. Our results also demonstrated that adenylate cyclase protein levels, but not RNA transcript levels, were reduced significantly in vitro at 39°C (Fig. 2) compared to 37°C. While the exact mechanism is unclear, several possible explanations for the reduced adenylate cyclase expression include less efficient translation of adenylate cyclase protein at 39°C than at 37°C or instability of the protein and partial unfolding at 39°C resulting in proteolysis by cellular proteases. The observation that the activity of purified adenylate cyclase is significantly reduced at 40°C relative to that at 37°C is consistent with the latter possibility (18). Together, the reduced growth rate and diminished expression of a key virulence factor at 39°C provide a plausible hypothesis as to why rhesus macaques are not easily infected with B. pertussis. If our temperature hypothesis proves true, it will suggest that most macaques and new-world monkeys will not provide a reliable model for pertussis, as they have normal body temperature ranges above 39°C. These temperature data are also intriguing given that pertussis-infected human patients lack significant fever (17). Pertussis-infected baboons exhibited only a minor and transient elevation in body temperature at the peak of infection (Fig. 3). In order to be an effective pathogen, a bacterium must either tolerate elevated temperatures in the host or avoid inducing fever. Our results suggest that B. pertussis utilizes the latter strategy.
Because the olive baboon has a normal body temperature range closer to that of humans, we evaluated the baboon as a model of pertussis disease. Our results led us to conclude that the baboon provides an excellent model of clinical pertussis. One hundred percent of baboons infected with a clinical isolate of B. pertussis exhibited all of the hallmark manifestations of human pertussis, including paroxysmal coughing, mucus production, and leukocytosis. The establishment of this model provides the opportunity to address unanswered questions about the natural progression of this disease in an extremely relevant model.
It has long been recognized that antibodies play an important role in protection against pertussis infection. While a correlate of protection has not been identified, household exposure studies suggest that titers of antibodies against PT and/or the B. pertussis adhesins pertactin and fimbriae may contribute to protection of vaccinated family members living with B. pertussis-infected patients (3, 26, 27). We measured serum anti-PT IgG titers before, during, and after infection. We chose PT because it is generally considered the most sensitive serological marker of pertussis infection (29). In our study, all seven animals tested showed a significant increase in anti-PT IgG. Several aspects of our ELISA data were of interest. The first was the range of peak amplitudes observed for different animals, as highlighted in Fig. 4. This phenomenon is also seen in humans with confirmed pertussis infections (6, 28). Also, as shown in Fig. 4, serum anti-PT follows a biphasic course, with a peak and initial rapid decrease followed by a much more gradual decline. The anti-PT response appears to be long-lived in the baboon model, with strong titers remaining after 7 months in the first two baboons. This is similar to the longevity seen in pertussis-infected humans, as anti-PT IgG levels remain elevated for 1 year or more following infection (6, 29). Another interesting observation was that the detection of serum anti-PT IgG roughly overlapped with clearance of the infection, with both occurring at about 3 weeks postinoculation. This suggests that the adaptive immune response is effective at clearing B. pertussis infection and is consistent with the observation that most human patients clear infection within a few weeks of the onset of symptoms.
Our results further demonstrate that infection results in protective anamnestic immunity. Antibody responses induced upon a second exposure peaked within the first week, and peak titers were 20-fold higher, on average, than those seen in the same animals following the primary exposure. The adaptive immune response prevented establishment of infection and the appearance of disease manifestations. The observation that it was not possible to recover bacteria from two of the four reinfected animals and that only very small numbers of bacteria were transiently recovered from the other two demonstrates that immunity following infection prevents not just disease but also reinfection of the immune individual. Given these data, we hypothesize that individuals who have recovered from a natural pertussis infection do not serve as effective vectors of disease for at least some period following their recovery. Indeed, clinical and epidemiological studies have described cases of pertussis reinfection in children. These studies suggest an estimate for the duration of immunity of between 3.5 and 12 years (30). The demonstration that the immunity conferred by infection in the baboon model is sufficient to protect from subsequent reinfection provides a starting point for the evaluation of pertussis vaccines and shows the utility of the baboon model as a tool to fill in the gaps in our understanding of vaccine-mediated protection from pertussis.
ACKNOWLEDGMENTS
We thank Lewis Shankle for technical assistance, Gary White and Roman Wolf for many helpful discussions, Erik Hewlett for helpful discussions and for providing anti-ACT antibody, and Jerry Keith for providing antibodies 1B7 and 1C6.
This work was funded by the Food and Drug Administration and NIH/NIAID through interagency agreement Y1-AI-1727-01. Baboons were obtained from the Oklahoma Baboon Research Resource. The Oklahoma Baboon Research Resource was supported by grants P40RR012317 and 5R24RR016556-10 from the National Institutes of Health National Center for Research Resources.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. CDC 2011. Pertussis, p 215–232 In Epidemiology and prevention of vaccine-preventable diseases, 12th ed Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 2. CDC 2011. Provisional cases of selected notifiable diseases. MMWR Morb. Mortal. Wkly. Rep. 59:1706–1716 [Google Scholar]
- 3. Cherry JD, Gornbein J, Heininger U, Stehr K. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901–1906 [DOI] [PubMed] [Google Scholar]
- 4. Cherry JD, Heininger U. 2009. Pertussis and other bordetella infections, p 1683–1706 In Feigin RD, Cherry JD, Demmler-Harrison GJ, Kaplan SL. (ed), Textbook of pediatric infectious diseases. W. B. Saunders, Philadelphia, PA [Google Scholar]
- 5. Culotta CS, Harvey DF, Gordon EF. 1935. Whooping cough. II. Experimental study. J. Pediatr. 6:743–752 [Google Scholar]
- 6. Dalby T, Petersen JW, Harboe ZB, Krogfelt KA. 2010. Antibody responses to pertussis toxin display different kinetics after clinical Bordetella pertussis infection than after vaccination with an acellular pertussis vaccine. J. Med. Microbiol. 59:1029–1036 [DOI] [PubMed] [Google Scholar]
- 7. Dalby T, Seier-Petersen M, Kristiansen MP, Harboe ZB, Krogfelt KA. 2009. Problem solved: a modified enzyme-linked immunosorbent assay for detection of human antibodies to pertussis toxin eliminates false-positive results occurring at analysis of heat-treated sera. Diagn. Microbiol. Infect. Dis. 63:354–360 [DOI] [PubMed] [Google Scholar]
- 8. Elahi S, Holmstrom J, Gerdts V. 2007. The benefits of using diverse animal models for studying pertussis. Trends Microbiol. 15:462–468 [DOI] [PubMed] [Google Scholar]
- 9. Friedrich MJ. 2011. Research aims to boost pertussis control. JAMA 306:27–29 [DOI] [PubMed] [Google Scholar]
- 10. Heininger U. 2010. Update on pertussis in children. Expert Rev. Anti Infect. Ther. 8:163–173 [DOI] [PubMed] [Google Scholar]
- 11. Hewlett EL, Kim KJ, Lee SJ, Gray MC. 2000. Adenylate cyclase toxin from Bordetella pertussis: current concepts and problems in the study of toxin functions. Int. J. Med. Microbiol. 290:333–335 [DOI] [PubMed] [Google Scholar]
- 12. Hozbor D, et al. 2009. Pertussis epidemiology in Argentina: trends over 2004–2007. J. Infect. 59:225–231 [DOI] [PubMed] [Google Scholar]
- 13. Huang CC, et al. 1962. Experimental whooping cough. N. Engl. J. Med. 266:105–111 [DOI] [PubMed] [Google Scholar]
- 14. Knapp S, Mekalanos JJ. 1988. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J. Bacteriol. 170:5059–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reference deleted.
- 16. Lin TM. 1958. Experimental whooping cough in monkey. J. Formos. Med. Assoc. 57:53–62 [Google Scholar]
- 17. Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murayama T, Hewlett EL, Maloney NJ, Justice JM, Moss J. 1994. Effect of temperature and host factors on the activities of pertussis toxin and Bordetella adenylate cyclase. Biochemistry 33:15293–15297 [DOI] [PubMed] [Google Scholar]
- 19. North EA, Keogh EV, Christie R, Anderson G. 1940. Experimental pertussis in the monkey. Aust. J. Exp. Biol. Med. Sci. 18:125–130 [Google Scholar]
- 20. Quinn HE, McIntyre PB. 2007. Pertussis epidemiology in Australia over the decade 1995–2005—trends by region and age group. Commun. Dis. Intell. 31:205–215 [DOI] [PubMed] [Google Scholar]
- 21. Rich AR, Long PH, Brown JH, Bliss EA, Holt LE. 1932. Experiments upon the cause of whooping cough. Science 76:330–331 [DOI] [PubMed] [Google Scholar]
- 22. Sauer LW, Hambrecht L. 1928. Experimental whooping cough. Am. J. Dis. Child. 37:732–744 [Google Scholar]
- 23. Schneider DR, Parker CD. 1982. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect. Immun. 38:548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shibley GS. 1934. Etiology of whooping cough. Proc. Soc. Exp. Biol. Med. 31:576–579 [Google Scholar]
- 25. Stibitz ES. 2006. The bvgASR locus of Bordetella pertussis, p 47–67 In Locht C. (ed), Bordetella molecular biology. Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 26. Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907–1916 [DOI] [PubMed] [Google Scholar]
- 27. Taranger J, et al. 2000. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J. Infect. Dis. 181:1010–1013 [DOI] [PubMed] [Google Scholar]
- 28. Teunis PF, et al. 2002. Kinetics of the IgG antibody response to pertussis toxin after infection with B. pertussis. Epidemiol. Infect. 129:479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watanabe M, Connelly B, Weiss AA. 2006. Characterization of serological responses to pertussis. Clin. Vaccine Immunol. 13:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wendelboe AM, Van Rie A, Salmaso S, Englund JA. 2005. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24:S58–S61 [DOI] [PubMed] [Google Scholar]
- 31. WHO 2010. Pertussis vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:385–400 [PubMed] [Google Scholar]