Abstract
Escherichia coli sequence type ST131 (O25b:H4) has emerged over the past decade as a globally disseminated, multidrug-resistant pathogen. Unlike traditional antimicrobial-resistant E. coli, ST131 derives from virulence-associated phylogenetic group B2 and exhibits extraintestinal virulence factors. This, plus preliminary evidence of virulence in experimental animals, has suggested that ST131's epidemic emergence may be due to high virulence potential, compared with other E. coli types. To test this hypothesis, we compared a large number of matched ST131 and non-ST131 E. coli clinical isolates, both fluoroquinolone resistant and susceptible, plus isolates from classic extraintestinal pathogenic E. coli (ExPEC) sequence types (STs) and case report ST131 household transmission isolates, for virulence in a mouse subcutaneous sepsis model. Overall, in mice, the study isolates produced a wide range of lethality and clinical illness. However, neither ST131 status nor fluoroquinolone phenotype correlated with this diversity of illness severity, which occurred within each of the 6 study groups. In contrast, multiple known or suspected ExPEC virulence genes, including pap (P fimbriae), vat (vacuolating toxin), kpsM II (group 2 capsule), ibeA (invasion of brain endothelium), and clbB/N (colibactin synthesis), plus molecularly defined ExPEC status, were significantly associated with virulence. These findings point away from ST131 isolates as having higher virulence potential compared with other E. coli types in causing invasive extraintestinal infections and suggest instead that ST131's epidemiological success may reflect enhanced fitness for upstream steps in pathogenesis or in colonization and transmission. Additionally, the extensive within-ST virulence diversity suggests an opportunity to compare closely related strains to identify the responsible genetic determinants.
INTRODUCTION
Escherichia coli strains of sequence type ST131 (O25b:H4) have emerged over the past decade as a globally disseminated cause of multidrug-resistant extraintestinal infections (41, 44). Most ST131 clinical isolates are fluoroquinolone (FQ) resistant; many also are coresistant to aminoglycosides and/or trimethoprim-sulfamethoxazole, and a minority produces extended-spectrum beta-lactamases (ESBLs) that confer resistance to extended-spectrum cephalosporins (16, 41, 44).
Unlike traditional antimicrobial-resistant E. coli (21, 22), ST131 derives from virulence-associated phylogenetic group B2 and exhibits extraintestinal virulence factors, including various adhesins, toxins, siderophores, and protectins, thereby qualifying molecularly as extraintestinal pathogenic E. coli (ExPEC) (5, 6, 16, 38). This, plus limited evidence of experimental virulence (5) and several case reports of unusually severe or fatal extraintestinal infections due to ST131 (8, 11, 40, 48), has suggested that ST131's rapid and extensive emergence may be due in part to a high virulence potential, compared with other E. coli types.
To test the high virulence potential hypothesis, we used a mouse subcutaneous sepsis model (42) to assess the virulence of 22 ST131 and 22 non-ST131 E. coli clinical isolates, divided as FQ resistant versus FQ susceptible, and matched for year of isolation and geographical locale. For comparison, we included 12 isolates from selected classic ExPEC sequence types (STs) (29) and 5 ST131 isolates from case reports of severe disease that involved suspected within-household transmission (8, 11, 40). We also compared the murine sepsis model results with the E. coli phylogenetic group and the presence of multiple proven or suspected ExPEC virulence genes.
MATERIALS AND METHODS
E. coli isolates.
The 62 E. coli study isolates included 61 human extraintestinal clinical isolates of diverse origins (Table 1) plus E. coli K-12 derivative MG1655 (3). Of the 61 clinical isolates, 44 were selected systematically as described below from larger published or unpublished collections, as 11 matched sets of 4 isolates each, to give a balanced distribution of ST131 and non-ST131 isolates. Source collections for these included (i) urine isolates from kidney transplant patients in Galveston, TX (2004 to 2005) (17), (ii) the SENTRY and TRUST (tracking resistance in the United States today) national surveillance programs (2003 to 2009) (16, 45), (iii) urine isolates from women across Canada with uncomplicated acute cystitis, from the NAUTICA program (2002 to 2004) (23), and (iv) consecutive E. coli isolates from the Minneapolis VA Medical Center clinical microbiology laboratory (October 2011 to December 2011). The members of these collections had already been tested for ST131 status by PCR detection of ST131-specific single-nucleotide polymorphisms in gyrB and mdh and/or full multilocus sequence typing (23) and for FQ phenotype by broth microdilution or disk diffusion, using Clinical and Laboratory Standards Institute-specified methods, control strains, and interpretive criteria.
Table 1.
Characteristics of 61 sequence type ST131 and non-ST131 extraintestinal E. coli clinical isolates studied in the murine sepsis model
| Study group | Strain identification no. | Epidemiological background |
Phylogenetic background |
Resistance trait |
Virulence trait |
Mouse result |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeara | Locale | Clinical contextb | ST/CC (O:K:H serotype)c | Phylod | ESBLe | FQRf | VF scoreg | ExPECh | Lethalityi | Mean statusj | ||
| ST131 (FQ-R) | JJ1899 | 2004 | Galveston, TX | Post-renal transplant bacteriuria | ST131 | B2 | Yes | 8.00 | Yes | 0.10 | 2.00 | |
| JJ1904 | 2005 | Galveston, TX | Post-renal transplant bacteriuria | ST131 | B2 | Yes | 9.00 | Yes | 0.00 | 1.33 | ||
| JMI-185 | 2009 | Louisville, KY | Bacteremia | ST131 | B2 | Yes | Yes | 12.00 | Yes | 0.00 | 1.67 | |
| JMI-268 | 2006 | Little Rock, AR | n/ak | ST131 | B2 | Yes | 11.00 | Yes | 1.00 | 5.00 | ||
| SaT-049 | 2007 | Stamford, CT | Wound | ST131 | B2 | Yes | 9.00 | Yes | 0.00 | 1.00 | ||
| SaT-158 | 2003 | New York, NY | Bacteremia | ST131 | B2 | Yes | 9.00 | Yes | 0.00 | 1.00 | ||
| Z-164 | 2004 | Winnipeg, MB | Cystitis | ST131 | B2 | Yes | 9.00 | Yes | 0.20 | 2.00 | ||
| Z-193 | 2004 | Moncton, NB | Cystitis | ST131 | B2 | Yes | 10.00 | Yes | 1.00 | 4.67 | ||
| MV0084 | 2010 | Minneapolis, MN | Asymptomatic bacteriuria | ST131 | B2 | Yes | 8.00 | Yes | 0.20 | 1.50 | ||
| MV0158 | 2010 | Minneapolis, MN | Asymptomatic bacteriuria | ST131 | B2 | Yes | 10.00 | Yes | 0.30 | 1.83 | ||
| MV0179 | 2011 | Minneapolis, MN | Febrile urinary tract infection | ST131 | B2 | Yes | 9.00 | Yes | 1.00 | 4.67 | ||
| ST131 (FQ-S) | JJ1897 | 2004 | Galveston, TX | Post-renal transplant bacteriuria | ST131 | B2 | 11.00 | Yes | 1.00 | 4.17 | ||
| JJ1901 | 2004 | Galveston, TX | Post-renal transplant bacteriuria | ST131 | B2 | 12.00 | Yes | 0.50 | 3.00 | |||
| JMI-178 | 2006 | Little Rock, AR | Bacteremia | ST131 | B2 | 10.00 | Yes | 0.00 | 1.67 | |||
| JMI-237 | 2009 | Louisville, KY | Bacteremia | ST131 | B2 | 9.00 | Yes | 1.00 | 4.67 | |||
| SaT-040 | 2007 | Burlington, VT | Bacteriuria | ST131 | B2 | 12.00 | Yes | 0.20 | 2.00 | |||
| SaT-142 | 2003 | New York, NY | n/a | ST131 | B2 | 12.00 | Yes | 1.00 | 4.67 | |||
| Z-063 | 2002 | Winnipeg, MB | Cystitis | ST131 | B2 | 12.00 | Yes | 1.00 | 3.67 | |||
| Z-071 | 2002 | Moncton, NB | Cystitis | ST131 | B2 | 10.00 | Yes | 1.00 | 4.33 | |||
| MV0130 | 2010 | Minneapolis, MN | Cystitis | ST131 | B2 | 12.00 | Yes | 0.30 | 2.00 | |||
| MV0131 | 2010 | Minneapolis, MN | Asymptomatic bacteriuria | ST131 | B2 | 8.00 | Yes | 0.00 | 2.00 | |||
| MV0167 | 2011 | Minneapolis, MN | Bacteriuria | ST131 | B2 | 8.00 | Yes | 0.00 | 1.33 | |||
| Non-ST131 (FQ-R) | JJ2106 | 2004 | Galveston, TX | Post-renal transplant bacteriuria | Non-ST131 | A | Yes | 3.00 | Yes | 0.00 | 1.33 | |
| JMI-172 | 2006 | Kansas City, MO | Bacteremia | Non-ST131 | B2 | Yes | 12.75 | Yes | 0.10 | 1.33 | ||
| SaT-034 | 2007 | Burlington, VT | Bacteriuria | Non-ST131 | B2 | Yes | 13.00 | Yes | 0.40 | 2.50 | ||
| Z-064 | 2002 | Winnipeg, MB | Cystitis | Non-ST131 | B2 | Yes | 8.00 | Yes | 1.00 | 4.67 | ||
| JJ2112 | 2005 | Galveston, TX | Post-renal transplant bacteriuria | Non-ST131 | D | Yes | 5.00 | 0.00 | 1.33 | |||
| JMI-216 | 2009 | Louisville, KY | Bacteremia | Non-ST131 | D | Yes | 6.00 | Yes | 0.40 | 3.00 | ||
| SaT-157 | 2003 | New York, NY | Bacteriuria | Non-ST131 | D | Yes | 7.00 | Yes | 1.00 | 5.00 | ||
| Z-136 | 2004 | Moncton, NB | Cystitis | Non-ST131 | D | Yes | 8.00 | Yes | 1.00 | 4.67 | ||
| MV0037 | 2010 | Minneapolis, MN | Urosepsis | Non-ST131 | D | Yes | 6.00 | Yes | 1.00 | 4.67 | ||
| MV0095 | 2010 | Minneapolis, MN | Urosepsis | Non-ST131 | D | Yes | 6.25 | Yes | 0.00 | 1.33 | ||
| MV0117 | 2010 | Minneapolis, MN | Urinary tract infection | Non-ST131 | D | Yes | 8.00 | Yes | 0.10 | 1.50 | ||
| Non-ST131 (FQ-S) | JJ2111 | 2005 | Galveston, TX | Post-renal transplant bacteriuria | Non-ST131 | A | 8.00 | Yes | 0.70 | 4.00 | ||
| JMI-235 | 2009 | Lexington, KY | Bacteremia | Non-ST131 | A | 2.00 | 0.00 | 1.67 | ||||
| Z-004 | 2002 | Winnipeg, MB | Cystitis | Non-ST131 | A | 7.25 | Yes | 0.00 | 2.00 | |||
| SaT-038 | 2007 | Burlington, VT | Bacteriuria | Non-ST131 | B1 | 3.00 | 0.00 | 1.00 | ||||
| Z-011 | 2002 | Moncton, NB | Cystitis | Non-ST131 | B1 | 8.00 | Yes | 1.00 | 3.67 | |||
| JJ2101 | 2004 | Galveston, TX | Post-renal transplant bacteriuria | Non-ST131 | B2 | 12.00 | Yes | 1.00 | 4.67 | |||
| MV0009 | 2010 | Minneapolis, MN | Pyelonephritis | Non-ST131 | B2 | 17.00 | Yes | 1.00 | 4.67 | |||
| MV0021 | 2010 | Minneapolis, MN | Surgical wound infection | Non-ST131 | B2 | 13.00 | Yes | 0.00 | 2.00 | |||
| MV0112 | 2010 | Minneapolis, MN | Cystitis | Non-ST131 | B2 | 12.50 | Yes | 1.00 | 4.67 | |||
| JMI-145 | 2006 | Little Rock, AR | Bacteremia | Non-ST131 | D | 6.00 | Yes | 0.90 | 3.67 | |||
| SaT-156 | 2003 | New York, NY | Bacteremia | Non-ST131 | D | Yes | 10.00 | Yes | 0.00 | 1.33 | ||
| Classic ExPEC | 536 | n/a | n/a | Pyelonephritis | ST127 (O6:K15:H31) | B2 | 11.00 | Yes | 1.00 | 5.00 | ||
| CFT073 | n/a | n/a | Pyelonephritis with bacteremia | ST73 (O6:K2:H1) | B2 | 17.00 | Yes | 0.74 | 4.00 | |||
| ECOR 64 | n/a | n/a | Cystitis | ST81 (O75:K5:H−) | B2 | 11.00 | Yes | 0.40 | 2.67 | |||
| NU14 | n/a | n/a | Cystitis | ST95 (O18:K1:H7) | B2 | 17.00 | Yes | 0.00 | 1.33 | |||
| RS218 | n/a | n/a | Neonatal meningitis | ST95 (O18:K1:H7) | B2 | 17.00 | Yes | 1.00 | 5.00 | |||
| U8 | n/a | n/a | Cystitis | ST95 (O18:K1:H7) | B2 | 17.00 | Yes | 1.00 | 5.00 | |||
| IA2 | n/a | n/a | Urinary tract infection | ST12/CC12 (O4:K12:H−) | B2 | 15.00 | Yes | 0.00 | 3.00 | |||
| CP9 | n/a | n/a | Bacteremia | ST599/CC12 (O4:K54:H5) | B2 | 15.00 | Yes | 1.00 | 5.00 | |||
| PY76 | n/a | n/a | Pyelonephritis | ST69 (O17:K52:H18) | D | 9.00 | Yes | 0.21 | 1.67 | |||
| 2P9 | n/a | n/a | Urosepsis | ST598/CC31 (O15:K52:H1) | D | 8.00 | Yes | 0.90 | 4.67 | |||
| PY14 | n/a | n/a | Pyelonephritis | ST59 (O1:K1:H−) | D | 10.00 | Yes | 0.20 | 2.67 | |||
| ECOR 40 | n/a | n/a | Pyelonephritis | ST62 (O7:K1:H−) | D | 6.00 | Yes | 0.80 | 4.00 | |||
| ST131 case reports | CU758 | 2007 | Columbia, MO | Septic arthritis, osteomyelitis | ST131 | B2 | Yes | 10.00 | Yes | 1.00 | 5.00 | |
| JJ1886 | 2008 | Portland, ME | Fatal urosepsis (see JJ1887) | ST131 | B2 | Yes | Yes | 9.00 | Yes | 0.70 | 4.67 | |
| JJ1887 | 2008 | Portland, ME | Recurrent cystitis (see JJ1886) | ST131 | B2 | Yes | Yes | 9.00 | Yes | 0.50 | 3.17 | |
| JJ2546 | 2008 | Bethlehem, PA | Renal abscesses (see JJ2547) | ST131 | B2 | Yes | Yes | 11.00 | Yes | 0.40 | 2.33 | |
| JJ2547 | 2008 | Bethlehem, PA | Pyelonephritis (see JJ2546) | ST131 | B2 | Yes | Yes | 11.00 | Yes | 0.00 | 1.67 | |
Year, year of isolation (shown only for recent clinical isolates).
Clinical context based on patient's clinical presentation, if known; otherwise, on specimen type. Paired same-household isolates JJ1886 and JJ1887 and JJ2546 and JJ2547 involved presumed within-household transmission, as did CU758 (which matched a fecal isolate from the 4-month-old patient's mother that was not studied here).
CC, clonal complex (= ST complex); includes STs that differ from the complex's index ST at a single locus. O:K:H serotype shown only for classic extraintestinal pathogenic E. coli (ExPEC) isolates.
Phylo, major phylogenetic group (A, B1, B2, D).
ESBL, extended-spectrum β-lactamase production. “No” is indicated by blank cells.
FQR, fluoroquinolone resistance. “No” is indicated by blank cells.
VF score, virulence factor score; calculated as the sum of all virulence genes detected, with adjustment for multiple detection of the pap (P fimbriae), sfa/foc (S and F1C fimbriae), and kpsM II (group 2 capsule) operons.
ExPEC, extraintestinal pathogenic E. coli, defined as presence of ≥2 of papAH and/or papC (P fimbriae structural subunit and assembly), sfa/foc, afa/dra (Dr family adhesins), iutA (aerobactin system), and kpsM II. “No” is indicated by blank cells.
Lethality, fraction of mice challenged with isolate (among ≥5 mice per isolate) that became moribund or died by 3 days postchallenge.
Median value for mouse mean status (according to daily observations over 3 days postchallenge), among the ≥5 mice challenged per isolate, where 1 = healthy, 2 = mildly ill, 3 = moderately ill, 4 = severely ill/moribund, and 5 = dead.
n/a, not available or not applicable.
The 11 matched sets of 4 were assembled by identifying, within each collection, for each of the collection's FQ-susceptible ST131 isolates, 3 geographically and temporally matched comparison isolates. The 3 comparison isolates per set were the representatives of the 3 alternate FQ/ST131 groups, i.e., FQ-resistant ST131 isolates, FQ-susceptible non-ST131 isolates, and FQ-resistant non-ST131 isolates, that most closely matched the index FQ-susceptible ST131 isolate by year of isolation and locale. Temporal matching was within 2 years. Geographical matching was by city when possible, although by state or adjacent states was allowed when necessary. This gave 4 temporally and geographically matched comparison groups of 11 isolates each, representing the 4 possible combinations of ST131 status and FQ phenotype. Only 2 of these isolates (one ST131, one non-ST131) were ESBL positive (Table 1). The isolates represented such clinical syndromes as cystitis, pyelonephritis, febrile urinary tract infection, urosepsis, post-renal transplant bacteriuria, asymptomatic bacteriuria, and surgical wound infection (Table 1).
To supplement these generic clinical isolates, a set of 12 urine and blood isolates representing 9 classic ExPEC clonal groups, 1 to 3 representatives per clonal group, was assembled from the investigator's collection (29) (Table 1). Included were 8 strains from phylogenetic group B2, representing 5 different clonal groups, i.e., CC12 (O4:K+:H5/H−) (31), ST73 (O6:K2:H1) (18), ST81 (O75:K5:H−) (9), ST95 (O18:K1:H7) (14), and ST127 (O6:K15:H31) (18), and 4 strains from phylogenetic group D, representing 4 different clonal groups, i.e., ST59 (O1:K1:H−) (1), ST62 (O7:K1:H−) (1), ST69 (also known as “clonal group A”; O17:K52:H18) (24), and ST598/CC31 (O15:K52:H1) (39). The specific clinical syndromes represented by these strains included cystitis, pyelonephritis, urosepsis, and neonatal meningitis (Table 1).
Finally, 5 FQ-resistant (+/− ESBL positive) ST131 isolates that had been the subject of case reports because of their distinctive clinical behavior and seeming within-household transmission were also studied (8, 11, 40) (Table 1). All represented pulsotype 968, the predominant pulsotype among recent ST131 isolates in the United States (27). Of the 5 isolates, 4 were paired isolates from epidemiologically associated adults (a father-daughter pair and 2 sisters) from 2 different families in widely separated locales (8, 40). Within each such isolate pair, one isolate had caused a mild-to-moderately severe infection in one family member, whereas the other had caused a severe or fatal infection in a different family member. The fifth ST131 case report isolate was from a 4-month-old girl with protracted septic arthritis and contiguous osteomyelitis (11). This strain likewise was identified in another household member (the patient's mother), as a fecal colonizer, which was not studied here.
Mouse sepsis model.
Isolates were tested for extraintestinal virulence using an established mouse model of systemic sepsis (13, 42). Approximately 108 CFU of log-phase organisms (from shaking broth cultures), suspended in saline, were injected subcutaneously into the nape of the neck of female Swiss-Webster mice (mean weight, 23 g; range, 20 to 30 g). Mice were observed twice daily over the following 3 days for health status, which was scored on a 5-step scale (1 = healthy, 2 = minimally ill, 3 = moderately ill, 4 = severely ill, 5 = dead), with the worst score on a given day being used as the score for that day. Mice were euthanatized if observed in stage 4 illness or at the end of the 72-h observation period, whichever came first. Mice euthanatized on day 1 or 2 received a score of 5 for the subsequent day(s). The mean of the three daily health status scores was used to summarize quantitatively each mouse's infection experience over the 3-day observation period. In addition, mice were scored as “dead” or “alive,” with any daily status score of 4 or 5 qualifying as “dead,” since in pilot experiments all mice that reached stage 4 illness died spontaneously either later that day or overnight (not shown).
For each isolate, two main outcome measures were assigned. The lethality score was the proportion of mice challenged with the isolate (0 to 1.0) that died within 3 days postinoculation, whereas the median mouse mean status score (1.0 to 5.0) was the group median for mouse mean status for all mice inoculated with the isolate. Additionally, isolates were classified as rapidly lethal if ≥50% of mice challenged with them died within 24 h postinjection, which corresponds with a median mean status score of 5.0.
On each day of mouse injections, the test isolates included an equal number of representatives of each of the 4 matched isolate groups (groups 1 to 4), +/− classic ExPEC strains or case report ST131 isolates (groups 5 to 6). Test isolates were administered to 5 mice each, in random sequence. In parallel, 5 mice each also received strains CFT073 (positive control) (32) and E. coli K-12 derivative MG1655 (negative control) (3). Test isolates that exhibited disproportionate variability of effect (i.e., standard error [SE] > 0.20 for mouse mean status) among the first 5 mice challenged were tested subsequently in 5 additional mice, and these results were then pooled with those from the initial 5 mice.
To confirm specificity of effect, in pilot experiments, postmortem quantitative cultures of cardiac puncture blood and spleen homogenates were done at the time of euthanasia or for mice found dead. Consistently, healthy mice had sterile or very low-count cultures, whereas cultures from ill mice were uniformly positive and yielded heavier growth, with bacterial densities correlating significantly with clinical severity (not shown). Likewise, for each of several dozen mice challenged with various isolates, postmortem spleen and blood isolates matched the corresponding challenge isolate according to random amplified polymorphic DNA profiling (2), whereas the different challenge isolates exhibited distinctive profiles (not shown).
Virulence genotyping and phylotyping.
Isolates were characterized for 43 virulence-associated genes and variants thereof by using established multiplex PCR-based assays (Table 2) (23). Major E. coli phylogenetic group was assessed by triplex PCR, using the initially described interpretive criteria (4). Testing was done in duplicate, using as template DNA boiled lysates prepared separately from two different colonies of each isolate. The virulence score was the number of virulence markers detected, adjusted for multiple detection of the pap (P fimbriae), sfa/foc (S and F1C fimbriae), and kps II (group 2 capsule) operons. The molecular criterion for ExPEC was detection of ≥2 of the following 5 markers: papA and/or papC (P fimbria structural subunit and assembly; counted as 1), sfa/focDE, afa/draBC (Dr-binding adhesins), kpsM II, and iutA (aerobactin system) (26).
Table 2.
Distribution of virulence-associated traits and phylogenetic group among 6 groups of extraintestinal E. coli isolates
| Trait category | Specific trait | Prevalence of traita,b within group, no. (column %) |
P valued (across all 6 groups) | |||||
|---|---|---|---|---|---|---|---|---|
| Matched setsc |
Classic ExPEC (n = 12) | ST131 case reports (n = 5) | ||||||
| ST131, FQ-R (n = 11) | ST131, FQ-S (n = 11) | Non-ST131, FQ-R (n = 11) | Non-ST131, FQ-S (n = 11) | |||||
| Adhesin | papAHe | 0 (0) | 1 (9) | 3 (27) | 3 (27) | 11 (92) | 0 (0) | <0.001 |
| papG allele II | 1 (9) | 0 (0) | 4 (36) | 1 (9) | 6 (50) | 0 (0) | 0.01 | |
| papG allele III | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 5 (42) | 0 (0) | 0.005 | |
| sfa/focDE | 0 (0) | 0 (0) | 1 (9) | 3 (27) | 8 (67) | 0 (0) | <0.001 | |
| sfaS | 0 (0) | 0 (0) | 0 (0) | 2 (18) | 4 (33) | 0 (0) | 0.03 | |
| iha | 11 (100) | 10 (91) | 6 (55) | 1 (9) | 7 (58) | 5 (100) | <0.001 | |
| Toxin | hlyD | 1 (9) | 0 (0) | 0 (0) | 3 (27) | 8 (67) | 0 (0) | <0.001 |
| sat | 11 (100) | 9 (82) | 6 (55) | 1 (9) | 4 (33) | 5 (100) | <0.001 | |
| vat | 0 (0) | 0 (0) | 3 (27) | 5 (46) | 7 (58) | 0 (0) | 0.001 | |
| Siderophore | iroN | 0 (0) | 1 (9) | 1 (9) | 5 (46) | 8 (67) | 0 (0) | <0.001 |
| fyuA | 11 (100) | 11 (100) | 8 (73) | 8 (73) | 12 (100) | 5 (100) | 0.03 | |
| ireA | 0 (0) | 0 (0) | 0 (0) | 2 (18) | 4 (33) | 0 (0) | 0.03 | |
| iutA | 11 (100) | 10 (91) | 8 (73) | 4 (36) | 6 (50) | 5 (100) | 0.002 | |
| Capsule | K1 | 0 (0) | 0 (0) | 1 (9) | 4 (36) | 5 (42) | 0 (0) | 0.009 |
| Misc.f | usp | 11 (100) | 11 (100) | 4 (36) | 5 (46) | 9 (75) | 5 (100) | <0.001 |
| ibeA | 0 (0) | 6 (55) | 2 (18) | 2 (18) | 3 (25) | 0 (0) | 0.04 | |
| H7 fliC | 0 (0) | 0 (0) | 0 (0) | 2 (18) | 4 (33) | 0 (0) | 0.03 | |
| malX | 11 (100) | 10 (91) | 5 (46) | 4 (36) | 9 (75) | 5 (100) | 0.001 | |
| clbB/N | 0 (0) | 0 (0) | 2 (18) | 4 (36) | 7 (58) | 0 (0) | 0.001 | |
| Phylo. gp.g | B2 | 11 (100) | 11 (100) | 3 (27) | 4 (36) | 8 (67) | 5 (100) | <0.001 |
| D | 0 (0) | 0 (0) | 7 (64) | 2 (18) | 4 (33) | 0 (0) | 0.001 | |
Traits shown are those that yielded a P value of <0.05 (by Fisher's exact test) in the 6-group comparisons. papAH (P fimbria structural subunit), papG alleles II and III (P adhesin variants), sfa/focDE (S and F1C fimbriae), sfaS (S fimbriae), iha (adhesin-siderophore), hlyD (alpha hemolysin), sat (secreted autotransporter toxin), vat (vacuolating toxin), iroN (catecholate siderophore receptor), fyuA (yersiniabactin receptor), ireA (siderophore), iutA (aerobactin receptor), K1 (group 2 capsule variant), ibeA (invasion of brain endothelium), usp (uropathogenic-specific protein), H7 fliC (flagellar variant), malX (pathogenicity island marker), clbB/N (polyketide synthesis).
Traits detected in ≥1 isolate but not yielding a P value of <0.05: papG allele I (P adhesin variant), focG (F1C fimbriae), afa/draBC (Dr-binding adhesins), fimH (type 1 fimbria adhesin), hra (heat-resistant agglutinin), hlyF (variant hemolysin), cnf1 (cytotoxic-necrotizing factor 1), pic (secreted autotransporter), tsh (temperature-sensitive hemagglutinin), kpsM II (group 2 capsules), K5 (group 2 capsule variant), K15 (group 2 capsule variant).
Sets were matched for collection, year of isolation, and locale.
P values are by Fisher's exact test (two-tailed).
papC, papEG, and papG (P fimbria assembly, tip pilins, and adhesin) yielded results similar to those for papAH.
Misc., miscellaneous traits.
Phylo. gp., E. coli phylogenetic group (A, B1, B2, D).
Statistical analysis.
Comparisons of proportions were tested using Fisher's exact test (two-tailed). Comparisons of virulence scores, percent mouse lethality, and mean mouse status were tested using the Mann-Whitney U test or the Kruskal-Wallis test for nonnormally distributed data, and a two-tailed t test or analysis of variance (ANOVA) for normally distributed data. In some comparisons, to account for clustering by isolate and isolate group, a generalized estimating equation model was used, with mice clustered by isolate and nested within isolate group (the between-subject grouping variable at the next higher hierarchical level). The criterion for statistical significance was a P value of <0.05.
RESULTS
Performance of the sepsis model.
In the mouse sepsis model, within each week's experiment, a median of all 5 mice challenged with positive-control strain CFT703 became moribund or died, compared with none of 5 mice challenged with negative-control strain MG1655, which uniformly remained completely healthy appearing. This excluded important week-to-week or within-experiment variation in the sepsis model.
With the 61 test clinical isolates, sepsis model outcomes were fairly consistent among the mice challenged with a given isolate. However, 16 isolates (26%) required repeat testing due to excessive initial mouse-to-mouse variability.
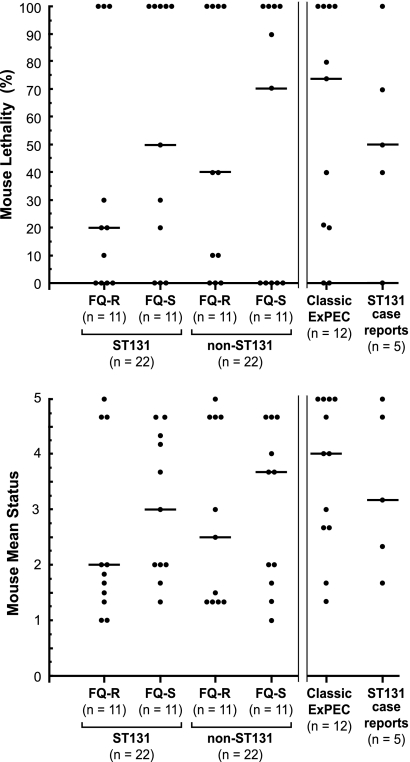
In contrast to the general consistency of effect observed for individual isolates, outcomes varied dramatically among isolates. That is, the different test isolates exhibited a wide range of experimental virulence, ranging from universal lethality (n = 21 isolates) and even rapid lethality (i.e., ≥50% of mice dead within 24 h of inoculation; n = 6), to no lethality (n = 18) and even no evident effect whatsoever (n = 3), comparable to negative-control strain MG1655 (Fig. 1).
Fig 1.
Mouse lethality (top) and mean clinical status (bottom) in the murine sepsis model for 61 extraintestinal Escherichia coli clinical isolates. Top, percentage of mice challenged with a given isolate that became moribund or died by day 3 postchallenge. Bottom, mean daily clinical status, over the 3-day observation period, among the ≥5 mice each challenged with a given isolate, where 1 = completely healthy and 5 = dead. Each dot represents a separate E. coli isolate. Number of isolates per group is shown below the x axes. Horizontal bars are the group medians. Parallel vertical lines separate the 4 groups on the left (which are the systematically selected, matched comparison groups) from the 2 groups on the right (which are, respectively, a convenience sample of non-ST131 ExPEC isolates and ST131 case report isolates). FQ-R, fluoroquinolone resistant; FQ-S, fluoroquinolone susceptible. P values (not shown), as calculated using the generalized estimating equation model and ANOVA, were >0.10 for all among-group comparisons but within each group were <0.001 for comparisons among the particular group's multiple isolates.
Experimental outcomes versus isolate group, ST131 status, and FQ phenotype.
Comparisons for percent mouse lethality and mean mouse status were made initially among the 6 prespecified isolate groups, which included 4 temporally and geographically matched sets of generic clinical isolates representing the 4 possible combinations of ST131 status and FQ phenotype (n = 11 isolates each), representatives of 9 classic ExPEC clonal groups (n = 12 isolates), and household transmission case report ST131 isolates (n = 5 isolates). Although the median values varied somewhat by isolate group for both main outcome measures, i.e., percent lethality and mean mouse status, within-group variation was extremely high, with one or more isolates from each group occupying either extreme of the scale (Fig. 1). Accordingly, the 6 groups did not differ significantly from one another for either outcome measure (P > 0.10), whether this was assessed using the generalized estimating equation (to account for clustering of mice by isolate) or ANOVA (which regards each mouse as an independent observation). Likewise, although rapid lethality was somewhat more common among the classic ExPEC strains (4/12) than within any other study group (0 to 1 rapidly lethal isolate per group), these between-group differences were not statistically significant (not shown).
In contrast, for both main outcome measures, highly statistically significant differences were evident within each isolate group (Fig. 1). This was true both for each group as a whole (Fig. 1) and for at least one (and usually multiple) possible pairwise comparisons between different group members (not shown). Additionally, differences in virulence were observed between members of certain non-ST131 clonal groups, e.g., within ST95 (where cystitis isolate NU14 appeared much less virulent than cystitis isolate U8 or neonatal meningitis isolate RS218) and CC12 (where urinary tract infection isolate IA2 appeared much less virulent than bacteremia isolate CP9) (Table 1).
Because of this apparent lack of association of isolate group or clonal group membership with infection outcomes, the 6 isolate groups were combined, allowing statistical comparisons according to ST131 status and FQ phenotype within the total population. Despite the increased statistical power from the larger sample size, neither ST131 status nor FQ phenotype was significantly associated with either percent lethality or mean mouse status, regardless of whether by-isolate clustering was taken into account in the analysis (not shown). Collectively, these analyses showed that although the mouse sepsis model clearly identified striking differences in virulence among the study isolates, these differences did not correspond with isolate group, ST131 status, or FQ phenotype.
Experimental outcomes versus virulence genotype and phylogenetic group.
To identify alternate explanations for the observed virulence differences, we next compared virulence, as reflected in percent lethality and mouse mean status, with bacterial characteristics other than ST131 status and FQ phenotype. These included phylogenetic group, individual virulence genes, ExPEC status, and aggregate virulence score, for many of which characteristics the 6 isolate groups differed substantially (Tables 2 and 3).
Table 3.
Distribution of aggregate virulence gene scores among 6 groups of extraintestinal E. coli isolates
| Study group | Virulence score, median (range)a | P valueb |
|---|---|---|
| ST131 (FQ-R) | 9 (8–12) | |
| ST131 (FQ-S) | 11 (8–12) | |
| Non-ST131 (FQ-R) | 7 (3–13) | 0.004 |
| Non-ST131 (FQ-S) | 8 (2–17) | |
| Classic ExPEC | 12 (6–17) | 0.01 |
| ST131 case reports | 10 (9–11) |
Virulence score is the number of virulence-associated genes detected in each isolate, adjusted for multiple detection of the pap (P fimbriae), sfa/foc (S and F1C fimbriae), and kps II (group 2 capsule) operons.
P values versus all other isolates (as calculated using the Mann-Whitney U test) are shown where P < 0.05.
These comparisons identified significant associations of 7 individual virulence genes, plus the derived variable of molecularly inferred ExPEC status, with one or both virulence outcome variables (Table 4). The 7 outcome-associated virulence genes represented diverse functional categories, including adhesins (papAH, papG allele III) (15), toxins (vat) (7), protectins (kpsM II, K1 capsule) (28), invasins (ibeA) (10), and miscellaneous traits (clbB/N) (19). The two outcome measures (lethality, mean status) exhibited overlapping yet distinctive patterns of association with the 7 virulence genes, 5 of which were significantly associated with only one outcome measure or the other. Additionally, aggregate virulence score was significantly or borderline significantly associated with both lethality (Spearman's rho = 0.22, P = 0.09) and mean mouse status (Spearman's rho = 0.28, P = 0.03). In contrast, neither phylogenetic group nor any other individual virulence gene was significantly associated with infection outcome (not shown).
Table 4.
Association of virulence-associated genes with mouse sepsis model outcomes among 61 extraintestinal E. coli isolates
| Traita | No. of isolates with trait (% of 61) | Percent lethality, median (range)b |
P valuec | Mean status, median (range)b |
P valuec | ||
|---|---|---|---|---|---|---|---|
| Trait absent | Trait present | Trait absent | Trait present | ||||
| papAH | 18 (30) | 30 (0–100) | 95 (0–100) | 0.06 | 2.0 (1.0–5.0) | 4.7 (1.3–5.0) | 0.007 |
| papG III | 6 (10) | 40 (0–100) | 48 (0–100) | 0.05 | 2.7 (1.0–5.0) | 5.0 (1.3–5.0) | 0.01 |
| vat | 15 (25) | 26 (0–100) | 100 (0–100) | 0.02 | 2.2 (1.0–5.0) | 4.7 (1.3–5.0) | 0.03 |
| kpsM II | 45 (74) | 0 (0–100) | 70 (0–100) | 0.008 | 1.6 (1.0–5.0) | 3.7 (1.3–5.0) | 0.008 |
| K1 | 10 (16) | 30 (0–100) | 100 (0–100) | 0.03 | 2.3 (1.0–5.0) | 4.3 (1.0–5.0) | 0.05 |
| ibeA | 13 (21) | 26 (0–100) | 100 (0–100) | 0.005 | 2.4 (1.0–5.0) | 4.2 (1.3–5.0) | 0.06 |
| clbB/N | 13 (21) | 35 (0–100) | 100 (0–100) | 0.21 | 2.5 (1.0–5.0) | 4.7 (1.3–5.0) | 0.048 |
| ExPEC | 58 (95) | 0 (0–0) | 45 (0–100) | 0.02 | 1.3 (1.0–1.7) | 3.0 (1.0–5.0) | 0.02 |
Traits shown are those that yielded a P value of <0.05 for one or both endpoints. clbB/N, polyketide (colibactin) synthesis; ExPEC, extraintestinal pathogenic E. coli (defined as ≥2 of papAH and/or papC [P fimbria structural subunit and assembly], sfa/foc [S and F1C fimbriae], afa/draBC [Dr-binding adhesins], kpsM II [group 2 capsule synthesis], and iutA [aerobactin operon]); ibeA, invasion of brain endothelium; K1, kpsM II variant (K1 capsule); papG III, variant P fimbria adhesin; vat, vacuolating toxin.
Outcomes were the percent mouse lethality and mean mouse status (over 3 days) among all mice challenged with a particular strain (median, n = 5 mice per strain; range, 5 to 39 mice).
P values were calculated using the Mann-Whitney U test (two-tailed).
DISCUSSION
In this assessment of 61 ST131 and non-ST131 E. coli clinical isolates for extraintestinal virulence in a murine sepsis model, we found a broad range of virulence within the study population. In conflict with our prior hypothesis, neither ST131 status nor FQ phenotype was significantly associated with virulence. Moreover, each isolate group (as defined based on ST131 status and FQ phenotype, classic ExPEC clonal group status, or ST131 case report status) exhibited a broad range of virulence, evidence of pathogenic diversity within these isolate groups and against a direct link between virulence and the group-defining variables. In contrast, multiple putative or proven ExPEC virulence genes, plus molecularly defined ExPEC status, correlated significantly with virulence.
These findings contradict the assumptions that ST131 isolates have a higher virulence potential than other E. coli isolates, that case report ST131 isolates are more virulent than other ST131 isolates, and that, irrespective of ST131 status, FQ-resistant E. coli isolates, per se are less virulent than FQ-susceptible E. coli isolates (49). In contrast, they implicate accessory traits (e.g., virulence factors) as the primary determinants of extraintestinal virulence (13, 20). They thus indicate a need to discover the presumed unidentified accessory traits that accounted for the observed within-group variation, including the residual virulence differences that remain after accounting for known virulence genes.
Our findings can be compared with previous related work. The authors of one of the first reports of the ST131 clonal group observed that all 4 ST131 isolates they studied in the murine sepsis model yielded 100% lethality among the 10 mice challenged per isolate (5). This seeming discrepancy with the present finding of considerable virulence diversity among ST131 isolates might be due to undefined differences in experimental conditions, use of different ST131 strains, or chance variation related to small numbers. Additionally, an earlier study that used the murine sepsis model to test a large number of clinical and fecal non-ST131 E. coli isolates found, in agreement with the present study, that specific accessory traits (virulence factors) were more predictive of lethality than were phylogenetic background or clinical source (13). Differences between that and the present study regarding which specific accessory traits were predictive of lethality and whether group B2 status is associated with virulence likely relate to the very different study populations, since the present study included only clinical isolates and was heavily biased toward (group B2-derived) ST131.
Thus, the preponderance of experimental animal model evidence indicates that ST131 isolates are neither uniformly virulent nor, as a group, discernibly more virulent than other extraintestinal E. coli isolates. How can this conclusion be reconciled with ST131's strikingly rapid emergence and current dominance as an extraintestinal pathogen (16, 41, 44)? One possibility is that the experimental models used to date, which involve direct parenteral inoculation of the test isolate into the host, bypass key earlier steps in pathogenesis at which ST131 excels. These include the transition from intestinal colonizer to invasive pathogen, with the corresponding requirement for adherence to vaginal and urinary epithelial cells, and entry into and persistence within normally sterile sites such as the urinary tract (47).
Alternatively (or additionally), ST131's striking epidemiological success may not be related to its pathogenic potential per se but rather to an enhanced ability to disseminate among hosts and/or to establish and maintain intestinal colonization upon encountering a new host. Both phenomena predictably would increase the number of hosts colonized with an ST131 strain, thereby increasing the likelihood of ST131 causing an infection, even if only by chance. This harkens back to the classic dichotomy between prevalence (within the intestinal microbiota) versus special pathogenicity as the underlying explanation for why certain E. coli genotypes tend to cause urinary tract infection (37). In support of the enhanced dissemination hypothesis, abundant molecular epidemiological evidence indicates that ST131 strains move rapidly and extensively among locales, to a greater extent than non-ST131 E. coli (16, 27, 38, 41, 44). In contrast, very little is known about the intestinal colonization dynamics of ST131 versus non-ST131 E. coli (11, 25, 33), making this an area ripe for future investigation.
Another notable study finding was that the ST131 case report isolates, despite having caused serious or fatal disease in their respective source patients, were not demonstrably more virulent in the mouse sepsis model than were the generic ST131 clinical isolates, which in contrast were selected based strictly on their ST131 status, FQ phenotype, and date and locale of isolation. Presuming the experimental system used provides a valid indication of a strain's intrinsic virulence potential, this finding suggests that the case report source patients' severe outcomes (8, 11, 40) may have been more attributable to host or environmental factors, or to chance, than to bacterial factors. Also of interest was the seeming variation in virulence within each of the 2 pairs of presumably closely related (within-household-transmitted) ST131 isolates. Possible explanations for this, aside from experimental artifact, include subtle virulence-relevant genetic differences such as minor sequence variation in conserved genes (35, 50) and/or mosaicism for relevant accessory traits (40).
Related to this, the observed variation in experimental virulence within both ST131 and certain other ExPEC clonal groups, i.e., ST95 (O18:K1:H7) and CC12 (O4:K+:H5/H−), invites an investigation of the underlying mechanisms. Whole-genome comparisons could be used to identify genetic differences that correspond with the observed virulence differences between these otherwise highly similar strains (34, 35, 36, 43, 50). This could be followed by molecular epidemiological studies assessing the distribution of these sequences in relevant comparison populations (e.g., clinical versus fecal isolates or isolates from distinct clinical syndromes or host groups) (46) and direct experimental assessment of their role in pathogenesis (12).
Study limitations include the possibly unrepresentative nature of the study population (with isolate selection focusing on source collections containing all 4 combinations of ST131 status and FQ phenotype), the mouse sepsis model's artificiality and possible unsuitability for mimicking the pathogenesis of ST131-related human infections, lack of attention to upstream colonization and transmission steps, and attention to only a subset of known/suspected extraintestinal virulence genes (30). Study strengths include the comparatively large number of ST131 isolates and controls, use of matched sets of comparison ST131/non-ST131 and FQ-resistant versus FQ-susceptible isolates, randomization of mice and isolates to reduce experimental artifacts, assessment of multiple bacterial characteristics in relation to virulence, and use of advanced statistical methods that address clustering.
In summary, we utilized a mouse subcutaneous sepsis model to compare the virulence of a large number of ST131 and non-ST131 E. coli clinical isolates, both FQ resistant and susceptible, plus isolates from classic ExPEC clonal groups and case report E. coli ST131 household transmission isolates. Although the study isolates displayed considerable diversity of virulence behavior, neither ST131 status nor FQ phenotype corresponded with this diversity. In contrast, multiple known or suspected ExPEC virulence genes, and molecularly defined ExPEC status, were significantly associated with experimental virulence. These findings point away from ST131 isolates as having heightened virulence potential compared with other E. coli types in causing invasive infections, suggesting instead that ST131's epidemiological success may reflect an enhanced fitness for upstream steps in pathogenesis, including possibly colonization and transmission. Additionally, the observed extensive within-ST virulence variation suggests an opportunity to compare closely related strains to identify the responsible genetic determinants.
ACKNOWLEDGMENTS
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (J.R.J.).
Dave Prentiss (Minneapolis VA Medical Center) prepared the figure. Mariana Castanheira (JMI Laboratories, Liberty, IA), James Rice (Scripps Institute, La Jolla, CA), and Daniel Sahm (Eurofins Medinet, Chantilly, VA) provided study isolates and associated data.
Footnotes
Published ahead of print 6 February 2012
REFERENCES
- 1. Achtman M, et al. 1983. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect. Immun. 39:315–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg DE, Akopyants NS, Kersulyte D. 1994. Fingerprinting microbial genomes using the RAPD or AP-PCR method. Methods Mol. Cell. Biol. 5:13–24 [Google Scholar]
- 3. Blattner FR, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474 [DOI] [PubMed] [Google Scholar]
- 4. Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clermont O, et al. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J. Antimicrob. Chemother. 61:1024–1028 [DOI] [PubMed] [Google Scholar]
- 6. Coque TM, et al. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dozois C, Johnson J. 2000. The place of P fimbriae in the emergence of Escherichia coli extraintestinal pathotypes. ASM News 66:262–263 [Google Scholar]
- 8. Ender PT, et al. 2009. Transmission of extended-spectrum beta-lactamase-producing Escherichia coli (sequence type ST131) between a father and daughter resulting in septic shock and emphysematous pyelonephritis. J. Clin. Microbiol. 47:3780–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goluszko P, et al. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Invest. 99:1661–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang S-H, Wan Z-S, Chen Y-H, Jong AY, Kim KS. 2001. Further characterization of Escherichia coli brain microvascular endothelial cell invasion gene ibeA by deletion, complementation, and protein expression. J. Infect. Dis. 183:1071–1078 [DOI] [PubMed] [Google Scholar]
- 11. Johnson JR, Anderson JT, Clabots C, Johnston B, Cooperstock M. 2010. Within-household sharing of a fluoroquinolone-resistant Escherichia coli sequence type ST131 strain causing pediatric osteoarticular infection. Pediatr. Infect. Dis. J. 29:474–475 [DOI] [PubMed] [Google Scholar]
- 12. Johnson JR, Clabots C, Rosen H. 2006. Effect of inactivation of the global oxidative stress regulator oxyR on the colonization ability of Escherichia coli O1:K1:H7 in a mouse model of ascending urinary tract infection. Infect. Immun. 74:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson JR, et al. 2006. Experimental mouse lethality of Escherichia coli isolates, in relation to accessory traits, phylogenetic group, and ecological source. J. Infect. Dis. 194:1141–1150 [DOI] [PubMed] [Google Scholar]
- 14. Johnson JR, Delavari P, O'Bryan T. 2001. Escherichia coli O18:K1:H7 isolates from acute cystitis and neonatal meningitis exhibit common phylogenetic origins and virulence factor profiles. J. Infect. Dis. 183:425–434 [DOI] [PubMed] [Google Scholar]
- 15. Johnson JR, Johnson CE, Maslow JN. 1999. Clinical and bacteriologic correlates of the papG alleles among Escherichia coli strains from children with acute cystitis. Pediatr. Infect. Dis. J. 18:446–451 [DOI] [PubMed] [Google Scholar]
- 16. Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States (2007). Clin. Infect. Dis. 51:286–294 [DOI] [PubMed] [Google Scholar]
- 17. Johnson JR, et al. 2010. Escherichia coli sequence type ST131 as an emerging fluoroquinolone-resistant uropathogen among renal transplant recipients Antimicrob. Agents Chemother. 54:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson JR, et al. 2008. Virulence genotypes and phylogenetic background of Escherichia coli serogroup O6 isolates from humans, dogs, and cats. J. Clin. Microbiol. 46:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson JR, Johnston B, Kuskowski MA, Nougayrede J-P, Oswald E. 2008. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J. Clin. Microbiol. 46:3906–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson JR, Kuskowski M, Denamur E, Elion J, Picard B. 2000. Clonal origin, virulence factors, and virulence (letter and reply). Infect. Immun. 68:424–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson JR, Kuskowski MA, Gajewski A, Sahm DF, Karlowsky JA. 2004. Virulence characteristics and phylogenetic background of multidrug-resistant and antimicrobial-susceptible clinical isolates of Escherichia coli from across the United States, 2000–2001. J. Infect. Dis. 190:1739–1744 [DOI] [PubMed] [Google Scholar]
- 22. Johnson JR, Kuskowski MA, Owens K, Gajewski A, Winokur PL. 2003. Phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J. Infect. Dis. 188:759–768 [DOI] [PubMed] [Google Scholar]
- 23. Johnson JR, et al. 2009. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002–2004. Antimicrob. Agents Chemother. 53:2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson JR, et al. 2011. Global distribution and epidemiological associations of Escherichia coli clonal group A, 1998–2007. Emerg. Infect. Dis. 17:2001–2009 [DOI] [PubMed] [Google Scholar]
- 25. Johnson JR, Miller S, Johnston B, Clabots C, Debroy C. 2009. Sharing of Escherichia coli sequence type ST131 and other multidrug-resistant and urovirulent E. coli strains among dogs and cats within a household. J. Clin. Microbiol. 47:3721–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson JR, et al. 2003. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 47:2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson JR, et al. Pulsed-field gel electrophoresis analysis of 579 Escherichia coli sequence type ST131 isolates (1967–2009) in relation to year, geography, ecological source, and antimicrobial resistance. Emerg. Infect. Dis., in press [Google Scholar]
- 28. Johnson JR, O'Bryan TT. 2004. Detection of the Escherichia coli group 2 polysaccharide capsule synthesis gene kpsM by a rapid and specific PCR-based assay. J. Clin. Microbiol. 42:1773–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson JR, Owens KL, Clabots CR, Weissman SJ, Cannon SB. 2006. Phylogenetic relationships among clonal groups of extraintestinal pathogenic Escherichia coli as assessed by multi-locus sequence analysis. Microbes Infect. 8:1702–1713 [DOI] [PubMed] [Google Scholar]
- 30. Johnson JR, Russo TA. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int. J. Med. Microbiol. 295:383–404 [DOI] [PubMed] [Google Scholar]
- 31. Johnson JR, et al. 1997. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect. Immun. 65:2153–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kao J-S, Stucker DM, Warren JW, Mobley HLT. 1997. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect. Immun. 65:2812–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leflon-Guibout V, et al. 2008. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J. Clin. Microbiol. 46:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Gall T, et al. 2007. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol. Biol. Evol. 24:2373–2384 [DOI] [PubMed] [Google Scholar]
- 35. Levert M, et al. 2010. Molecular and evolutionary bases of within-patient genotypic and phenotypic diversity in Escherichia coli extraintestinal infections. PLoS pathog. 6:e1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lieberman TD, et al. 2011. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 43:1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moreno E, et al. 2006. Relationship between Escherichia coli strains causing urinary tract infection in women and the dominant faecal flora of the same hosts. Epidemiol. Infect. 134:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicolas-Chanoine M-H, et al. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281 [DOI] [PubMed] [Google Scholar]
- 39. Olesen B, et al. 2009. Three-decade epidemiological analysis of Escherichia coli O15:K52:H1. J. Clin. Microbiol. 47:1857–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Owens RC, et al. 2011. Community transmission in the United States of a CTX-M-15-producing sequence type ST131 Escherichia coli strain resulting in death. J. Clin. Microbiol. 49:3406–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peirano G, Pitout JDD. 2010. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 35:316–321 [DOI] [PubMed] [Google Scholar]
- 42. Picard B, et al. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reeves PR, et al. 2011. Rates of mutation and host transmission for an Escherichia coli clone over 3 years. PLoS One 6:e26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66:1–14 [DOI] [PubMed] [Google Scholar]
- 45. Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. 2001. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob. Agents Chemother. 45:1402–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sannes MR, Kuskowski MA, Owens K, Gajewski A, Johnson JR. 2004. Virulence factor profiles and phylogenetic background of Escherichia coli isolates from veterans with bacteremia and uninfected control subjects. J. Infect. Dis. 190:2121–2128 [DOI] [PubMed] [Google Scholar]
- 47. Stamm WE, et al. 1989. Urinary tract infections: from pathogenesis to treatment. J. Infect. Dis. 159:400–405 [DOI] [PubMed] [Google Scholar]
- 48. Vigil KJ, et al. 2010. Escherichia coli pyomyositis: an emerging infectious disease among patients with hematologic malignancies. Clin. Infect. Dis. 50:374–380 [DOI] [PubMed] [Google Scholar]
- 49. Vila J, et al. 2002. Are quinolone-resistant uropathogenic Escherichia coli less virulent? J. Infect. Dis. 186:1039–1042 [DOI] [PubMed] [Google Scholar]
- 50. Weissman SJ, et al. 2007. Differential stability and trade-off effects of pathoadaptive mutations in the Escherichia coli FimH adhesin. Infect. Immun. 75:3548–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]