Abstract
Several studies reported increased numbers of mucosa-associated Escherichia coli strains in patients with inflammatory bowel disease (IBD), encompassing Crohn's disease (CD) and ulcerative colitis (UC). The majority of E. coli strains possess type 1 fimbriae, whose tip fibrillum protein, FimH, naturally undergoes amino acid replacements, an important process in the adaptation of commensal E. coli strains to environmental changes, like those observed in IBD and urinary tract infections. In this study, we analyzed mutational patterns in the fimH gene of 52 mucosa-associated E. coli strains isolated from IBD and non-IBD pediatric patients, in order to investigate microevolution of this genetic trait. FimH-positive strains were also phylogenetically typed and tested for their adhesive ability on Caco-2 cells. Specific FimH alleles for each grouping feature were found. Mutations G66S and V27A were related to CD, while mutations A242V, V163A, and T74I were attributed to UC. Otherwise, the G66S, N70S, and S78N mutations were specifically attributed to B2/D phylogroups. The N70S and A119V mutations were related to adhesive E. coli strains. Phylogroup B2, adhesive, and IBD E. coli strains showed a higher site substitution rate (SSR) in the fimH gene, together with a higher number of mutations. The degree of naïve mucosal inflammation was related to specific FimH alleles. Moreover, we could suggest that the V27A mutation is pathoadaptive for the CD intestinal habitat, while we could also suggest that both the N70S and S78N mutations are related to the more virulent E. coli B2 phylogroup. In conclusion, we found some FimH variants that seem to be more involved than others in the evolution of IBD pathogenesis.
INTRODUCTION
The inflammatory bowel disease (IBD) spectrum, of which Crohn's disease (CD) and ulcerative colitis (UC) are the two main phenotypes (21), is the result of a complex interaction between elements such as host susceptibility, mucosal immunity, and the intestinal milieu. Several lines of evidence support the hypothesis that intestinal bacteria play a role in the pathogenesis of adult IBD and pediatric IBD (6, 12, 18, 32, 36, 37, 42). Increased numbers of mucosa-associated Escherichia coli isolates were observed in both CD and UC (17, 25). The E. coli dominant genotype associated with strains isolated from IBD patients was also described. In addition, a new E. coli pathotype referred to as adherent-invasive E. coli (AIEC) has been recognized (2, 4, 5, 13, 14, 29, 30). Strong adherence of bacteria to surfaces is an essential preliminary stage in colonization (24, 27) and an integral and essential step in infection. The adherence is mediated by structures called adhesins (28). E. coli expresses a number of different adhesive organelles, including P, type 1, S, F1C, and long polar fimbriae (1, 8, 22). A mean of 95% of all isolates of E. coli express type 1 fimbriae, also called mannose-sensitive fimbriae or, commonly, fimbriae. It was reported how pilus type 1 is the major factor responsible for the enhanced adhesive and invasive properties of E. coli (3, 7, 26, 39). The type 1 pilus consists of five parts. They are, starting from the cellular body, FimD (inserted in the outer membrane), FimA (pilus rod), and FimF, FimG, and FimH (tip fibrillum). Recently, it has become clear that pilus type 1 exhibits several different phenotypes, due to allelic variation of the genes fimA and fimH, and that these phenotypes are differentially distributed among E. coli isolates (15, 33). The 273-amino-acid-long FimH protein is structured in two main domains: the mannose-binding lectin domain (Ld; residues 1 to 156) and the fimbria-incorporating pilin domain (Pd; residues 160 to 273), which are connected via a 3-amino-acid interdomain linker peptide chain (residues 157 to 159) (31). Naturally occurring phenotypic variants of the FimH protein have recently been recognized among intestinal and uropathogenic E. coli strains (10). More precisely, these natural variations confer stronger monomannose and three-mannose binding (33), as seen in urinary tract infections, depending on the shear stress which E. coli cells undergo (1, 43). Thus, FimH mutations may be adaptive in secondary habitats of E. coli. The aim of this work was to study the fimH gene (903 nucleotides) mutational patterns of 52 mucosa-associated E. coli strains isolated from pediatric patients diagnosed with IBD and those without IBD, in order to evaluate potential associations between fimH mutational profiles and particular features, such as disease status, E. coli phylogroup, and in vitro static adhesion levels of the isolated strains.
MATERIALS AND METHODS
Patients.
Thirty-eight pediatric patients (age range, 8 to 17 years) referred to the Pediatric Gastroenterology and Liver Unit of the Sapienza University of Rome, Italy, for suspected IBD were studied: active CD was diagnosed in 12, and active UC was diagnosed in 7. The remaining 19 subjects with functional gastrointestinal disorders (undetermined colitis, lymphonodular hyperplasia) and normal colonoscopy and histology findings served as controls. As reported in Table S1 in the supplemental material, the baseline demographic characteristics were similar in the three groups. The patient groups did not differ significantly by age and disease duration. All children with CD had ileocolonic involvement, and all had disease activity in the moderate to severe range. All UC patients had endoscopic evidence of pancolitis, showing a backwash ileitis, and the disease was classified severe. The diagnostic workup of UC and CD was done according to international protocols. Children with CD were assessed using the Pediatric Crohn's Disease Activity Index (PCDAI), which is a multi-item score based on recall of the preceding week's symptoms, laboratory parameters (erythrocyte sedimentation rate, hematocrit and albumin levels), and physical examination. A score of 10 implies inactive disease, one of 11 to 30 implies mild disease, and one of >30 implies moderate to severe disease. It is noteworthy that our CD pediatric patients did not have a previous endoscopic assessment (see Table S1 in the supplemental material), nor any kind of treatment (sulfasalazine was given to 2/12 CD patients after biopsy sampling for this study), so their naïve mucosal inflammation reflected the actual selective pressure on the E. coli strains isolated. Patients with UC were evaluated using the Pediatric Ulcerative Colitis Activity Index (PUCAI), which is based only on clinical symptoms. A score of 10 indicates inactive disease, one of 11 to 34 implies mild disease, one of 35 to 64 implies moderate disease, and one of ≥65 implies severe disease. Only one UC patient out of seven received a previous treatment with steroids as enema; thus, also for the UC cohort, the naïve mucosal inflammation reflected a naïve habitat from which E. coli strains were isolated. All patients underwent ileocolonoscopy after parental informed written consent was provided. During ileocolonoscopy, two biopsy samples were taken from each region (ileum and descending colon) for routine histological assessment and bacteriological study. Specimens were collected in 2-ml screw-cap vials filled with 0.85 ml of brain heart infusion broth (Oxoid, Cambridge, United Kingdom) and 0.15 ml of glycerol (Sigma-Aldrich, St. Louis, MO) and immediately stored at −80°C.
Treatment of biopsy specimens and bacterial culture.
For E. coli species isolation, biopsy specimens (15 mg each) from CD, UC, and control patients and from different regions (ileum and colon) were first washed in 500 μl of physiologic saline with 0.016% dithioerythritol to remove the mucus and then shaken 3 times in 500 μl of physiological saline for 30 s. After a fourth wash, the biopsy specimens were hypotonically lysed by vortexing for 30 min in 500 μl distilled water. One hundred microliters of the cell debris left after hypotonic lysis was plated in 10-fold dilution steps onto MacConkey agar. After 24 h of incubation at 37°C, all bacterial colonies were isolated and subcultured onto nutrient agar and successively identified by use of the API 32 ID system (bioMérieux, Milan, Italy), in order to recognize and isolate E. coli species. Sixty E. coli strains were randomly chosen (2 to 3 from each patient), taking into account both biopsy specimens (from the ileum and descending colon). E. coli strains from controls were retrieved only from descending colon biopsy specimens.
Amplification of E. coli fimH gene.
Total DNA from E. coli strains was obtained by putting a single colony picked up from a petri dish in a 0.2-ml tube containing 50 μl of 0.22-μm-pore-size-filtered and autoclaved double-distilled H2O. A subsequent cell-disrupting step was achieved by disruption for 10 min at 95°C in an Eppendorf thermocycler, followed by a 4°C step and a centrifugation step for 10 min at 10,000 rpm. Supernatant containing genomic and plasmid DNA was collected in new 0.2-ml tubes and stored at −20°C until PCR amplification. The fimH gene (903 bp) was amplified in a 25-μl-final-volume PCR mixture containing 1× Phusion High-Fidelity PCR master mix with HF buffer (New England BioLabs, Ipswich, MA), primers AdH_FWnoGC (5′-ATGAAACGAGTTATTACCCTGTTTGCTG-3′) and Pil_RW (5′-ATTGATAAACAAAAGTCACGCCAATAATCG-3′) at 0.5 μM, and 3 μl of supernatant, used as DNA template. Run conditions were as follows: 95°C for 5 min; 25 cycles at 94°C for 20 s, 59°C for 20 s, and 72°C for 40 s; and then a final step at 72°C for 1 min. The whole PCR mixture was run on a 1% agarose gel, and the resulting band was excised and purified with a MinElute gel extraction kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The eluted DNA was sequenced in both directions by the MWG-Biotech (Eurofins) service to have coverage of the entire fimH gene. Electropherograms for all singleton mutations were also inspected through Geneious (version 4.8.3) software (Biomatters Ltd.) for consistency between strands, and any ambiguous nucleotides were resolved by resequencing.
Analysis of fimH gene sequences.
Upon receipt, sequences underwent a BLAST search (www.ncbi.nih.gov/blast) to confirm that they belonged to the fimH gene of E. coli and were then aligned with Geneious (version 4.8.3) software (Biomatters Ltd.) for DNA mutational pattern analysis. The fimH gene sequence of E. coli strain MG1655 was taken as the reference one (GeneID 948847). The silent and nonsilent mutations were transformed in a binary matrix of presence/absence of such point mutations, so as to have a comprehensive view of mutational patterns. We subaligned all 52 fimH sequences, plus those of strains MG1655 and LF82, according to status (CD, UC, control), phylogenetic group (A, B1, B2, D), and adhesion class (class 1 and class 2), performing various match-by-match analyses on subalignments.
(i) Mutational pattern analysis.
A nonsupervised method, agglomerative hierarchical clustering (AHC), was made to test the clustering behavior of the nucleotide and amino acid sequences of the 52 strains plus those of MG1655 and LF82. Dendrograms were generated on mean-centered and variance-scaled data by means of the partial least-squares discriminant analysis (PLS-DA) algorithm implemented in Simca-P+ software (Umetrics), taking into account Euclidean distances and the agglomeration method of Ward. PSL-DA is a regression analysis that correlates a number of variables x (point mutations) to a set of variables y (fimH sequences), obtaining the covariance between x and y. The PSL-DA approach creates a predictive model, validated through Fisher's test, useful to classify E. coli strains according to the mutational patterns in the fimH gene. The two-tailed χ2 test was used to discover whether the distribution of E. coli strains differed between the clusters identified by the dendrogram. A P value of less than or equal to 0.05 was considered statistically significant.
(ii) Discrimination of nucleotides and amino acids.
PLS-DA was also used to find nucleotide and amino acid mutations that made a major contribution in dividing subalignments. Data were automatically mean centered and unit variance (UV) scaled by the statistical software. Each nucleotide mutation was hierarchically classified on the basis of a software-assigned variable importance (VIP) value. The variables with VIP values of >1 were chosen as being discriminatory.
(iii) Transitions, transversions, and dN/dS ratio.
Calculation of transitions (AG/GA, CT/TC) and transversions was performed on each subalignment by means of the on-line software Los Alamos National Laboratory Highlighter tool (http://hcv.lanl.gov/content/sequence/HIGHLIGHT/highlighter.html), to visualize transition and transversion mutations in a set of nucleotide sequences that are aligned and in frame. Computation of silent (synonymous [dS]) and nonsilent (nonsynonymous [dN]) mutations, along with the dN/dS ratio, was performed on each subalignment by means of the online software Los Alamos National Laboratory SNAP tool (http://hcv.lanl.gov/content/sequence/SNAP/SNAP.html), useful to give a quantification of positive selection for pathoadaptive changes in the fimH gene. A dN/dS ratio of ≤1 indicates purifying or neutral selection favoring amino acid conservation, while a dN/dS ratio of >1 indicates positive selection favoring amino acid substitutions (20, 38). To assess differences between dN/dS ratios, a two-tailed t test was performed on means and standard deviations (SDs), and a P value of less than or equal to 0.05 was considered statistically significant.
(iv) Dice index.
The Dice similarity index (mean percent ± SD) was calculated within each subalignment to assess intersequence similarity by the formula (2nAB)/(nA + nB), where nA is the total number of nucleotide mutations in pattern A, nB is the total number of nucleotide mutations in pattern B, and nAB is the number of nucleotide mutations common to patterns A and B. Next, a mean intragroup Dice index was calculated in order to compare all patient groups. A two-tailed t test was performed to find differences, and a P value of ≤0.05 was considered statistically significant.
(v) Phylogenetic tree.
Phylogenetic analysis of the fimH gene sequence (799 bp) was achieved by means of Geneious (version 4.8.3) software (Biomatters Ltd.), and an unconstrained-branch-length Bayesian phylogenetic tree was a posteriori constructed after generation of 5,501 raw trees with nucleotide substitution model JC96 and a gamma-rate variation. The site substitution rate (SSR) was also computed for each fimH sequence, taking the sum of distances from the root. The two-tailed χ2 test was used to assess differences between SSR groups belonging to a defined subalignment, and a P value of ≤0.05 was considered statistically significant.
(vi) Clustered image mapping (CIM).
Cross-correlations of fimH mutational patterns between subalignments were obtained by the online software CIMminer (http://discover.nci.nih.gov/cimminer), based on a scaled and centered set of data on the presence/absence of nucleotide mutations. Weight coefficients were computed by the PLS-DA algorithm with Simca-P+ software (Umetrics) for each mutated nucleotide (70 x variables) on each classification group (9 y variables). x and y variables were two-dimensionally clustered on the basis of the Euclidean distance dissimilarity matrix and the agglomeration method of Ward.
Static adhesion assay.
Monolayers of differentiated Caco-2 (human colonic adenocarcinoma) cells were cultured in 24-well plates in minimum essential medium (MEM; Euroclone, Milan, Italy) supplemented with NaHCO3 at 1.2 g/liter, 2 mM glutamine, penicillin at 100 IU/ml, streptomycin at 0.1 mg/ml, and 10% heat-inactivated fetal calf serum (Euroclone) in a 5% CO2 incubator. For static adhesion assays, the cells were seeded at a density of 2 × 104 cells/well and cultured to complete differentiation for 15 days before infection. For maximal fimbrial expression, bacterial colonies were grown overnight in nutrient agar, resuspended in sterile saline solution, and left for 48 h at room temperature. Each monolayer was infected with 1 × 108 bacteria per well. After 2 h of incubation at 37°C, monolayers were washed 3 times with phosphate-buffered saline (PBS), cells were lysed with 0.1% Triton X-100, and the numbers of CFU were determined by plating. For each experiment, the mean number of Caco-2 cells after 15 days of culture was also determined. Bacterial adhesion was defined as the percentage of attached bacteria compared with the initial inoculum, which was taken to be 100%, and a cutoff value equal to or greater than 0.8% was used to define a strain as adhesive (class 2), while a cutoff value of less than 0.8% defined a strain as nonadhesive (class 1). Adhesion assays were performed in triplicate.
Phylogenetic PCR grouping.
The phylogenetic groups of E. coli strains were identified using a multiplex PCR, as already described (11). The PCR products were 279 bp for the chuA gene, 211 bp for the yjaA gene, and 152 bp for the TspE4C2 fragment. Phylogenetic grouping was made on the basis of the presence of specific PCR-amplified fragments: group A was chuA negative, yjaA positive or negative, and TspE4C2 negative; group B1 was chuA negative, yjaA positive or negative, and TspE4C2 positive; group B2 was chuA positive, yjaA positive, and TspE4C2 positive or negative; and group D was chuA positive, yjaA negative, and TspE4C2 positive or negative.
RESULTS
Mucosa-associated E. coli strain characterization.
A total of 60 mucosal E. coli strains were isolated by cultural techniques: 28 from patients with CD, 19 from patients with UC, and 13 from non-IBD controls. The prevalence of E. coli in each cohort was 83% in CD patients (10/12), 100% in UC patients (7/7), and 53% in controls (10/19) (χ2 = 6.35, P = 0.04). The E. coli bacterial load varied from patient to patient and ranged from 1 × 104 to 1 × 106 CFU/ml in IBD patient specimens and from 0 to 1 × 103 CFU/ml in control specimens. Higher bacterial concentrations were found in biopsy specimens from patients with IBD. Among all isolates of the E. coli strains, 52/60 (86.7%) were positive for the fimH gene and underwent further characterization. The adhesive properties of the fimH-positive E. coli isolates were investigated in the Caco-2 cell model. The mean adhesion rate of the referent nonadhesive strain, E. coli DH5α, was 0.4% ± 0.3% of the original inoculum, whereas the referent adhesive strain enteropathogenic E. coli 32O55 had a mean adhesion index of 2.7% ± 0.7%. E. coli strains tested in this study were considered adherent when the mean adhesion index was 0.8% or more of the original inoculum. Table S2 in the supplemental material reports the results of the E. coli adhesion assays. E. coli strains were separated into two adhesion classes. Class 1, with adhesion levels ranging from 0 to 0.8% (nonadhesive strains), included 16 strains: 8/23 (34.8%) from patients with CD, 2/16 (12.5%) from patients with UC, and 6/13 (46.2%) from controls. Class 2, with adhesion levels of ≥0.8% (adhesive strains), comprehended 36 strains: 15/23 (65.2%) from patients with CD, 14/16 (87.5%) from patients with UC, and 7/13 (53.8%) from controls. The strains isolated from CD patients and controls were randomly distributed between the two classes, while the strains from patients with UC were significantly present in adhesion class 2 (χ2 = 15.1, P = 0.0001). Table S2 in the supplemental material also reports the phylogroup distributions among the patients. We decided, supported by literature data (23), to join together phylogenetic groups B2 and D, typically shown by virulent E. coli types. Therefore, phylogenetic groups A and B1 were joined, too. The B2/D group included 29 strains: 15/23 (65.2%) from patients with CD, 9/16 (56.2%) from patients with UC, and 5/13 (38.5%) from controls. The A/B1 group included 23 strains: 8/23 (34.8%) from patients with CD, 7/16 (43.7%) from patients with UC, and 8/13 (61.5%) from controls. The four phylogroups were randomly distributed among the cohorts (χ2 = 2.41, P = 0.21), and, in particular, the B2 group was exclusively present, but not significantly, in overall IBD strains.
FimH protein harbors characteristic mutations shaped from selective habitats.
All 52 FimH sequences were compared to the E. coli MG1655 FimH sequence, and we observed 15 distinct FimH protein allelic variants, but only alleles retrieved two or more times were reported (Table 1). FimH sequences did not differ by source (ileum or descending colon), as assessed by the Wilcoxon signed-rank test on the mean intragroup Dice index (P = 0.145), meaning a high intrapatient similarity of FimH protein sequence. By PLS-DA, performed on FimH sequences, we generated predictive models based on different grouping features, as stated in Materials and Methods. The patients' status was well predicted (78.5%, Fisher's P = 5.9 × 10−8), with G66S and V27A mutations specifically ascribed to the CD cohort, while A242V, V163A, and T74I mutations were attributed to the UC cohort (Fig. 1A). Mutation Y195F was considered predictive for controls (datum not shown). E. coli phylogroups (A/B1 and B2/D) were correctly predicted by FimH sequences (85%, Fisher's P = 5.1 × 10−4), with the G66S, N70S, and S78N mutations specifically being attributed to B2/D, while A119V was ascribed to A/B1 (Fig. 1B). Finally, E. coli adhesion class 2, as stated in Materials and Methods, was properly predicted (81.7%, Fisher's P = 9.2 × 10−3) by amino acid sequences, with N70S and A119V mutations (Fig. 1C) being distinctive for the adhesive class. The nonadhesive class (class 1) gave no significant results (data not shown). By PLS-DA, different FimH mutational patterns were found for each E. coli characteristic (status, phylogroup, adhesion class); thus, it could be hypothesized that, under specific selective pressure, the FimH protein undergoes selective amino acid mutations.
Table 1.
FimH alleles of the pediatric E. coli isolates
| Allelea | No. (%) of strains |
Mutation at amino acid residue: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD (n = 23) | UC (n = 16) | Controls (n = 13) | B2/D (n = 29) | A/B1 (n = 23) | Class 2 (n = 35) | Class 1 (n = 16) | V27 | G66 | N70 | T74 | S78 | A119 | V163 | Y195 | A202 | A242 | |
| 1 (n = 16) | 10 (43.5) | 2 (12.5) | 4 (30.8) | 11 (37.9) | 5 (21.7) | 6 (17.1) | 10 (62.5) | A | |||||||||
| 2 (n = 12) | 4 (17.4) | 6 (37.5) | 2 (15.4) | 4 (13.8) | 8 (34.8) | 11 (31.4) | 1 (6.2) | A | V | ||||||||
| 3 (n = 3) | 3 (13.0) | 0 | 0 | 3 (10.3) | 0 | 3 (8.6) | 0 | A | S | S | N | ||||||
| 4 (n = 2) | 2 (8.7) | 0 | 0 | 2 (6.9) | 0 | 2 (5.7) | 0 | A | S | V | |||||||
| 5 (n = 3) | 1 (4.3) | 1 (6.2) | 1 (7.7) | 2 (6.9) | 1 (4.3) | 3 (8.6) | 0 | A | S | N | |||||||
| 6 (n = 2) | 0 | 2 (12.5) | 0 | 2 (6.9) | 0 | 1 (2.9)b | 0 | A | S | I | N | A | |||||
| 7 (n = 3) | 0 | 3 (18.7) | 0 | 2 (6.9) | 1 (4.3) | 2 (5.7) | 1 (6.2) | A | N | V | |||||||
| 8 (n = 2) | 0 | 0 | 2 (15.4) | 1 (3.4) | 1 (4.3) | 1 (2.9) | 1 (6.2) | A | F | ||||||||
Alleles are defined as combinations of amino acid mutations. Only alleles retrieved two or more times were reported.
Strain 41, harboring FimH allele 6, was cytotoxic for Caco-2 cells, so it was not referred to any adhesion class.
Fig 1.
Discriminating amino acid mutations in FimH protein. A three-dimensional graphical representation of the low-affinity state of FimH protein (Protein Data Bank accession number 3JWN) showed amino acid residues with the power to discriminate a patient's status (A) (red, CD; blue, UC), the E. coli phylogroup (B) (red, B2/D; blue, A/B1), and the E. coli for adhesion capability (C) (red, class 2). The donor strand of the FimG protein, which incorporates into the pilin β-barrel domain, is depicted in green.
FimH protein mutations arise from a fine-tuned microevolution on different parts of fimH gene.
In order to evaluate microevolution in the fimH gene, which is responsible for selective amino acid mutations, we analyzed all 52 gene sequences collected. Among the 52 isolates, 26 distinct fimH allelic variants were observed, with 72 unique single nucleotide polymorphisms (SNPs) found at 70 polymorphic sites. Fourteen of the 72 SNPs (19.4%) were singletons (observed in only one fimH type) with 9 amino acid replacements; thus, 64.3% of singletons had phenotypic effects. All 72 mutations were point substitutions; 17 (23.6%) were transversions, and 55 (76.4%) were transitions. More precisely, we found 33 C/T transitions (45.8%), 22 A/G transitions (30.6%), and 17 transversions (23.6%). The codon distribution of the 72 SNPs was 11/72 (15.3%) in the 1st base, 14/72 (19.4%) in the 2nd base, and 47/72 (65.3%) in the 3rd base. Out of 72 mutations, 21 resulted in amino acid replacements and 51 were silent substitutions. Among the amino acid replacements, only four were caused by transversion.
fimH sequences did not differ by source (ileum or descending colon), as assessed by the Wilcoxon signed-rank test on the mean intragroup Dice index (P = 0.362), meaning a high intrapatient similarity of the fimH gene sequence. By means of Dice index (see supplemental material and Fig. S2 in the supplemental material) we found that CD and UC strains had roughly 50% similarity in fimH mutational profiles, while controls had only 36% (Mann-Whitney U test, P < 0.0001). In addition, B2 phylogroup fimH sequences were similar at 63%, against roughly 47% of the other phylogroups (Mann-Whitney U test, P < 0.0001).
To find out similarity patterns in the fimH gene, we generated hierarchical dendrograms by PLS-DA analysis, based on different grouping features, as stated in Materials and Methods. As in the case of the PLS-DA based on protein sequences, fimH mutational patterns were able to predict the characteristics of fimH-harboring E. coli strains (see supplemental material and Fig. S1 in the supplemental material). Mutated nucleotides with discriminatory power are reported in Table 2. Nucleotide mutation T80C, corresponding to the amino acid variation V27A, was distinctive for UC and for the overall IBD group (Fisher's P = 1.1 × 10−11), while A209G (corresponding to FimH mutation N70S) and G233A (corresponding to FimH mutated residue S78N) were both related to the B2 phylogroup (Fisher's P = 3.6 × 10−8). These results could suggest a pathoadaptive effect of the V27A mutation on E. coli fitness to the inflamed intestinal habitat, while the combination of N70S and S78N was linked to the phylogroup which typically presents virulent E. coli types (11, 23).
Table 2.
Mutated nucleotides with discriminatory power highlighted by PLS-DA
| Grouping feature | Discriminating NT mutations (PLS-DA)a |
|---|---|
| Disease | |
| CD | C369T, C426T |
| UC | T80C (V27A), T471C |
| Controls | C525T |
| Phylogenetic group | |
| A/B1 | T255G, C276G |
| B2 | G345T, A249T, A209G (N70S), G233A (S78N) |
| D | G639C, C408T |
| Adhesion class 2 | T144C, T471C |
Underlined mutations are nonsilent, mutations between parentheses are the corresponding FimH mutated amino acids, and mutations in bold are transversions.
The latter analysis provided only fimH nucleotides with a higher power to divide E. coli features, such as status, phylogroup, and adhesion class, but it gave no information about the fine-tuned modulation of the fimH gene. Trying to understand the underlying molecular strategies used by E. coli to microevolve the fimH gene under different conditions, we computed the mean numbers of transitions and transversions and the dN/dS ratio, as reported in Table 3. The mean dN/dS ratio was always less than 1, indicating a purifying selection (34) on the overall fimH gene, although we found a higher mutation number in specific hot-spot sites (T80C, T144C, T255G, C276G, T471C, A540G, T651A/C, A654G, G744A, T768C), in which we retrieved a mutation more than 50% of the time. Ninety percent (9/10) of these hot spots were silent, due to their presence in the 3rd base; thus, a positive selection (a dN/dS ratio of more than 1) for the overall fimH gene would be masked, even if a selective pressure acted on specific nucleotides. It was noteworthy that fimH from patients with UC showed higher mean values for all parameters, especially for the dN/dS ratio, in comparison to fimH from patients with CD (t = 2.327, P = 0.0218) and controls (t = 2.425, P = 0.0253). Moreover, adhesive strains (class 2) had a significantly higher dN/dS ratio than nonadhesive ones (t = −6.252, P < 0.0001), together with an average number of mutations. These results could suggest a tendency for E. coli strains from patients with IBD, mainly from patients with UC, to enhance adhesion by increasing the number of mutations and the number of nonsynonymous substitutions per nonsynonymous site (dN). In fact, UC strains were significantly present in adhesion class 2 (χ2 = 15.1, P = 0.0001), as indicated above. Interestingly, the fimH gene underwent a domain-specific mutational pattern (see Fig. S3 in the supplemental material). The lectin domain (nucleotides 1 to 468) showed a higher number of mutations (χ2 = 32.33, P < 0.0001) and hot spots of C/T transitions and transversions compared with the pilin domain (nucleotides 478 to 902). The latter had a tendency to harbor A/G transitions rather than transversions (χ2 = 10.33, P < 0.008).
Table 3.
Mutation parameters for each subalignmenta
| Feature | Total no. of mutations | No. of AG/GA transitions | No. of transversions | No. of CT/TC transitions | dN/dS (mean ± SD) |
|---|---|---|---|---|---|
| CD | 13.78 | 3.48 | 2.87 | 7.43 | 0.0580 ± 0.0204 |
| UC | 15.25 | 4.00 | 3.25 | 8.00 | 0.0761 ± 0.0296 |
| Control | 11.23 | 3.31 | 2.69 | 5.23 | 0.0526 ± 0.0232 |
| A/B1 | 12.22 | 3.22 | 2.87 | 6.13 | 0.0672 ± 0.0254 |
| B2/D | 14.69 | 3.90 | 3.00 | 7.79 | 0.0574 ± 0.0204 |
| Class 1 | 11.99 | 3.25 | 3.12 | 5.62 | 0.0301 ± 0.0131 |
| Class 2 | 14.12 | 3.69 | 2.83 | 7.60 | 0.0689 ± 0.0239 |
Values for total numbers of mutations, transitions, and transversions are reported as the average for each fimH sequence within a specific subalignment.
In order to have a snapshot of such fimH mutational profiles, we calculated how well each one of the 70 polymorphic sites was important in predicting E. coli habitat source (CD, UC, or control). We performed a cross-correlation between the patients' status and nucleotide mutations by means of CIMminer online software, based on coefficient loadings collected from Simca-P+ software (Umetrics) (Fig. 2). The existence of particular red zones (coefficient values higher than 5.0) within each status group evidenced specific mutational patterns as a result of fine-tuned microevolution in different regions of the fimH gene. It was noteworthy that, within each red zone, CD E. coli strains had a fimH mutability biased toward both C/T transitions (47.4%) and transversions (31.6%), while UC strains had a preponderance of C/T transitions (61.5%) (χ2 = 8.78, P = 0.003). These results could suggest that the intestinal habitat of CD, UC, and control patients exerted a status-specific stress-response on the fimH gene of E. coli, thus leading to a specific mutagenic profile.
Fig 2.
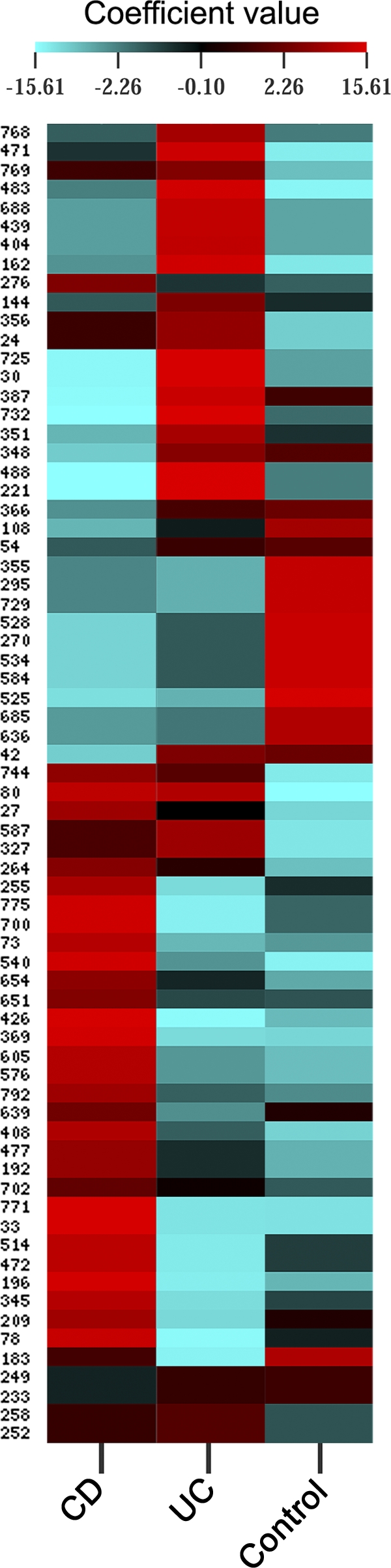
Clustered image mapping of fimH gene mutation weights. Simca-P+ software was used to compute weight coefficients for each mutation (70 x variables) on a patient's status (3 y variables) with a scaled and centered data set. These coefficients were useful to interpret the influence of the x variables on the y ones. Coefficients for different responses (y variables) were also comparable, as the y variables were normalized (scaled). As depicted in the color-coded legend, the higher that the coefficient value is, the higher the weight (red), while the lower that the value is, the lower the weight (turquoise).
fimH gene from B2 and CD E. coli strains has enhanced mutational rate.
Trying to understand the fimH gene mutability, we then calculated the site substitution rate (SSR) for all fimH sequences, and a Bayesian phylogenetic tree was generated (Fig. 3). Three main nucleotide (NT) clusters were obtained: NT1 (encompassing NT1a and NT1b; mean SSR = 0.712 ± 0.028), NT2 (mean SSR = 0.567 ± 0.017), and NT3 (mean SSR = 0.236 ± 0.018). Differences in mean SSR values between the three clusters were all significant (P < 0.0001). A fourth cluster (strains 1, 14, and 21) was considered an outgroup. FimH mutation V27A was a common background for all clusters, and it was the first to appear in the phylogenetic tree (Fig. 3). Then, two separate clades evolved the mutations A119V (cluster NT2) and A202V (cluster NT1b), while the ultimate clade raised FimH mutations N70S and S78N (cluster NT1a). We also analyzed the percent distribution of E. coli strains in the phylogenetic tree in relation to the aforementioned classification criteria (status, phylogroup, adhesion class). CD strains were significantly present in NT1 (mainly NT1b; χ2 = 5.12, P = 0.024), while UC strains were equally distributed. Considering the UC and CD strains as an IBD group, we found a significant presence (43.5%) of IBD strains in NT1 and NT2, which had higher SSRs than NT3 (χ2 = 4.82, P = 0.0282). In addition, 57% of E. coli phylogroup B2/D strains were present in cluster NT1 (χ2 = 7.18, P = 0.0074), while strains belonging to the phylogenetic groups A and B1 were significantly present in NT2 (χ2 = 7.54, P = 0.006). The strain distribution in relation to E. coli adhesive classes was found to be random (data not shown). These overall data could suggest a tendency for CD and B2 strains to develop a higher SSR in response to a definite intestinal habitat, with specific FimH mutations emerging.
Fig 3.
Bayesian phylogenetic tree. A Bayesian tree generated by Geneious (version 4.8.3) software as described in Materials and Methods is depicted. This rooted phylogenetic tree of the fimH sequences of 52 strains (plus the fimH sequences of strains MG1655 and LF82) showed four distinct groups: NT1a, NT1b (NT1a and NT1b were joined into NT1 in the text for SSR analysis), NT2, and NT3. These groups were progressively denoted from higher SSRs to lower ones. Inside each group, depicted with an ellipse, were reported the amino acid mutations found in ≥90% of strains belonging to the group itself. The black circle indicates the root.
Specific FimH alleles are related to mucosal inflammation.
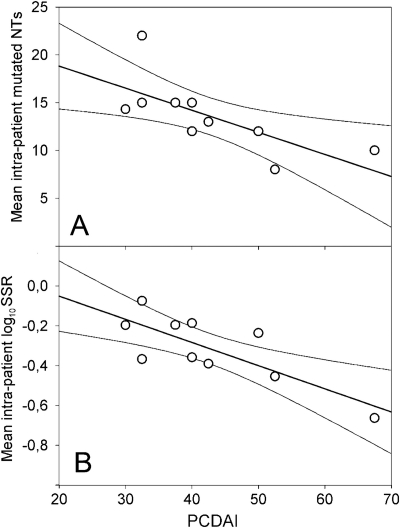
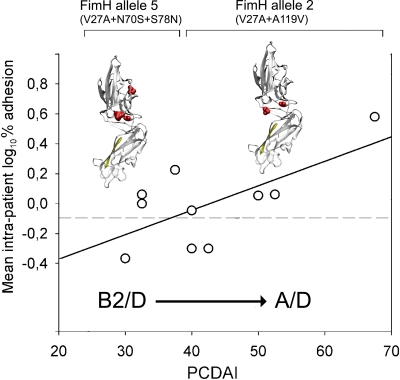
Trying to understand the underlying molecular strategy of an E. coli strain to better fit and adhere to a CD patient naïve inflamed epithelium (that is, one that never underwent pharmacological treatment), we set a graphical relation between PCDAI and the mean intrapatient number of mutations, SSRs, and adhesion levels (Fig. 4). The equation y = mx + q, where m is the slope and q is the intercept on the y axis, was implemented. Taking into account that two or three E. coli strains were isolated from each CD patient (see Materials and Methods), we computed the mean intrapatient values of numbers of fimH mutations, SSRs, and E. coli adhesion percentages. The results showed an inverse correlation for both mean intrapatient number of mutations (r2 = 0.9677; m = −0.23, P = 0.0234; q = 23.44, P = 0.0002) and mean intrapatient log10 SSR (r2 = 0.9192; m = −0.0116, P = 0.0073; q = 0.1821, P = 0.2385) in respect to PCDAI (Fig. 4). Within the PCDAI interval from 30 to 37.5, akin to a mild inflammation, the mean SSR was 0.656 ± 0.063, while within the PCDAI range from 40 to 67.5, akin to a moderate to severe inflammation, the mean SSR was 0.374 ± 0.065, and this difference was statistically significant (Mann-Whitney U test, P = 0.0016). A direct correlation between mean intrapatient E. coli adhesion level and PCDAI was found (r2 = 0.4398; m = 0.0164, P = 0.0366; q = −0.6985, P = 0.0406) (Fig. 5). Considering the overall FimH mutational patterns, we found that the A119V mutation was absent when both N70S and S78N were present, and vice versa, presenting a mutual exclusion. FimH allele 2 (V27A and A119V) was preponderant in strains from patients with PCDAI values ranging from 40 to 67.5, reciprocally excluding FimH allele 5 (V27A, N70S, and S78N), which was present in strains from patients with a range of PCDAIs of from 30 to 37.5 (χ2 = 16.9, P < 0.0001) (Table 4). Phylogroup B2 was prevalent among strains from patients with PCDAI values ranging from 30 to 37.5, while phylogroup A was entirely comprised of strains from patients within the PCDAI interval from 40 to 67.5 (χ2 = 8.20, P = 0.017). Phylogroup D was equally distributed among strains from patients along the PCDAI range. From these results, it could be argued that V27A is the primary pathoadaptive FimH mutation arising in pediatric patients with CD covering the entire spectrum of mucosal inflammation. In addition, B2 E. coli strains, harboring N70S and S78N mutations together with V27A, could take advantage of only a mildly inflamed mucosa, because we did not find any B2 phylogroup in strains from patients with PCDAIs ranging from 40 to 67.5. Finally, it could be suggested that in a naïve pediatric patient with CD (with no previous pharmacological treatment) with a moderately to severely inflamed mucosa, strong adhesion can be achieved by an E. coli strain of the A/D phylogroup harboring FimH allele 2 (V27A and A119V).
Fig 4.
Correlation of fimH gene mutability and PCDAI. PCDAI was correlated to the mean intrapatient number of fimH mutations (A) and the mean intrapatient logarithmic SSR (B). Solid thin lines at both sides of the straight line of correlation are the 95% confidence interval.
Fig 5.
Correlation of E. coli adhesion capability and PCDAI. PCDAI was correlated to the mean intrapatient logarithmic percent adhesion value. Three-dimensional FimH protein representations above the straight line of correlation depicted FimH alleles mostly found within defined PCDAI ranges. FimH allele 2 was found within strains from patients with PCDAIs ranging from 30 to 37.5, while FimH allele 5 was found within strains from patients with PCDAIs ranging from 40 to 67.5. The dashed line depicts the cutoff adhesion value of 0.8%, as described in Materials and Methods.
Table 4.
E. coli FimH alleles and phylogroups related to mucosal inflammation degree
| Inflammation (PCDAI) | Phylogroup | FimH allele | Mutations |
|---|---|---|---|
| Mild inflamed mucosa (30–37.5) | B2 | 5 | N70S, S78N, V27A |
| Moderate-severe inflamed mucosa (40–67.5) | A/D | 2 | V27A, A119V |
DISCUSSION
Adhesion capability is a critical step for bacterial fitness in natural environments. E. coli species were found to be enormously abundant in the intestinal mucosa of pediatric patients with IBD, thus leading to the hypothesis of an enhanced adhesion capability of such strains. It is well-known from the literature that naturally occurring amino acid replacements in the FimH protein, the main E. coli adhesive hook, can modify its tropism toward epithelial tissue (43). In this study, we found that fimH gene and FimH protein variants were able to predict a patient's disease status, the E. coli phylogroup, and the percent adhesion to Caco-2 cells. The significantly higher similarity observed between fimH alignments from patients with CD could be explained by a reduced intestinal allowance, with a lower number of ecological niches being available. A higher homogeneity between fimH alignments in the B2 group and adhesive E. coli strains was also found. Moreover, alignments of fimH from CD and B2 strains showed a significantly higher mean SSR. In this context, only a few E. coli FimH variants will survive and colonize. This could also explain why E. coli B2 strains are the ones almost always recovered in a pathological context and related to the virulent type (11, 23). Those strains could be part of E. coli subpopulations with higher SSRs that, in a changing habitat, will be better able to survive.
We observed an inverse correlation both for the mean intrapatient number of mutations and the mean intrapatient log10 SSR in respect to PCDAI (Fig. 4), while a direct correlation between the mean intrapatient E. coli adhesion level and PCDAI was found (Fig. 5). It was proposed not only how rapidly pathoadaptive FimH mutations could arise (positive selection) but also how rapidly they could be lost (purifying selection), once selective pressure is weakened (35). Table 4 reports the phylogroups and FimH variants predominant in mucosa with different degrees of inflammation. From these results, it could be argued that V27A is the primary pathoadaptive FimH mutation arising in pediatric patients with CD with mucosal inflammation covering the entire spectrum. In addition, B2 E. coli strains, harboring N70S and S78N mutations together with V27A, could take advantage of only a mildly inflamed mucosa, because we did not find any B2 phylogroup in strains from patients with PCDAIs ranging from 40 to 67.5. Finally, it could be suggested that, in a naïve pediatric patient with CD (with no previous treatment) with moderately to severely inflamed mucosa, a strong adhesion can be achieved by an E. coli strain of the A/D phylogroup harboring FimH allele 2 (V27A and A119V). These results could imply that a shift is occurring in the intestinal habitat when the inflammation level is indicated by a PCDAI greater than 40, and a new naïve selective pressure would bring about a different FimH variant. It has been shown in a mouse model (16) that bacteria with increased mutation rates take advantage when colonizing new or changing habitats: this is due to their capacity to generate adaptive mutations rapidly, allowing them to exploit the ecosystem resources more quickly than wild-type bacteria. This advantage is reduced to little or nothing once adaptation is achieved, as proposed in the source-sink model of virulence evolution (9); thus, specific pathoadaptive mutations different for each group (patients with CD, patients with UC, controls, etc.) would be selected.
It is noteworthy that our CD pediatric patients did not have a previous endoscopic assessment (see Table S1 in the supplemental material) or any kind of treatment (sulfasalazine was given to 2/12 patients with CD after biopsy sampling for this study), so their naïve mucosal inflammation reflected the actual selective pressure on the E. coli strains isolated. Due to a prolonged disease duration (calculated as the number of weeks from early symptoms to biopsy sampling), the E. coli species evolves a niche adaptation by means of FimH mutability. The LF82 prototype AIEC strain, associated with CD in adult patients, could be considered the one carrying the genetic combination resulting from a persistent mild inflammation state able to select, in a fine-tuned fashion, specific amino acid variations in the FimH protein. Even though AIEC strains have different genomic profiles, they probably possess phenotypic characters important to prevalently colonize the inflamed mucosa, such as mannose-bonding FimH protein.
By PLS-DA and phylogenetic Bayesian tree analysis, we highlighted 11 E. coli strains (strains 2, 3, 4, 23, 30, 34, 36, 39, 41, 45, 61) showing a high sequence similarity (82.4% ± 1.4%) with strain LF82 (Fig. 3; see Fig. S3 in the supplemental material). All these strains belonged to the IBD group (except strain 61, isolated from a patient with undetermined colitis) and shared 3 out of 4 LF82 amino acid mutations in the adhesin domain (V27A, N70S, S78N), all of which were derived from transitions in the 2nd position (T80C, A209G, and G233A, respectively). This pattern of amino acid mutations was previously found in a fecal E. coli strain (F-18) and a uropathogenic one (CI4), and it is able to mediate patterns of enhanced adhesion to epithelial layers (34). Interestingly, the V27A mutation was significantly found in 100% of our E. coli strains belonging to the IBD group, while the N70S and A119V mutations were found to be significantly prevalent in the more adhesive E. coli strains. These two amino acid mutations were located near the hinge domain, and a reduction in steric crowdedness could probably be taken advantage of in order to reduce interactions between adhesin and pilin domains, thus enhancing bacterial adhesion to mannose residues (41).
In respect to the other grouping features considered in this study, 4 out of 11 (36.4%) of the aforementioned strains also belonged to the B2/D phylogenetic group, like strain LF82, and had the highest SSR. A similar correlation to the B2 group of N70S/S78N mutations, which are harbored by LF82, was previously reported (19): our results were in agreement with those describing the major pathoadaptability of B2/D strains harboring higher SSRs (Fig. 3). The distribution of E. coli strains in the Bayesian tree in respect to disease status showed the presence of E. coli strains isolated from patients with IBD mainly in the groups with higher SSRs. Similar results were found for phylogroups B2 and D, while in respect to adhesive classes, the distribution of E. coli strains occurred in a random fashion. The SSR could be considered an important issue in the ability to adapt to environmental changes. Only bacterial strains with high SSRs could develop the skills to be positively selected in the newly forming habitat (16).
The E. coli mutagenesis response to a specific environmental stress led to specific transitions and transversions, leading to distinct effects on fimH mutational patterns (Table 1; see Fig. S3 in the supplemental material). We found only a few fimH gene variants in the intestine in the context of IBD, and one may think that a specific stress (inflammation context) led to a specific mutagenic profile. Our data could be correlated with the already accepted idea that the frequency of transition and transversion mutation is a function of both environmental factors and the stress-responses of organisms (depending on the single-locus SSR value of the strains). Interestingly, in agreement with the findings of Sokurenko et al., the dN/dS ratio, calculated to determine fimH variation across our E. coli isolates, was much less than 1, probably due to an overwhelming purifying selection above the nonsynonymous mutations (35). Strains from patients with UC showed increased numbers of mutations and dN/dS ratios, and strains from patients with CD had an enhanced mutational rate as a common strategy to survive in a pathological habitat, but different FimH mutations arose. This phenomenon could imply a different behavior adopted by E. coli to survive under different shear stresses and different inflammation conditions. Further studies should be performed to find out if the specific mutagenic profile found could lead to distinct and predictable changes in protein geometry and functionality within the same pediatric patient (40). Genetic variation in an originally commensal trait could change the adhesiveness of E. coli, shifting it toward a phenotype of potential intestinal virulence. Specific allelic variants could be a hint of specific E. coli adaptation to the inflammatory state of the IBD intestine, thus representing promising new targets for therapeutic intervention in the early stages of the disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Broad Medical Research Program (BMRP) grant IBD-0225R3-2 and university MIUR grants to S. Schippa and M. P. Conte.
AIEC strain LF82 was kindly provided by Arlette Darfeuille-Michaud.
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Aprikian P, et al. 2007. Interdomain interaction in the FimH adhesin of Escherichia coli regulates the affinity to mannose. J. Biol. Chem. 282:23437–23446 [DOI] [PubMed] [Google Scholar]
- 2. Barnich N, et al. 2007. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J. Clin. Invest. 117:1566–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boudeau J, Barnich N, Darfeuille-Michaud A. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272–1284 [DOI] [PubMed] [Google Scholar]
- 4. Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bringer MA, Glasser AL, Tung CH, Méresse S, Darfeuille-Michaud A. 2006. The Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell. Microbiol. 8:471–484 [DOI] [PubMed] [Google Scholar]
- 6. Cartun RW, Van Kruiningen HJ, Pedersen CA, Berman MM. 1993. An immunocytochemical search for infectious agents in Crohn's disease. Mod. Pathol. 6:212–219 [PubMed] [Google Scholar]
- 7. Carvalho FA, et al. 2009. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J. Exp. Med. 206:2179–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chassaing B, et al. 2011. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J. Clin. Invest. 121:966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chattopadhyay S, et al. 2009. High frequency of hotspot mutations in core genes of Escherichia coli due to short-term positive selection. Proc. Natl. Acad. Sci. U. S. A. 106:12412–12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen SL, et al. 2009. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. U. S. A. 106:22439–22444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conte MP, et al. 2006. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55:1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Darfeuille-Michaud A, et al. 2004. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127:412–421 [DOI] [PubMed] [Google Scholar]
- 14. Darfeuille-Michaud A, et al. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115:1405–1413 [DOI] [PubMed] [Google Scholar]
- 15. Dias RC, Moreira BM, Riley LW. 2010. Use of fimH single-nucleotide polymorphisms for strain typing of clinical isolates of Escherichia coli for epidemiologic investigation. J. Clin. Microbiol. 48:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giraud A, et al. 2001. Costs and benefits of high mutation rates: adaptive evolution of bacteria in the mouse gut. Science 291:2606–2608 [DOI] [PubMed] [Google Scholar]
- 17. Glasser AL, et al. 2001. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect. Immun. 69:5529–5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartley MG, et al. 1992. The rectal mucosa-associated microflora in patients with ulcerative colitis. J. Med. Microbiol. 36:96–103 [DOI] [PubMed] [Google Scholar]
- 19. Hommais F, et al. 2003. The FimH A27V mutation is pathoadaptive for urovirulence in Escherichia coli B2 phylogenetic group isolates. Infect. Immun. 71:3619–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes AL, Nei M. 1989. Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc. Natl. Acad. Sci. U. S. A. 86:958–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaser A, Zeissig S, Blumberg RS. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28:573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klemm P. 1994. Fimbriae, adhesion, genetics, biogenesis and vaccines. CRC Press, Boca Raton, FL [Google Scholar]
- 23. Kotlowski R, Bernstein CN, Sepehri S, Krause DO. 2007. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 56:610–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin HM, et al. 2004. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 127:80–93 [DOI] [PubMed] [Google Scholar]
- 25. Masseret E, et al. 2001. Genetically related Escherichia coli strains associated with Crohn's disease. Gut 48:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mossman KL, et al. 2008. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J. Immunol. 181:6702–6706 [DOI] [PubMed] [Google Scholar]
- 27. Ofek I, Doyle RJ. 1994. Bacterial adhesion to cells and tissues, p 536–546 Chapman and Hall, New York, NY [Google Scholar]
- 28. Rendón MA, et al. 2007. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad. Sci. U. S. A. 104:10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rolhion N, Darfeuille-Michaud A. 2007. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm. Bowel Dis. 13:1277–1283 [DOI] [PubMed] [Google Scholar]
- 30. Sasaki M, et al. 2007. Invasive Escherichia coli are a feature of Crohn's disease. Lab. Invest. 87:1042–1054 [DOI] [PubMed] [Google Scholar]
- 31. Schembri MA, Pallesen L, Connell H, Hasty DL, Klemm P. 1996. Linker insertion analysis of the FimH adhesin of type 1 fimbriae in an Escherichia coli fimH-null background. FEMS Microbiol. Lett. 137:257–263 [DOI] [PubMed] [Google Scholar]
- 32. Seksik P, et al. 2006. The role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment. Pharmacol. Ther. 24:11–18 [DOI] [PubMed] [Google Scholar]
- 33. Sokurenko EV, et al. 1998. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc. Natl. Acad. Sci. U. S. A. 95:8922–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sokurenko EV, Courtney HS, Maslow J, Siitonen A, Hasty DL. 1995. Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes. J. Bacteriol. 177:3680–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sokurenko EV, et al. 2004. Selection footprint in the FimH adhesin shows pathoadaptive niche differentiation in Escherichia coli. Mol. Biol. Evol. 21:1373–1383 [DOI] [PubMed] [Google Scholar]
- 36. Subramanian S, Campbell BJ, Rhodes JM. 2006. Bacteria in the pathogenesis of inflammatory bowel disease. Curr. Opin. Infect. Dis. 19:475–484 [DOI] [PubMed] [Google Scholar]
- 37. Swidsinski A, et al. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka T, Nei M. 1989. Positive Darwinian selection observed at the variable-region genes of immunoglobulins. Mol. Biol. Evol. 6:447–459 [DOI] [PubMed] [Google Scholar]
- 39. Tchesnokova V, et al. 2011. Type 1 fimbrial adhesin FimH elicits an immune response that enhances cell adhesion of Escherichia coli. Infect. Immun. 79:3895–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tchesnokova V, et al. 2008. Integrin-like allosteric properties of the catch bond-forming FimH adhesin of Escherichia coli. J. Biol. Chem. 283:7823–7833 [DOI] [PubMed] [Google Scholar]
- 41. Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. 2002. Bacterial adhesion to target cells enhanced by shear force. Cell 109:913–923 [DOI] [PubMed] [Google Scholar]
- 42. Thompson-Chagoyán OC, Maldonado J, Gil A. 2005. Aetiology of inflammatory bowel disease (IBD): role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin. Nutr. 24:339–352 [DOI] [PubMed] [Google Scholar]
- 43. Weissman SJ, et al. 2007. Differential stability and trade-off effects of pathoadaptive mutations in the Escherichia coli FimH adhesin. Infect. Immun. 75:3548–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.