Abstract
Cytolethal distending toxins (CDTs), which block eukaryotic cell proliferation by acting as inhibitory cyclomodulins, are produced by diverse groups of Gram-negative bacteria. Active CDT is composed of three polypeptides—CdtA, CdtB, and CdtC—encoded by the genes cdtA, cdtB, and cdtC, respectively. We developed a PCR-restriction fragment length polymorphism assay for the detection and differentiation of five alleles of cdtB (Cdt-I through Cdt-V) in Escherichia coli and used the assay to investigate the prevalence and characteristic of CDT-producing E. coli in children with diarrhea (A. Hinenoya et al., Microbiol. Immunol. 53:206–215, 2009). In these assays, two untypable cdtB genes were detected and the organisms harboring the cdtB gene were identified as Providencia alcalifaciens (strains AH-31 and AS-1). Nucleotide sequence analysis of the cdt gene cluster revealed that the cdtA, cdtB, and cdtC genes of P. alcalifaciens are of 750, 810, and 549 bp, respectively. To understand the possible horizontal transfer of the cdt genes among closely related species, the presence of cdt genes was screened in various Providencia spp. by colony hybridization assay, and the cdt gene cluster was found in only limited strains of P. alcalifaciens. Genome walking revealed that the cdt gene cluster of P. alcalifaciens is located adjacent to a putative transposase gene, suggesting the locus might be horizontally transferable. Interestingly, the CDT of P. alcalifaciens (PaCDT) showed some homology with the CDT of Shigella boydii. Whereas filter-sterilized lysates of strains AH-31 and AS-1 showed distention of CHO but not of HeLa cells, E. coli CDT-I exhibited distention of both cells. This activity of PaCDT was confirmed by generating recombinant PaCDT protein, which could also be neutralized by rabbit anti-PaCdtB antibody. Furthermore, recombinant PaCDT was found to induce G2/M cell cycle arrest and phosphorylation of host histone H2AX, a sensitive marker of DNA double-strand breaks. To our knowledge, this is the first report showing that certain clinical P. alcalifaciens strains could produce variants of the CDTs compared.
INTRODUCTION
Cytolethal distending toxin (CDT) was first discovered as a new type of toxin in Escherichia coli strains isolated from patient with diarrhea in 1987 (29). CDT has a unique activity, which differs from heat-labile enterotoxin (LT). Although LT causes only cell elongation, CDT causes not only cell elongation but also cell distention and blocking of eukaryotic cell cycle at G2/M phase, leading to cell death. Since 1987, the presence of CDTs has been reported in various Gram-negative bacteria, such as Aggregatibacter actinomycetemcomitans, Campylobacter spp., Escherichia albertii, Haemophilus ducreyi, Helicobacter spp., and Shigella spp. (55). CDTs are composed of three polypeptides, namely, CdtA, CdtB, and CdtC, which form a complex structure needed for the toxin activity (55). CdtA and CdtC subunits bind the eukaryotic cell surface, followed by entry of the CdtB subunit into the cell (55). After entering the cell, the CdtB was transferred in the nucleus (55), and ultimately it causes DNA double-strand breaks by using its DNase I activity (15, 32). Therefore, CDT has recently been recognized as a new family of bacterial toxins, called genotoxin (37) or cyclomodulin (38).
In E. coli, at least five different types of EcCDTs have been reported thus far. EcCDT-I and EcCDT-II were initially identified in enteropathogenic E. coli (EPEC) strains isolated from patients with diarrhea (45, 46). EcCDT-III was discovered in an E. coli strain isolated from a calf with septicemia (43). EcCDT-IV was detected in pathogenic E. coli strains isolated from human or animal intestinal and extraintestinal sources (50), whereas EcCDT-V was identified in Shiga toxin-producing E. coli (STEC) or enterohemorrhagic E. coli (EHEC) strains (28). Among five different EcCDTs, it has been reported that the EcCDT-III gene is carried by a conjugative plasmid, called pVir (43), whereas EcCDT-IV and EcCDT-V genes are carried by the lambdoid and P2 phages, respectively (28, 51). Asakura et al. (5) has recently reported that EcCDT-I produced by certain strains of EPEC was encoded on an inducible lambdoid phage.
Although a number of studies regarding the isolation and characterization of CDT-producing bacteria from patients with diarrhea have been reported (2, 4, 9, 20, 24, 34, 39, 41), the role of CDT in human diseases, including diarrhea, has not yet been established. However, it has been demonstrated that shedding of Helicobacter hepaticus cdt-negative mutant was much shorter, and there was also mild inflammation of intestine compared to that of its isogenic wild-type strain (57). Recombinant S. dysenteriae CDT could cause diarrhea in the suckling mouse model (40). Furthermore, coadministration of H. ducreyi and purified H. ducreyi CDT could induce more severe inflammation than H. ducreyi alone (54). It is noteworthy that high amount of CDT-producing E. coli (CTEC) strains have been isolated from patients with bloody diarrhea in India (41). CTEC was also isolated from patients with bloody diarrhea as a sole pathogen in Japan (23). It has been reported that EcCDT-V genes in EHEC O157:H7 strains were significantly more frequent in isolates from patients with diarrhea than in isolates from asymptomatic carriers (18) and that cdt in eae (E. coli attaching and effacing)-negative STEC was significantly more frequent in patients with hemolytic-uremic syndrome and in patients with diarrhea than in asymptomatic carriers (7). These data suggest that CDT could possibly be a virulence factor and may contribute to persistence of infection or it could enhance pathogenicity in host. To elucidate the relevance of CTEC in diarrhea, we developed a PCR-restriction fragment length polymorphism (RFLP) assay for the detection and differentiation of cdt genes in E. coli and examined the prevalence and characteristics of CTEC among children with diarrhea in Japan (24). Untypeable cdtB genes were detected directly from two stool samples of patients with diarrhea by the PCR-RFLP assay, and the bacteria harboring these untypeable cdtB genes were isolated and identified as Providencia alcalifaciens (24; unpublished data).
In the present study we attempted to analyze the cdt gene cluster and its flanking region in the genome of P. alcalifaciens. To examine the possible horizontal transfer of the cdt gene cluster among closely related species, the distribution of cdt genes in various Providencia spp. was also checked. In addition, the biological activity and possible mechanism of action of these CDTs produced by the P. alcalifaciens strains were examined and discussed.
(This study was performed in partial fulfillment of the requirements of a Ph.D. thesis for A.S. from Graduate School of Life and Environmental Sciences, Osaka Prefecture University, Osaka, Japan.)
MATERIALS AND METHODS
Bacterial strains and growth condition.
The bacterial strains used in the present study are listed in Table 1. Two P. alcalifaciens isolated from children with diarrhea during surveillance of cdt gene harboring E. coli and identified by biochemical test using API 20E and adonitol and galactose utility tests (24, unpublished) were examined. Furthermore, eight P. alcalifaciens strains, six P. rettgeri strains, two P. rustigianii strains, one P. heimbachae strain, and one P. stuartii strain were also investigated for the presence of cdt genes. Bacteria were grown aerobically in Luria-Bertani (LB) medium (Becton Dickinson, Franklin Lakes, NJ), in brain heart infusion (BHI) medium (Becton Dickinson) or on LB agar (Becton Dickinson) containing 30 μg of kanamycin (Nacalai Tesque, Inc., Kyoto, Japan)/ml when appropriate.
Table 1.
Bacterial strains used in this study
| Bacterium | Strain | Characteristic | Source or reference |
|---|---|---|---|
| P. alcalifaciens | AH-31 | Clinical isolate (PaCDT) | 24 |
| AS-1 | Clinical isolate (PaCDT) | Diarrheal children (this study) | |
| F90-2004 | Clinical isolate | ICDDR,B | |
| 24717 | Clinical isolate | ICDDR,B | |
| GTC2020 | Clinical isolate | Purchased from Gifu University | |
| 18H253 | Clinical isolate | Osaka Prefectural Institute of Public Health | |
| 18H399 | Clinical isolate | Osaka Prefectural Institute of Public Health | |
| 19H270 | Clinical isolate | Osaka Prefectural Institute of Public Health | |
| P2556 | Clinical isolate | Diarrheal adult (unpublished) | |
| RME362 | Human isolate | Specimen from regular medical examination (unpublished) | |
| P. rettgeri | GTC1263 (ATCC 29944) | Unknown | Purchased from Gifu University |
| P2234 | Clinical isolate | Diarrheal children (unpublished) | |
| P2253 | Clinical isolate | Diarrheal children (unpublished) | |
| P2312 | Clinical isolate | Diarrheal children (unpublished) | |
| P2536 | Clinical isolate | Diarrheal children (unpublished) | |
| RME220 | Human isolate | Specimen from regular medical examination (unpublished) | |
| P. rustigianii | GTC1504 (ATCC 33673) | Human isolate | Purchased from Gifu University |
| RME3 | Human isolate | Specimen from regular medical examination (unpublished) | |
| P. heimbachae | GTC1501 (ATCC 35613) | Penguin isolate | Purchased from Gifu University |
| P. stuartii | GTC1444 (ATCC 29914) | Human isolate | Purchased from Gifu University |
| E. coli | GB1371 | Clinical isolate (EcCDT-I) | 41 |
| C600 | NAa | Laboratory strain (C. Sasakawa) | |
| BL21(DE3) | NA | Laboratory strain (Promega) | |
| BL21(DE3) | With pET28a | This study | |
| BL21(DE3)/TAS-1 | With pAS-1 (rPaCdtB) | This study | |
| BL21(DE3)/TAS-2 | With pAS-2 (rPaCDT) | This study |
NA, not applicable.
Isolation of Providencia spp.
Rectal swabs collected from patients with diarrhea were plated on polymyxin-mannitol-xylitol medium for Providencia (PMXMP) agar (56). In addition, stool specimens obtained from a regular medical examination of 3- to 9-year-old children (kindergarten and primary school) were also screened by plating on PMXMP agar. Suspected bacterial colonies were isolated and identified as Providencia spp. by the API 20E system, and adonitol and galactose utility tests.
Colony hybridization assay for detection of cdt genes.
To examine the presence of cdt genes in P. alcalifaciens strains and in other Providencia spp., including P. rettgeri, P. rustigianii, P. heimbachae, and P. stuartii, the cdtB DNA fragment was generated by PCR using the Cdt-Bcomu and Cdt-Bcomd primers, and the fragment was used as a probe in colony hybridization assay (24). Strain AH-31 was always used as a positive control.
PCR.
PCR for the detection of cdtB gene was performed as described previously (24).
Nucleotide sequence analysis.
To determine the nucleotide sequence of the cdt gene cluster, PCR products of cdtB gene and its flanking region were sequenced either by a standard method or by genome walking (6). Briefly, PCR product was purified by QIAquick PCR products purification kit (Qiagen, GmbH, Hilden, Germany), and the nucleotide sequence of the PCR product was determined by using a BigDye terminator cycle sequencing kit on an ABI Prism 3100 genetic analyzer (Applied Biosystems, Foster City, CA) essentially as described by the manufacturer. Synthetic primers were designed on the basis of obtained sequence and genome walking was performed as described previously (6). Nucleotide and amino acid sequences were analyzed and compared by using GenBank, DDBJ (the DNA Data Bank of Japan), and DNASIS software (Hitachi Software Engineering Co., Ltd., Tokyo, Japan). Dendrogram analysis was performed by using the software CLUSTAL W of MegAlign (DNASTAR, Inc., Madison, WI).
Preparation of recombinant proteins.
The P. alcalifaciens cdt and cdtB genes (Pacdt and PacdtB) were amplified from the genomic DNA of P. alcalifaciens strain AH-31 by PCR using the primer set PacdtABC-F (5′-ATATGGATCCATGAATAATAAACGCACAT-3′) and PacdtABC-R (5′-ATATCTCGAGTTTAAATAACGGGTGACTC-3′) and the primer set PacdtB-F (5′-GAGAGGATCCGTGTTTTTATCGTTTTACGC-3′) and PacdtB-R (5′-GAGACTCGAGTTTACCTTCTGAATACGCC-3′), respectively. PCR was carried out in a 50-μl reaction mixture for each tube containing 2.5 μl of DNA template, 1× ExTaq PCR buffer (Takara Bio, Inc., Shiga, Japan), 0.2 mM deoxynucleoside triphosphate mixture, 0.5 μM concentrations of each primer set, and 1.25 U of ExTaq polymerase (Takara Bio, Inc.). DNA template was prepared from an overnight culture, which was diluted 10-fold in sterile TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) and boiled for 10 min, followed by centrifugation at 12,000 × g at 4°C for 5 min. The PCR conditions were optimized as an initial denaturation of 5 min at 94°C, followed by 30 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 55°C, and extension for 90 s or 60 s at 72°C, with a final extension step for 5 min at 72°C in GeneAmp PCR System 9700 (Perkin-Elmer, Waltham, MA). Each PCR product was digested with BamHI and XhoI and ligated into pET-28a (pAS-1 for PacdtB, pAS-2 for Pacdt), and pAS-1 and pAS-2 were transformed into E. coli BL21(DE3) (strain TAS-1 with pAS-1 and strain TAS-2 with pAS-2). E. coli strain BL21(DE3) with pET-28a was similarly prepared and used as a vector control. Recombinant PaCdtB (rPaCdtB) was purified from crude rPaCdtB expressed in E. coli strain TAS-1. Briefly, E. coli strain TAS-1 was grown at 37°C overnight in LB broth containing kanamycin (30 μg/ml). The culture was diluted 1:100 in the fresh medium and incubated at 37°C until the optical density at 600 nm of the culture reached 0.6. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 0.1 mM, and the culture was further incubated at 18°C for 16 h with vigorous shaking. The bacterial cells were then collected by centrifugation at 6,000 × g at 4°C for 15 min. The cells were suspended in 50 mM Tris-HCl (pH 8.0) containing 150 mM NaCl and 50 mM imidazole and then sonicated using an Astrason ultrasonic processor (Heat-System Ultrasonics, Farmingdale, NY). The lysates were centrifuged at 15,000 × g at 4°C for 15 min, and the supernatants were collected and used for further purification. The rPaCdtB was purified by using an Ni-Sepharose column (GE Healthcare UK, Ltd., Buckinghamshire, England). The purity of the rPaCdtB was confirmed by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis (SDS–15% PAGE) (31). For the preparation of crude rPaCDT, E. coli strain TAS-2 was cultured at 37°C overnight with vigorous shaking. The culture was sonicated as described above, the lysates were centrifuged at 12,000 × g at 4°C for 5 min, and the supernatants were filtrated using a 0.22-μm-pore-size filter (Asahi Glass Co., Ltd., Tokyo, Japan). The filter-sterilized lysate of E. coli strain BL21(DE3)/pET28a was similarly prepared as a vector control. The filtrate was either directly used for cytotoxicity assay or used for suckling mouse assay after concentration by ammonium sulfate precipitation. For ammonium sulfate precipitation, solid ammonium sulfate was first added to 40% saturation, and then the precipitate was removed by centrifugation at 20,000 × g at 4°C for 20 min. Finally, the supernatant solution was brought to 60% saturation with respect to ammonium sulfate. After centrifugation at 20,000 × g at 4°C for 20 min, the precipitate was dialyzed against phosphate-buffered saline (PBS; pH 7.4) until ammonium sulfate was completely removed. The crude rPaCDT was then used for suckling mouse assay. The endotoxin content of rPaCDT was determined by using the ToxinSensor endotoxin detection system (GenScript USA, Inc., Piscataway, NJ) and confirmed to be similar to the preparation from negative control [E. coli BL21(DE3) harboring pET28a].
Preparation of antisera against rPaCdtB.
Purified rPaCdtB was immunized against 8-week-old male New Zealand White rabbit (Oriental Yeast Co., Ltd., Tokyo, Japan). Briefly, 300 μg of purified rPaCdtB was injected into four sites: subcutaneously into the shoulders and intramuscularly into the thighs every 2 weeks interval with Freund complete adjuvant (Becton Dickinson) first and subsequently with Freund incomplete adjuvant (Becton Dickinson) for 8 weeks. The rabbits were anesthetized with ketamine (35 mg/kg [body weight]) and xylazine (5 mg/kg [body weight]), blood was collected, and serum was obtained by centrifugation at 6,000 × g for 10 min.
Determination of titer.
The titer was determined by using an Ouchterlony double gel diffusion test as described previously (58). Briefly, the double gel diffusion test was carried out with 1.2% Noble agar (Difco) in 50 mM Tris-HCl buffer (pH 8.0) containing 150 mM NaCl. Each sample was applied into a hole, and the plate was placed in a humidified chamber at room temperature for about 16 to 24 h. The plate was then washed extensively with a solution of 0.4% NaCl and 0.4% sodium borate and dried. The plate was stained with 0.5% Coomassie brilliant blue (CBB) dissolved in a solution of 50% methanol, 10% acetic acid, and 40% water and destained with the same solution without CBB. The antibody titer was defined as the highest dilution of serum that yielded a visible precipitation line by CBB staining.
Western blotting.
P. alcalifaciens strains AH-31 and AS-1 were cultured at 37°C for 16 h in BHI medium, respectively. A 1-ml portion of the culture was centrifuged at 6,000 × g for 10 min, and the cells were resuspended and sonicated in 200 μl of PBS for 1 min on ice using a handy sonicator UR-20P (Tomy Seiko Co., Ltd., Tokyo, Japan). Cell lysates were separated by SDS-15% PAGE as described above. The proteins were blotted to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA) using a Trans-Blot SD semidry electrophoretic transfer cell essentially as described by the manufacturer (Bio-Rad) and probed with anti-rPaCdtB antiserum, followed by goat anti-rabbit IgG tagged with horseradish peroxidase (HRP) as a secondary antibody (Invitrogen, Carlsbad, CA). Color development was performed with 4CN-PLUS (Perkin-Elmer) at room temperature.
Cell culture.
HeLa, Vero, Int407, HEp2, and Caco-2 cells were cultured in minimum essential medium (MEM; Invitrogen). CHO, Y-1, or NIH/3T3 was cultured either in MEM-α (Invitrogen), Ham F-12 (Invitrogen), or Dulbecco's modified Eagle medium (Invitrogen), respectively. All media contained 10% fetal bovine serum (Invitrogen) and 1% antibiotic, including antimycotic (×100) liquid (penicillin G sodium [10,000 U/ml], streptomycin sulfate [10,000 U/ml], and 25 μg of amphotericin B/ml as Fungizone in 0.85% saline [Invitrogen]). In addition, 1% nonessential amino acids solution (×100; Invitrogen) was added to the MEM for Caco-2 cells. The cells were cultured at 37°C under 5% CO2 in air.
Cytotoxicity assay.
P. alcalifaciens strains (AH-31 and AS-1) harboring Pacdt genes (Table 1) were cultured at 37°C for 16 h in an appropriate medium, and the culture was sonicated as described above. The lysates were passed through a sterile disposable filter with a 0.22-μm pore size, and filter-sterilized bacterial lysates were examined for the ability to cause the distension and death of CHO, HeLa, Vero, HEp2, Int407, Caco-2, Y-1, and NIH/3T3 cells. Filter-sterilized lysates of E. coli strain TAS-2 (rPaCDT) were also included. Filter-sterilized lysates of E. coli strains GB1371 (EcCDT-I) and C600 were used as positive and negative controls, respectively. The cells were seeded at a density of 5 × 103 cells in a 96-well plate (Asahi Glass Co., Ltd.). After 24 h of incubation, 20 μl of 2-fold serially diluted crude PaCDT was added. For the neutralization assay, 20 μl of 2-fold serially diluted rabbit anti-rPaCdtB serum, as well as preimmune serum, was added with filter-sterilized bacterial lysates into the cell culture. Cell morphology was observed after 72 h of incubation under microscopy. A neutralizing titer was defined as the highest dilution of serum that inhibits the 50% cytotoxicity caused by PaCDT.
Plasmid isolation.
Plasmid DNA was extracted from 100 ml of overnight bacterial culture by the alkaline lysis method (8) and electrophoresed in 0.7% agarose.
Analysis of cell cycle inhibition.
To measure cell cycle arrest induced by PaCDT, HeLa or CHO cells were seeded at density of 2 × 105 cells in a 25-cm2 flask (Corning, NY). After 24 h of incubation, 1 ml of a 5-fold serially diluted filter-sterilized lysate of P. alcalifaciens strain AH-31 or AS-1 (PaCDT) or E. coli TAS-2 strain (rPaCDT) was added to the flask. Filter-sterilized lysates of E. coli strains GB1371 (EcCDT-I) and C600 were used as positive and negative controls, respectively. After 24 h of incubation, the medium was replaced, and incubation continued for another 24 h. Cells were collected and fixed for 1 h on ice with 70% ethanol. The cells were then stained with propidium iodide (50 μg/ml) in PBS containing 0.25 mg of RNase A (Sigma, St. Louis, MO)/ml at 4°C for 20 min in the dark. For each flask, 104 cells were analyzed by using FACSCalibur (Becton Dickinson). Cell cycle analysis was performed using BD CellQuest Pro software (Becton Dickinson).
Fluorescence microscopy.
CHO cells (104) were seeded on a glass slide (Nalge Nunc International, Rochester, NY) and allowed to adhere for 24 h. The cells were incubated with the filter-sterilized bacterial lysate of strain AH-31 for 16 h. Intoxicated cells were fixed in 3.7% formaldehyde for 10 min, treated with ice-cold methanol for 20 min at −30°C, and then treated with 0.5% Triton X-100 for 20 min. Cells were stained with Alexa Fluor 546-conjugated phalloidin (Invitrogen). For immunostaining, the cells were blocked in PBS containing 0.3% Triton X-100 and 1% bovine serum albumin for 1 h and then treated with Alexa Fluor 488-conjugated anti-phospho-histone H2AX (phosphor-Ser139) antibodies (Cell Signaling Technology, Danvers, MA) at 4°C overnight.
Purification of recombinant cholera toxin.
Recombinant cholera toxin (rCT) used as a positive control for suckling mice assay was purified as described previously (52).
Suckling mouse assay and diarrhea score.
The enterotoxic activity of CDT produced by P. alcalifaciens was examined by using suckling mouse assay and diarrhea score was calculated as described previously (40). Either rPaCDT, which showed a high titer (50% cytotoxic dose [CD50] = 2,560) to CHO cell cytotoxicity, or 1010 CFU of live bacteria such as P. alcalifaciens strains AH-31 and AS-1 and E. coli C600 (negative control), respectively, was given orally to each mouse.
Nucleotide sequence accession number.
The nucleotide sequences of cdt genes and its flanking region of P. alcalifaciens strains AH-31 and AS-1 have been registered in DDBL under accession numbers AB583184 and AB583185, respectively.
Statistical analysis.
To compare the effect of CDT-producing strains [strains AH-31, AS-1, GB1371, and BL21(DE3)/TAS-2] and nontoxigenic control strains [strains C600 and BL21(DE3)] on CHO cells (G1 and G2/M), statistical analysis was performed using the Student t test. A P value of <0.05 was considered significant (n = 3).
RESULTS
Nucleotide sequence of the P. alcalifaciens cdt genes.
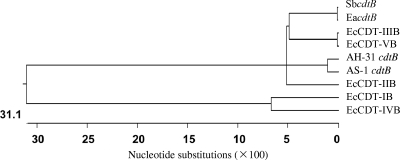
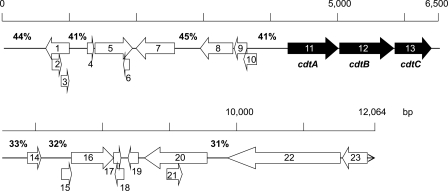
We sequenced 12,064 bp, including the cdt genes and its flanking region of P. alcalifaciens strains AH-31 and 2,186 bp of the cdt genes of the strain AS-1. These two DNA fragments contained three open reading frames (ORFs) termed PacdtA (750 bp, 249 amino acids [aa]), PacdtB (810 bp, 269 aa), and PacdtC (549 bp, 182 aa). Dendrogram analysis indicated that PacdtB is highly homologous to the Shigella boydii cdtB, E. albertii cdtB, and EcCDT-IIIB and EcCDT-VB genes (Fig. 1). Furthermore, ORFs 1 to 10 were found in the upstream region of Pacdt genes in the AH-31 strain, although ORFs 14 to 23 were found in the downstream region, as shown in Fig. 2 and Table 2. Detailed data regarding percent G+C (%GC) content and homologous proteins are summarized in Table 2 (10, 12, 14, 25, 30, 42, 44, 49, 53, 59). Although genes related to prophage were not found and the functions of some gene products are unknown, genes partly homologous to transposase and the IS element were found in the flanking region as shown in Table 2 (14, 42, 49). In addition, no plasmid was found in P. alcalifaciens strains AH-31 and AS-1. These data indicated that the Pacdt genes in AH-31 strain are most likely located in the chromosome and probably acquired by horizontal gene transfer mechanism through phage(s) or transposon(s).
Fig 1.
Dendrogram analysis of cdtB genes. Dendrogram analysis was performed using CLUSTAL W of MegAlign. Genes: AH-31 cdtB, cdtB of P. alcalifaciens strain AH-31 (AB583184); AS-1 cdtB, cdtB of P. alcalifaciens strain AS-1 (AB583185); EcCDT-IB (U03293), EcCDT-IIB (U04208), EcCDT-IIIB (U89305), EcCDT-IVB (AY578329), EcCDT-VB (AJ508930), EacdtB (AT696755), SbcdtB (AT696753).
Fig 2.
Schematic representation of the Pacdt genes and its flanking regions of the strain AH-31. Closed and open arrows indicate Pacdt genes and ORFs, respectively, located in the flanking region. The number indicated above each bar represents the %GC content of a noncoding region.
Table 2.
Characteristics of the ORFs of the Pacdt gene and its flanking regions
| ORFa | Gene coordinates and direction | Gene product size (aa) | GC% | Related bacterial proteins |
Source or reference | ||
|---|---|---|---|---|---|---|---|
| Product(s) and origin | GenBank accession no. | BLAST E-value (identity), % | |||||
| 1 | 651←1001 | 117 | 47.86 | Transposase (fragment), Xenorhabdus nematophila ATCC 19061 | FN667742 | 4e–19 (64/110), 58% | Direct submission |
| 2 | 745→885 | 47 | 49.65 | Transposase (fragment), Xenorhabdus nematophila ATCC 19061 | FN667742 | 7e–05 (22/39), 56% | Direct submission |
| 3 | 882→1004 | 41 | 46.34 | No hits found | |||
| 4 | 1270→1368 | 33 | 36.36 | Unknown, Comamonas testosteroni PtL5 | AF076997 | 0.24 (15/22), 68% | Direct submission |
| 5 | 1387→1947 | 187 | 34.22 | Unknown, Photorhabdus luminescens subsp. laumondii TTO1 | BX571866 | 8e–65 (126/187), 67% | 53 |
| 6 | 1812←1898 | 29 | 42.53 | Putative DNA-binding protein, Proteus mirabilis HI4320 | AM942759 | 8e–06 (24/28), 85% | 42 |
| 7 | 1996←2574 | 193 | 43.01 | Transposase, Salmonella enterica subsp. salamae serovar Sofia | FJ496648 | 7e–86 (154/192), 80% | Direct submission |
| 8 | 2957←3445 | 163 | 47.65 | Putative exported protein, Citrobacter rodentium ICC168 | FN543503 | 1e–27 (55/100), 55% | 44 |
| 9 | 3464←3655 | 64 | 44.27 | Transposase, Proteus mirabilis HI4320 | AM942759 | 5e–24 (54/67), 80% | 42 |
| 10 | 3604←3801 | 66 | 41.92 | Putative putative IS element transposase, Proteus mirabilis HI4320 | AM942759 | 3e–10 (23/32), 71% | 42 |
| 11* | 4264→5013 | 750 | 42.80 | Cytolethal distending toxin A, Shigella boydii strain K-1 | AY696753 | 92% | 25 |
| 12* | 5034→5843 | 810 | 42.10 | Cytolethal distending toxin B, Shigella boydii strain K-1 | AY696753 | 95% | 25 |
| 13* | 5858→6406 | 549 | 37.34 | Cytolethal distending toxin C, Shigella boydii strain K-1 | AY696753 | 90% | 25 |
| 14 | 6884→7090 | 69 | 29.47 | Replication initiator and transcription repressor, Pantoea stewartii subsp. stewartii | L42524 | 3e–16 (47/94), 50% | 19 |
| 15 | 7390→7542 | 51 | 41.83 | Unknown, Escherichia coli strain CB853 | FM210347 | 0.044 (22/50), 44% | 10 |
| 16 | 7539→8168 | 210 | 45.40 | Hypothetical protein, Enterobacter cloacae | AY780889 | 4e–78 (142/205), 69% | 59 |
| 17 | 8168→8281 | 38 | 51.75 | Transposase, Escherichia coli strain BEN2908 | AY857617 | 1e–09 (30/41), 73% | 14 |
| 18 | 8186←8323 | 46 | 44.93 | Hypothetical ORF in IS2, Klebsiella pneumoniae | AY378100 | 0.020 (24/47), 51% | 12 |
| 19 | 8384←8542 | 53 | 35.85 | Transposase, Clostridium difficile CD196 | FN538970 | 1.7 (15/34), 44% | 49 |
| 20 | 8629←9567 | 313 | 37.49 | EspG protein, Escherichia coli strain 71074 | GQ338312 | 2e–44 (104/314), 33% | Direct submission |
| 21 | 8960→9190 | 77 | 37.66 | Pyridine nucleotide-disulfide oxidoreductase, Listeria monocytogenes HCC23 | CP001175 | 32.7 (17/50), 34% | Direct submission |
| 22 | 9861←11549 | 563 | 38.90 | Protein tyrosine phosphatase SptP, Salmonella enterica subsp. enterica serovar Typhimurium strain SL1344 | U63293 | 9e–82 (190/559), 33% | 30 |
| 23 | 11579←1195 | 124 | 31.45 | Chaperone protein SicP, Salmonella enterica subsp. salamae serovar Sofia | FJ496648 | 2e–16 (43/93), 46% | Direct submission |
*, Results obtained from the CLUSTAL W method.
The homology of the deduced amino acid sequences of the PaCdtA, PaCdtB, and PaCdtC proteins of two P. alcalifaciens strains was 96.4, 97.8, and 97.3%, respectively. The deduced amino acid sequences of PaCDT of the strain AH-31 were highly homologous to the CDT of S. boydii (accession no. AY696753) with 92.4, 94.8, and 90.2% identities for CdtA, CdtB, and CdtC, respectively, followed by E. albertii CDT (AY696755: CdtA, 91.6%; CdtB, 94.8%; CdtC, 89.6%), EcCDT-II (U04208: CdtA, 89.6%; CdtB, 93.7%; CdtC, 85.5%), EcCDT-III (U89305: CdtA, 89.2%; CdtB, 94.4%; CdtC, 87.4%), and EcCDT-V (AJ508930: CdtA, 89.2%; CdtB, 94.4%; CdtC, 88.5%), and homologous to other CDTs reported in A. actinomycetemcomitans CDT (AB011405), EcCDT-I (U03293), EcCDT-IV (AY578329), C. jejuni CDT (U51121), C. coli CDT (AB182109), C. fetus CDT (AB211058), H. ducreyi CDT (U53215), H. hepaticus CDT (AF163667), and S. dysenteriae CDT (55) (CdtA, 16.8% to 35.6%; CdtB, 45.2% to 55.6%; CdtC, 18.6% to 32.2%). The putative amino acid residues (H154, G191-N194, D229, and S259-V264) important for DNase I activity were perfectly conserved in PaCdtB. The nuclear localization signals (NLS1 and NLS2) detected in EcCDT-II were also almost conserved in PaCdtB, except for two amino acid substitutions in each of the regions (NLS1, A198D and R210N; NLS2, F262Y and S267F). The RR(X)10-20RR motif (33) in NLS2 was found to be completely conserved, suggesting that PaCDT may enter into the nucleus for its genotoxic activity.
Distribution of cdt genes among Providencia spp.
To examine the distribution of cdt genes among Providencia spp., 18 strains belonging to the genus Providencia, including eight P. alcalifaciens strains, six P. rettgeri strains, two P. rustigianii strains, one P. heimbachae strain, and one P. stuartii strain, were tested using the cdtB gene as a probe by colony hybridization assay. The probe was able to detect only a single strain of P. alcalifaciens, and further analysis revealed that the strain harbors a truncated cdt gene cluster, which was the most likely reason for the absence of CDT activity in this strain.
Expression of recombinant PaCDT.
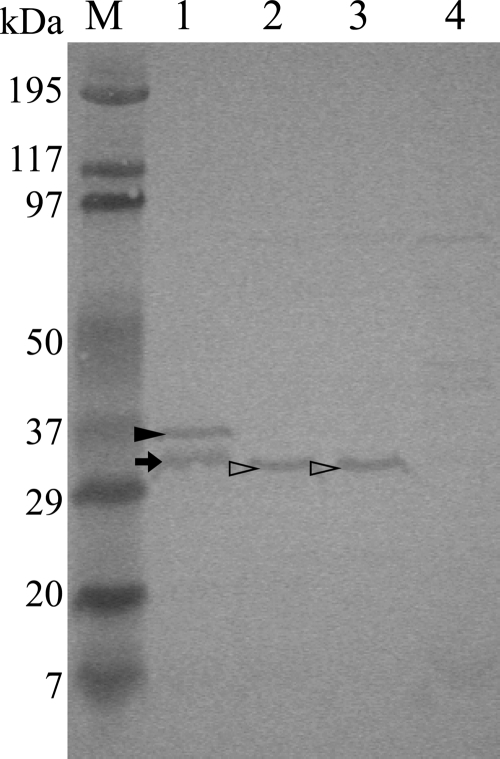
To confirm whether P. alcalifaciens strains AH-31 and AS-1 produced PaCDTs, we attempted to raise an antibody against PaCdtB for Western blotting and cytotoxic assay. For this purpose, a PacdtB gene was cloned and expressed in E. coli as rPaCdtB with His tag (see the details in Materials and Methods). Purified rPaCdtB was used to immunize rabbits and antiserum against rPaCdtB was successfully obtained. Western blotting revealed that antiserum against rPaCdtB was specific for PaCdtB (Fig. 3). Two bands (of about 35 and 33 kDa), which seem to be intact and degraded products of rPaCdtB, respectively, were obtained in lane 1 (rPaCdtB), while only one band (about 32 kDa) was obtained in lanes 2 and 3 with lysates of P. alcalifaciens strains AH-31 and AS-1, respectively. However, no reactive bands were obtained from the lysate of E. coli strain BL21(DE3) carrying the empty vector pET28a, indicating that P. alcalifaciens strains AH-31 and AS-1 produced PaCdtB and antibody against rPaCdtB was specifically reactive to the protein.
Fig 3.
Detection of PaCdtB of wild-type P. alcalifaciens by Western blotting. Whole-cell lysate of P. alcalifaciens was separated by SDS-PAGE (15%), transferred into a PVDF membrane, and probed with rabbit anti-rPaCdtB antiserum, followed by treatment with goat anti-rabbit IgG tagged with HRP as a secondary antibody. Color development was performed with 4CN-PLUS. Lanes: M, prestained SDS-PAGE broad-range marker (Bio-Rad);1, purified rPaCdtB; 2, bacterial lysate of AH-31 (P. alcalifaciens); 3, bacterial lysate of AS-1 (P. alcalifaciens); 4, bacterial lysate of E. coli strain BL21(DE3) carrying empty vector pET28a. The closed and open arrowheads and the arrow indicate rPaCdtB, PaCdtB, and degraded product of rPaCdtB, respectively. The experiment was carried out at least thrice.
Genotoxic activity of PaCDT.
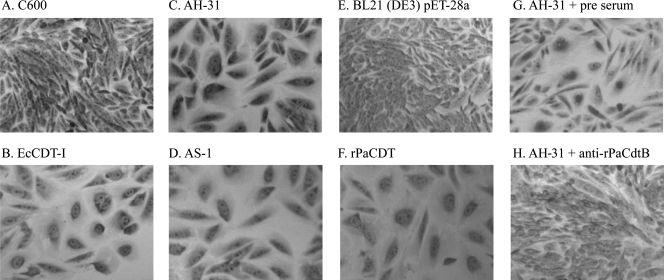
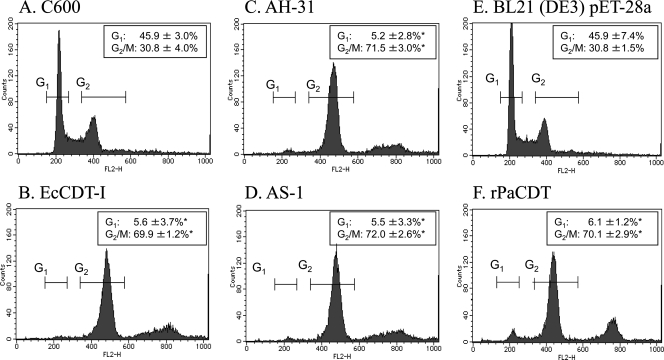
Since production of PaCdtB was confirmed in both P. alcalifaciens strains AH-31 and AS-1, we further examined whether biologically active CDT was produced by these strains. Filter-sterilized lysates of both P. alcalifaciens strains AH-31 (Fig. 4C) and AS-1 (Fig. 4D) induced cell distention on CHO cells. Although filter-sterilized lysate of E. coli strain GB1371 (EcCDT-I) used as a positive control showed cell distention in both CHO (Fig. 4B) and HeLa (data not shown) cells, the filter-sterilized lysates of P. alcalifaciens strains AH-31 and AS-1 (PaCDT) did not show any morphological changes of HeLa cells (data not shown). Since the activities of PaCDT and EcCDT-I to CHO and HeLa cells were different, an additional six cell lines, namely, Vero, HEp2, Int407, Caco-2, Y-1, and NIH/3T3, were also tested to examine any such differential activities by these two toxins. Although EcCDT-I showed cytotoxicity to Vero, HEp2, Int407, and Caco-2 cells, PaCDT was cytotoxic only to Vero and Caco-2 cells. Both toxins failed to show any cytotoxic effects on Y-1 and NIH/3T3 cells. To further examine whether the cytotoxic activity of PaCDT on CHO cells is specific, CHO cells were incubated with filter-sterilized lysate of P. alcalifaciens strains AH-31 or AS-1 in the presence of rabbit antiserum raised against rPaCdtB or filter-sterilized lysate of E. coli strain TAS-2 (rPaCDT) alone. CHO cell distention was not observed only when filter-sterilized lysate of P. alcalifaciens strain AH-31 or AS-1 was mixed with anti-rPaCdtB (Fig. 4H) but not with preimmunized rabbit serum (Fig. 4G). Furthermore, rPaCDT alone also caused morphological changes of CHO cells (Fig. 4F), which was similar to EcCDT-I, indicating that the CHO cell cytotoxic effect was most likely due to the CDT produced by P. alcalifaciens strain AH-31 or AS-1. We further explored the DNA contents of CHO cells treated with PaCDT. The filter-sterilized lysates of both parental P. alcalifaciens strains AH-31 (Fig. 5C) and AS-1 (Fig. 5D) caused G2/M cell cycle arrest on CHO cells (P < 0.05) compared to that of the lysate of E. coli strain C600, a CDT-negative wild-type strain used as a control (Fig. 5A). Similarly, filter-sterilized lysate of E. coli strain TAS-2 producing rPaCDT (Fig. 5F) caused G2/M cell cycle arrest on CHO cells (P < 0.05) compared to that of E. coli BL21(DE3) strain carrying the empty vector pET-28a (Fig. 5E).
Fig 4.
Cytotoxic effect of CDT produced by P. alcalifaciens on CHO cells. Whereas alteration of cellular morphology was not apparent at 72 h after exposure of the filter-sterilized samples from E. coli strain C600 (A) and E. coli strain BL21(DE3) carrying pET-28a as a vector control (E) to CHO cells, cytoplasmic distension was apparent by the filter-sterilized samples from E. coli strain GB1371 (EcCDT-I) (B), P. alcalifaciens strains AH-31 (C) and AS-1 (D), E. coli strain TSA-1 (rPaCDT) (F), and P. alcalifaciens strain AH-31 with preimmunization serum (G). The CDT activity of P. alcalifaciens strain AH-31 was neutralized in the presence of anti-rabbit rPaCdtB (H). The experiment was performed at least thrice.
Fig 5.
Analysis of CHO cell cycle after treatment with various preparations. The cell cycle distribution of 10,000 cells was determined by flow cytometry, and representative results are shown. The average percentages and the standard deviations of cells in each cell cycle phase calculated with three independent experiments are indicated. CHO cells were unaffected when exposed to lysates of nontoxigenic control E. coli strain such as C600 (A) or BL21(DE3) carrying the empty vector pET-28a (E) cells, but the cells were blocked in the G2/M cell cycle phase when they were exposed to EcCDT-I (B) or PaCDT (C, D, and F). DNA content of CHO cells was monitored by flow cytometry as described in Materials and Methods. The effects of EcCDT-I (B) and PaCDT (C and D) on the CHO cell cycle (G1 and G2/M) were compared to the lysate of E. coli strain C600 (A) used as a negative control. The effect of rPaCDT (F) on CHO cell cycle (G1 and G2/M) was compared to that of E. coli strain BL21(DE3) carrying the empty vector pET-28a (F). *, P < 0.05 (Student t test, n = 3).
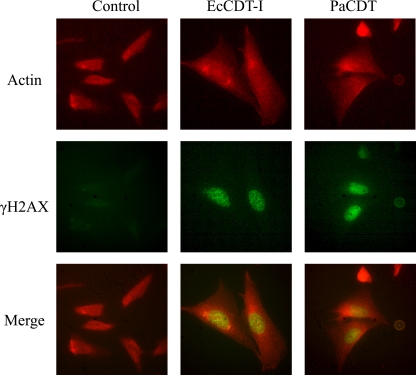
Since EcCDT-I has been shown to cause phosphorylation of the histone H2AX, a sensitive marker for double-strand DNA breaks, we also examined whether PaCDT is involved in phosphorylation of the histone H2AX (γH2AX). As shown in Fig. 6, phosphorylated H2AX was visualized by direct immunofluorescence using antibodies against γH2AX, and a strong nuclear signal was detected in PaCDT-treated cells such as EcCDT-I-treated cells but not in control cells, indicating that PaCDT entered into the nucleus and induced DNA double-strand breaks of CHO cells.
Fig 6.
Genotoxic effect of PaCDT. CHO cells were treated with filter-sterilized lysates of bacteria producing EcCDT-I (GB1371) or PaCDT (AH-31). After 16 h of treatment, the cells were stained as described in Materials and Methods with Alexa Fluor 546-conjugated phalloidin (upper panels) or with fluorescein isothiocyanate-conjugated anti-phospho-histone H2AX (H2AX) monoclonal antibody (middle panels). EcCDT-I- and PaCDT-treated cells exhibited nuclear H2AX indicating host DNA double-strand breaks, enlarged nuclei and cell bodies, and the absence of mitotic features. Filter-sterilized lysate of E. coli C600 was used as a negative control. The experiment was repeated at least three times.
Enterotoxic activity.
Enterotoxicity of CDT produced by P. alcalifaciens was examined by suckling mouse assay. Crude rPaCDT prepared from E. coli strain TAS-2, rCT, or PBS was orally administered to each mouse, followed by an evaluation of the diarrheal score for each sample. rCT and PBS showed 100% positive and negative results, respectively. However, crude rPaCDT did not show any enterotoxicity (data not shown).
Subsequently, live bacteria were orally inoculated into suckling mice to see whether there was any fluid accumulation. Although 1010 CFU of P. alcalifaciens strain AS-1 did not cause any diarrhea in 12 mice tested, the same dose of P. alcalifaciens strain AH-31 could cause diarrhea in 7 of 12 mice. Further study is needed to prove the enterotoxic activity of purified PaCDT.
DISCUSSION
Despite tremendous efforts toward understanding the pathogenesis of P. alcalifaciens, it is still unclear how P. alcalifaciens causes diarrhea in humans. In the present study we show that P. alcalifaciens strains isolated from patients with diarrhea could produce CDT, and this is the first report, to our knowledge, regarding the production of the toxin by the genus Providencia, including P. alcalifaciens.
Genus Providencia, belonging to the family Enterobacteriaceae, consists of five species: P. alcalifaciens, P. stuartii, P. rettgeri, P. rustigianii, and P. heimbachae (27). Among these, P. alcalifaciens has been described as a causative agent of diarrhea because a number of P. alcalifaciens strains were isolated from patients with diarrhea in developing countries (21, 22, 47, 48). Indeed, a case control study conducted by Albert et al. (3) demonstrated that P. alcalifaciens was associated with diarrhea in children in Bangladesh. Haynes and Hawkey (22) reported that P. alcalifaciens was associated with traveler's diarrhea. Yoh et al. (56) showed that not only P. alcalifaciens but also P. rettgeri in particular is an important pathogen for traveler's diarrhea. Furthermore, two large outbreaks of food poisoning caused by P. alcalifaciens have been reported from Japan and the Czech Republic (13, 36).
Several studies demonstrated that P. alcalifaciens is able to invade cultured epithelial cells (1, 21, 26). Invasion was also observed in intestinal tissues by using a removable intestinal tie adult rabbit diarrhea (RITARD) model and an adult rabbit ileal loop model (1, 35). Although invasion was considered as one of the virulence mechanisms to cause diarrhea by P. alcalifaciens strains, noninvasive P. alcalifaciens were also isolated from patients with diarrhea (21, 48). In our study, one strain, P. alcalifaciens AH-31, showed invasiveness to HeLa cells; however, another strain, P. alcalifaciens strain AS-1, did not show any invasiveness (data not shown). This observation suggests that invasiveness could be one of several possible virulence mechanisms. Therefore, other mechanisms by which P. alcalifaciens is involved in diarrhea have been considered but are not fully understood. Until the present study, no toxin was reported to be the virulence factor of P. alcalifaciens. CDT produced by P. alcalifaciens may be a candidate virulence factor in these strains other than invasiveness. Colony hybridization assay revealed that cdt genes are present only in certain strains of P. alcalifaciens. In addition to the cytotoxicity test, we also attempted to examine the enterotoxic activity of PaCDT in a suckling mouse assay. However, the suckling mouse assay did not show any enterotoxicity by crude concentrated rPaCDT, but one of the P. alcalifaciens strains (the strain AH-31) showed enterotoxicity when live bacteria were orally administered (Table 3). Based on these findings, it is not clear whether PaCDT is indeed a virulence factor for this pathogen, and further studies are needed to shed light on this aspect.
Table 3.
Suckling mouse assay with Providencia alcalifaciens
| Sample | Diarrhea score (no. of positive animals/total no. of animals)a |
|---|---|
| E. coli C600 | 0 (0/12) |
| P. alcalifaciens AH-31 | 58 (7/12) |
| P. alcalifaciens AS-1 | 0 (0/12) |
| rCTb | 100 (9/9) |
The animals excreting stained loose and/or watery feces over 24 h after sample administration were judged positive.
Recombinant cholera toxin.
Some bacterial species, e.g., certain strains of E. coli carry cdt genes, including its several variants (55), and other species, e.g., C. jejuni and C. coli, ubiquitously carry cdt genes in a species-specific manner (6). Although cdt genes in C. jejuni and C. coli are not associated with any mobile genetic element, CDT produced by E. coli has been demonstrated to be encoded on bacteriophage or plasmid (5, 43, 50, 51). In order to understand whether the cdt gene cluster is horizontally transferred among closely related species, the distribution of cdt genes was explored in P. alcalifaciens, as well as other Providencia spp., including P. stuartii, P. rettgeri, and P. rustigianii, and it was shown that only limited strains of P. alcalifaciens harbored the cdt genes. This result prompted us to further examine the possibility of horizontal transfer of Pacdt genes. Initially, we attempted to isolate plasmid; however, no plasmid was detected in both P. alcalifaciens strains AH-31 and AS-1. Nucleotide sequence analysis of the flanking region of Pacdt genes, however, revealed the presence of sequence predicted to encode a protein homologous to a transposase. The %GC content of each ORF or the entire region varied from 30 to 50% (Fig. 2 and Table 2), respectively. The %GC content of the genus Providencia has been reported to be ca. 39 to 42% (17). However, the %GC content of Pacdt flanking regions showed mosaic structure of high and low %GC content, indicating that Pacdt genes might have been acquired by horizontal gene transfer events, and later on it evolved further by repeated homologous recombination in various bacterial species or even in various bacterial genera.
A number of Gram-negative bacteria have been reported to produce CDT. CDT is a very unique bacterial protein toxin, which inhibits the cell cycle at G2/M phase, leading to cell death and can enter into the nucleus and directly damage DNA. Thus, CDT is also called cyclomodulin or genotoxin. Comparative analysis of nucleotide and amino acid sequences of CDT revealed that CDT produced by P. alcalifaciens is closest to that produced by S. boydii. CDT thus far reported was generally active on CHO and HeLa cells (46, 51). However, PaCDT could cause cell distention and death of CHO, Vero, and Caco-2 cells, but it is not effective in other cells, including HeLa cells even when used at high concentration (Table 4). We cannot exclude the possibility that the toxin concentration was too low in our preparation to cause any cytotoxic effect to these cell lines. The receptor for EcCDT-I has been reported to be putative G protein-coupled receptor encoded by TMEM181 (11). These observations indicate that the receptor for PaCDT may be different from that of other CDTs, including EcCDT-I. Similar findings that CDTs produced from different bacteria, e.g., A. actinomycetemcomitans, H. ducreyi, C. jejuni, and E. coli, may display variable target cell tropism and may have different receptors as reported by Eshraghi et al. (16).
Table 4.
Cytotoxicity of EcCDT-1 and PaCDT on various cell lines
| Strain and sample | CD50a |
|||||||
|---|---|---|---|---|---|---|---|---|
| CHO | HeLa | Vero | HEp2 | Int407 | Caco-2 | Y-1 | NIH/3T3 | |
| E. coli | ||||||||
| GB1371 (EcCDT-I) | 64 (70) | 128 (140) | 32 (35) | 16 (18) | 16 (18) | 128 (140) | <1 (<1) | <1 (<1) |
| TAS-2 (rPaCDT) | 8 (7.7) | <1 (<0.97) | 1 (0.97) | <1 (<0.97) | <1 (<0.97) | 4 (3.9) | <1 (<0.97) | <1 (<0.97) |
| P. alcalifaciens | ||||||||
| AH-31 (PaCDT) | 2 (2.2) | <1 (<1.1) | <1 (<1.1) | <1 (<1.1) | <1 (<1.1) | 1 (1.1) | <1 (<1.1) | <1 (<1.1) |
| AS-1 (PaCDT) | 8 (12) | <1 (<1.5) | 1 (1.5) | <1 (<1.5) | <1 (<1.5) | 4 (6.0) | <1 (<1.5) | <1 (<1.5) |
The values indicate titers as the 50% cytotoxic dose (CD50). Numbers in parentheses indicate the titer per mg of protein of the sample (n = 3).
In conclusion, our data clearly demonstrated that P. alcalifaciens is a new member of CDT-producing bacterial family. However, in the present study, we were unable to demonstrate PaCDT as a possible virulence factor in P. alcalifaciens. Further investigation is necessary to determine the role of CDT in the pathogenesis of diarrhea caused by P. alcalifaciens and to clarify how the cdt genes was evolved and transferred among P. alcalifaciens strains.
ACKNOWLEDGMENTS
We thank Rupak K. Bhadra, Indian Institute of Chemical Biology, and Sucharit B. Neogi, Osaka Prefecture University, for critical reading the manuscript.
A.S. is a recipient of a Research Fellowship for Young Scientists of the Japan Society for the Promotion of Science (JSPS). This study was supported in part by a Grant-in-Aid for Scientific Research from the JSPS.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Albert MJ, et al. 1992. Pathogenesis of Providencia alcalifaciens-induced diarrhea. Infect. Immun. 60:5017–5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MJ, et al. 1996. Controlled study of cytolethal distending toxin-producing Escherichia coli infections in Bangladeshi children. J. Clin. Microbiol. 34:717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert MJ, Faruque AS, Mahalanabis D. 1998. Association of Providencia alcalifaciens with diarrhea in children. J. Clin. Microbiol. 36:1433–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ansaruzzaman M, et al. 2000. Clonal groups of enteropathogenic Escherichia coli isolated in case-control studies of diarrhoea in Bangladesh. J. Med. Microbiol. 49:177–185 [DOI] [PubMed] [Google Scholar]
- 5. Asakura M, et al. 2007. An inducible lambdoid prophage encoding cytolethal distending toxin (Cdt-I) and a type III effector protein in enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 104:14483–14488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asakura M, et al. 2007. Comparative analysis of cytolethal distending toxin (cdt) genes among Campylobacter jejuni, C. coli, and C. fetus strains. Microb. Pathog. 42:174–183 [DOI] [PubMed] [Google Scholar]
- 7. Bielaszewska M, et al. 2004. Characterization of cytolethal distending toxin genes and expression in Shiga toxin-producing Escherichia coli strains of non-O157 serogroups. Infect. Immun. 72:1812–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birnboim HC, Doly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouzari S, Oloomi M, Oswald E. 2005. Detection of the cytolethal distending toxin locus cdtB among diarrheagenic Escherichia coli isolates from humans in Iran. Res. Microbiol. 156:137–144 [DOI] [PubMed] [Google Scholar]
- 10. Burgos Y, Beutin L. 2010. Common origin of plasmid encoded alpha-hemolysin genes in Escherichia coli. BMC Microbiol. 10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carette JE, et al. 2009. Haploid genetic screens in human cells identify host factors used by pathogens. Science 326:1231–1235 [DOI] [PubMed] [Google Scholar]
- 12. Chen YT, et al. 2004. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337:189–198 [DOI] [PubMed] [Google Scholar]
- 13. Chlibek R, Jirous J, Beran J. 2002. Diarrhea outbreak among Czech Army Field Hospital personnel caused by Providencia alcalifaciens. J. Travel Med. 9:151–152 [DOI] [PubMed] [Google Scholar]
- 14. Chouikha I, et al. 2006. A selC-associated genomic island of the extraintestinal avian pathogenic Escherichia coli strain BEN2908 is involved in carbohydrate uptake and virulence. J. Bacteriol. 188:977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elwell C, Chao K, Patel K, Dreyfus L. 2001. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect. Immun. 69:3418–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eshraghi A, et al. 2010. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. J. Biol. Chem. 285:18199–18207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falkow S, Ryman IR, Washington O. 1962. Deoxyribonucleic acid base composition of Proteus and Providencia organism. J. Bacteriol. 83:1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedrich AW, et al. 2006. Cytolethal distending toxin in Escherichia coli O157:H7: spectrum of conservation, structure, and endothelial toxicity. J. Clin. Microbiol. 44:1844–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu JF, Chang HC, Chen YM, Chang YS, Liu ST. 1995. Sequence analysis of an Erwinia stewartii plasmid, pSW100. Plasmid 34:75–84 [DOI] [PubMed] [Google Scholar]
- 20. Ghilardi AC, Gomes TA, Trabulsi LR. 2001. Production of cytolethal distending toxin and other virulence characteristics of Escherichia coli strains of serogroup O86. Mem. Inst. Oswaldo Cruz 96:703–708 [DOI] [PubMed] [Google Scholar]
- 21. Guth BEC, Perrella E. 1996. Prevalence of invasive ability and other virulence-associated characteristics in Providencia alcalifaciens strains isolated in Sao Paulo, Brazil. J. Med. Microbiol. 45:459–462 [DOI] [PubMed] [Google Scholar]
- 22. Haynes J, Hawkey PM. 1989. Providencia alcalifaciens and traveller's diarrhoea. BMJ 299:94–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinenoya A, et al. 2007. Cytolethal distending toxin (Cdt)-producing Escherichia coli isolated from a child with bloody diarrhea in Japan. Microbiol. Immunol. 51:435–438 [DOI] [PubMed] [Google Scholar]
- 24. Hinenoya A, et al. 2009. Prevalence and characteristics of cytolethal distending toxin-producing Escherichia coli from children with diarrhea in Japan. Microbiol. Immunol. 53:206–215 [DOI] [PubMed] [Google Scholar]
- 25. Hyma KE, et al. 2005. Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains J. Bacteriol. 187:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janda JM, Abbott SL, Woodward D, Khashe S. 1998. Invasion of HEp-2 and other eukaryotic cell lines by Providenciae: further evidence supporting the role of Providencia alcalifaciens in bacterial gastroenteritis. Curr. Microbiol. 37:159–165 [DOI] [PubMed] [Google Scholar]
- 27. Janda JM, Abbott LS. 2006. The enterobacteria, 2nd ed, p 279–299 ASM Press, Washington, DC [Google Scholar]
- 28. Janka A, et al. 2003. Cytolethal distending toxin gene cluster in enterohemorrhagic Escherichia coli O157:H− and O157:H7: characterization and evolutionary considerations. Infect. Immun. 71:3634–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson WM, Lior H. 1987. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol. Lett. 43:19–23 [Google Scholar]
- 30. Kaniga K, Uralil J, Bliska JB, Galán JE. 1996. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21:633–641 [DOI] [PubMed] [Google Scholar]
- 31. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 32. Lara-Tejero M, Galan JE. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354–357 [DOI] [PubMed] [Google Scholar]
- 33. Leslie A, McSweeney LA, Dreyfus LA. 2004. Nuclear localization of the Escherichia coli cytolethal distending toxin CdtB subunit. Cell. Microbiol. 6:447–458 [DOI] [PubMed] [Google Scholar]
- 34. Marques LR, Tavechio AT, Abe CM, Gomes TA. 2003. Search for cytolethal distending toxin production among fecal Escherichia coli isolates from Brazilian children with diarrhea and without diarrhea. J. Clin. Microbiol. 41:2206–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathan MM, Mathan VI, Albert MJ. 1993. Electron microscopic study of the attachment and penetration of rabbit intestinal epithelium by Providencia alcalifaciens. J. Pathol. 171:67–71 [DOI] [PubMed] [Google Scholar]
- 36. Murata T, et al. 2001. A large outbreak of food-borne infection attributed to Providencia alcalifaciens. J. Infect. Dis. 184:1050–1055 [DOI] [PubMed] [Google Scholar]
- 37. Nesic D, Hsu Y, Stebbins CE. 2004. Assembly and function of a bacterial genotoxin. Nature 429:429–433 [DOI] [PubMed] [Google Scholar]
- 38. Nougayrede JP, Taibe F, Rycke JD, Oswald E. 2005. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol. 13:103–110 [DOI] [PubMed] [Google Scholar]
- 39. Okeke IN, Lamikanra A, Steinruck H, Kaper JB. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 38:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. 1997. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect. Immun. 65:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandey M, et al. 2003. Association of cytolethal distending toxin locus cdtB with enteropathogenic Escherichia coli isolated from patients with acute diarrhea in Calcutta, India. J. Clin. Microbiol. 41:5277–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearson MM, et al. 2008. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J. Bacteriol. 190:4027–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pérès SY, et al. 1997. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol. Microbiol. 24:1095–1107 [DOI] [PubMed] [Google Scholar]
- 44. Petty NK, et al. 2010. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J. Bacteriol. 192:525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pickett CL, Cottle DL, Pesci EC, Bikah G. 1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62:1046–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott DA, Kaper JB. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sen R. 1962. Isolation of strains of Providencia group from cases with diarrhoea in Ibadan, Nigeria, West Africa. Indian J. Med. Res. 50:622–626 [PubMed] [Google Scholar]
- 48. Sobreira M, Leal NC, Magalhães M, Guth BE, Almeida AM. 2001. Molecular analysis of clinical isolates of Providencia alcalifaciens. J. Med. Microbiol. 50:29–34 [DOI] [PubMed] [Google Scholar]
- 49. Stabler RA, et al. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tóth I, Herault F, Beutin L, Oswald E. 2003. Production of cytolethal distending toxins by pathogenic Escherichia coli strains isolated from human and animal sources: establishment of the existence of a new cdt variant (type IV). J. Clin. Microbiol. 41:4285–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tóth I, et al. 2009. Cytolethal distending toxin type I and type IV genes are framed with lambdoid prophage genes in extraintestinal pathogenic Escherichia coli. Infect. Immun. 77:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uesaka Y, et al. 1994. Simple method of purification of Escherichia coli heat-labile enterotoxin and cholera toxin using immobilized galactose. Microb. Pathog. 16:71–76 [DOI] [PubMed] [Google Scholar]
- 53. Williamson VM, Kaya HK. 2003. Sequence of a symbiont. Nat. Biotechnol. 21:1294–1295 [DOI] [PubMed] [Google Scholar]
- 54. Wising C, Molne L, Jonsson IM, Ahlman K, Lagergard T. 2005. The cytolethal distending toxin of Haemophilus ducreyi aggravates dermal lesions in a rabbit model of chancroid. Microbes Infect. 7:867–874 [DOI] [PubMed] [Google Scholar]
- 55. Yamasaki S, et al. 2006. Cytolethal distending toxin (CDT): genetic diversity, structure and role in diarrheal disease. Toxin Rev. 25:61–88 [Google Scholar]
- 56. Yoh M, et al. 2005. Importance of Providencia species as a major cause of travellers' diarrhoea. J. Med. Microbiol. 54:1077–1082 [DOI] [PubMed] [Google Scholar]
- 57. Young VB, et al. 2004. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect. Immun. 72:2521–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yutsudo T, Nakabayashi N, Hirayama T, Takeda Y. 1987. Purification and some properties of a Vero toxin from Escherichia coli O157:H7 that is immunologically unrelated to Shiga toxin. Microb. Pathog. 3:21–30 [DOI] [PubMed] [Google Scholar]
- 59. Yu YS, Du XX, Zhou ZH, Chen YG, Li LJ. 2006. First isolation of blaIMI-2 in an Enterobacter cloacae clinical isolate from China. Antimicrob. Agents Chemother. 50:1610–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]