Abstract
Interleukin 1 receptor antagonist (IL-1Ra)-deficient BALB/c mice develop spontaneous arthritis resembling human rheumatoid arthritis. We herein report that infection with Toxoplasma gondii, an intracellular protozoan, is capable of ameliorating the spontaneous development of arthritis in IL-1Ra-deficient mice. The onset of arthritis development was delayed and the severity score of arthritis was significantly suppressed in T. gondii-infected mice. Expression of IL-12p40 mRNA from CD11c+ cells of mesenteric lymph nodes (mLN) and spleen markedly increased at 1 week after peroral infection. While CD11c+ cells also produced IL-10, IL-1β, and IL-6, CD4+ T cells from T. gondii-infected mice expressed significantly high levels of T-bet and gamma interferon (IFN-γ) mRNA in both mLN and spleen. Levels of GATA-3/IL-4 mRNA or RORγt/IL-17 mRNA decreased in the infected mice, indicating Th1 cell polarization and the reduction of Th2 and Th17 cell polarization. The severity of arthritis was related to Th1 cell polarization accompanied by Th17 cell reduction, demonstrating the protective role of the T. gondii-derived Th1 response against Th17 cell-mediated arthritis in IL-1Ra-deficient mice.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite. In intermediate hosts such as humans and mice, perorally (p.o.) infected encysted T. gondii bradyzoites undergo stage conversion into rapidly dividing tachyzoites that are responsible for acute toxoplasmosis in immunocompromised individuals and fetuses infected within the uterus (43). Furthermore, chronic infection with T. gondii is one of the most common worldwide parasitic infections in humans, since T. gondii survives in hosts throughout their lives by forming tissue cysts in organs. Yet, the associations between chronic infection with T. gondii and human disease (and human behavior) have been underexplored.
We have previously demonstrated that T. gondii-infected mice produce anti-murine heat shock protein 70 (HSP70) autoantibody, which cross-reacts with T. gondii HSP70, a tachyzoite-specific virulent molecule that modulates the immune response of hosts (2, 10, 11, 24, 33), from VH1-JH1 B-1 cells of infected BALB/c (a resistant strain) and C57BL/6 (B6, a susceptible strain) mice (5, 7). Furthermore, we have revealed that T. gondii infection is capable of preventing the development of lupus nephritis in New Zealand Black × New Zealand White F1 mice (NZBW F1 mice), a mouse model of systemic lupus erythematosus (SLE), an autoimmune disease characterized by B cell hyperactivity (6).
On the other hand, gamma interferon (IFN-γ) production by CD4+ T, CD8+ T, and NK cells has been proven to be essential in inducing and maintaining host-protective immunity against T. gondii infection (13, 21, 38, 43), and antigen-presenting cells (APC), such as dendritic cells (DC), play a crucial role in initiating interleukin 12 (IL-12) synthesis and determining a highly polarized Th1-type response triggered by T. gondii infection (1, 23, 31, 42). Therefore, in this study, we attempted to examine the effects of T. gondii infection on T cell-mediated autoimmunity by using IL-1 receptor antagonist (IL-1Ra)-deficient BALB/c mice, a rheumatoid arthritis mouse model (19). IL-1 is a key mediator of a series of host responses to infection and inflammation known as the acute-phase response and affects a wide range of cells and organs (9). IL-1Ra competes with IL-1α and IL-1β in binding IL-1 receptors and has been considered to be a negative regulator of IL-1 signals (9, 22). Excess IL-1 signal due to a deficiency in IL-1Ra causes autoimmunity (19, 22). The evidence that the arthritis in IL-1Ra-deficient BALB/c mice starts at 4 to 5 weeks of age and that almost all mice are affected by 12 weeks (19) has prompted us to analyze the roles of T. gondii infection on the development of arthritis in IL-1Ra-deficient BALB/c mice.
Since spontaneous development of arthritis in IL-1Ra-deficient mice has been proven to be IL-17 dependent (17, 26, 36), effects of T. gondii infection on DC activation and successive Th cell polarization in IL-1Ra-deficient BALB/c mice have been evaluated. The application of a T. gondii HSP70 gene vaccine that induces DC cell activation and Th1 cell polarization (31) will be discussed.
MATERIALS AND METHODS
Mice and T. gondii strain.
A generation of IL-1Ra-deficient mice with a BALB/c background has previously been described (19). Wild-type (WT) BALB/c mice were purchased from SLC (Hamamatsu, Japan). Cysts of an avirulent Fukaya strain of T. gondii were prepared as described previously (30). Ten T. gondii cysts of the Fukaya strain were suspended in 200 μl of phosphate-buffered saline (PBS), and 2.5-week-old WT or IL-1Ra-deficient BALB/c mice were p.o. infected with 10 T. gondii cysts with a specially prepared small-size needle with a round head.
Clinical evaluation and histological examination.
Development of arthritis was examined weekly with a clinical macroscopic evaluation, and the arthritis severity score was determined as described previously (19). For a histological examination, ankle joints were fixed in 10% phosphate-buffered formalin, decalcified in 10% EDTA-4Na, and embedded in paraffin. Sections (4 mm) were stained with hematoxylin/eosin as described previously (19).
RT-PCR.
WT and IL-1Ra-deficient BALB/c mice with or without T. gondii infection were euthanized at 1 week postinfection (p.i.), and CD11c+ or CD4+ cells were fractionated from either the mesenteric lymph nodes (mLN) or spleens of the mice with a Vario magnetically activated cell sorting (MACS) separator system (Miltenyi Biotec, Auburn, CA) with microbead-conjugated anti-CD11c or anti-CD4 monoclonal antibody (MAb) (Miltenyi Biotec) according to the company's instructions. CD4+ cells were similarly fractionated from the LN (popliteal, inguinal, and axillary LN) or spleens of uninfected or infected IL-1Ra-deficient BALB/c mice at 8 weeks p.i. Total RNA from CD11c+ or CD4+ cells of the LN or spleen was isolated and transcribed to cDNA by reverse transcription (RT), and cDNA was used for real-time quantitative PCR using a TaqMan PCR system (Applied Biosystems, Tokyo, Japan). Primers and 6-carboxyfluorescein (FAM)-labeled probes (Applied Biosystems) specific for IL-12p40, IL-10, IL-6, and IL-1β were used for CD11c+cells, and those specific for IFN-γ, IL-4, IL-17, T-bet, GATA-3, and RORγt (nuclear hormone receptor retinoid-related orphan receptor γt) were used for CD4+ cells. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control. The primer/probe sets were purchased from Applied Biosystems. We normalized each set of samples using the difference in threshold cycles (ΔCT) between the sample gene and the internal control gene (GAPDH) as follows: ΔCT = CT sample − CT GAPDH. The calibrator sample (ΔCT calibrator) was assigned as the sample with the highest ΔCT in each set. The relative scale of mRNA measurement is represented on the y axes of figures by the expression of log-transformed 2−ΔΔCT, where ΔΔCT = ΔCT sample (n) − ΔCT calibrator (n). Each reaction was done in triplicate.
Intracellular cytokine staining.
LN or spleen cells from uninfected or infected IL-1Ra-deficient BALB/c mice were stimulated with 25 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, Tokyo, Japan) plus 1 μg/ml ionomycin (Sigma-Aldrich) for 6 h in the presence of Golgi-Stop (BD Biosciences), which was added for the last 4 h, and then stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 MAb (clone GK1.5), fixed, and permeabilized using Cytofix/Cytoperm (BD Biosciences), followed by staining with phycoerythrin (PE)-conjugated anti-IFN-γ (XMG1.2), anti-IL-4 (11B11), or anti-IL-17 (TC11-18H10) MAb. PE-conjugated isotype control IgG (rat IgG1, κ) was used for staining. All MAbs used for the staining were purchased from BD Biosciences. Intracellular cytokine expression was analyzed with a FACSCalibur flow cytometer (Becton Dickinson).
Statistics.
The significance of differences between groups was determined by a 2-by-2 contingency χ2 test or Student's t test. P values less than 0.05 were considered statistically significant.
RESULTS
Effects of T. gondii infection on the spontaneous development of arthritis in IL-1Ra-deficient mice.
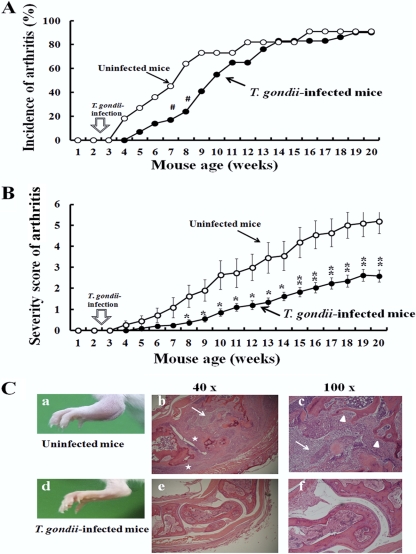
Almost all IL-1Ra-deficient mice with a BALB/c background, but not with a C57BL/6 background, develop spontaneous arthritis closely resembling rheumatoid arthritis by 12 weeks of age, without gender differences (19). As BALB/c mice are a strain resistant to T. gondii infection and do not die after infection, we took advantage of using IL-1Ra-deficient BALB/c mice for the study. Since arthritis in mice starts at 4 to 5 weeks of age (19), we p.o. infected the mice at 2.5 weeks with 10 T. gondii cysts. Compared with arthritis development in the uninfected mice, the incidence of spontaneous arthritis development was delayed for 2 to 3 weeks in the T. gondii-infected mice until 10 to 12 weeks of age (Fig. 1A). Also, the severity score of arthritis was significantly suppressed in T. gondii-infected mice of all ages (P < 0.05 at 8 to 14 weeks and P < 0.01 at 15 to 20 weeks of age in a comparison of infected and uninfected mice) (Fig. 1B).
Fig 1.
Effects of T. gondii infection on spontaneous development of arthritis in IL-1Ra-deficient mice. (A) IL-1Ra-deficient BALB/c mice were p.o. infected with 10 T. gondii cysts at 2.5 weeks, and the development of arthritis in uninfected (open circles) and infected (closed circles) mice was examined weekly as described in Materials and Methods. Incidence (percentage) was calculated from 11 uninfected and 29 T. gondii-infected mice. (B) The severity scores of arthritis in uninfected (open circles) and infected (closed circles) mice were determined weekly as described in Materials and Methods. The average scores ± standard deviations (SD) were obtained from 11 uninfected and 29 T. gondii-infected mice. Statistical differences between uninfected and T. gondii-infected groups are shown as follows: #, P was <0.05 by 2-by-2 contingency χ2 test; *, P was <0.05; and **, P was <0.01 by Student's t test. (C) Histopathology of the ankle joints of uninfected (a to c) and T. gondii-infected (d to f) IL-1Ra-deficient BALB/c mice. (a) Swelling and redness of the ankle joint of an uninfected IL-1Ra-deficient BALB/c mouse. (b, c) Paraffin-embedded tissue sections of ankle joints from uninfected IL-1Ra-deficient mice were stained with hematoxylin/eosin, and microscopic views magnified at ×40 and ×100 are shown. Marked infiltration of inflammatory cells (arrows), hyperplasia of synovial membrane (stars), and erosive destruction of the bone (arrowheads) were observed. (d) Ankle joint of a T. gondii-infected IL-1Ra-deficient mouse. Swelling and redness of the ankle joint are not observed. (e to f) Microscopic observation of the ankle joint of a T. gondii-infected IL-1Ra-deficient mouse without infiltration of inflammatory cells.
Consistent with a low degree of clinical severity, histological findings of ankle joints in uninfected IL-1Ra-deficient mice which demonstrated a remarkable infiltration of inflammatory cells, hyperplasia of the synovial membrane, and erosive destruction of the bone (Fig. 1Ca to c) were markedly reduced in the T. gondii-infected mice (Fig. 1Cd to f). Thus, T. gondii infection is capable of ameliorating the spontaneous development of arthritis in IL-1Ra-deficient mice.
Effect of T. gondii infection on DC activation in IL-1Ra-deficient mice.
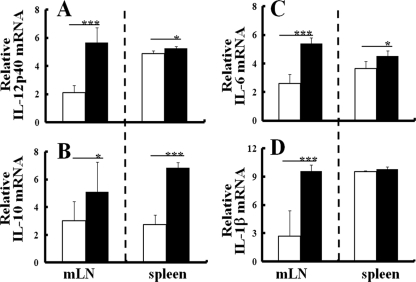
Since DC play a central role in determining Th cell polarization, which regulates acquired immunity, effects of T. gondii infection on DC activation in IL-1Ra-deficient mice were comparatively analyzed with those of WT mice at 1 week p.i. After T. gondii infection, production of IL-12p40 mRNA from CD11c+ cells of IL-1Ra-deficient mice markedly increased in both the mLN and the spleen (Fig. 2A), as well as in those of WT mice (see Fig. S1 in the supplemental material). Also, levels of surface expression of CD86, an accessory molecule for T cell activation, and major histocompatibility complex (MHC) class II molecules on CD11c+ cells in the mLN of T. gondii-infected IL-1Ra-deficient mice were upregulated at 1 week p.i. compared to those of uninfected mice (data not shown). Thus, in vivo maturation of DC was induced in the draining LN (dLN) after p.o. T. gondii infection at 1 week p.i.
Fig 2.
Effects of T. gondii infection on DC activation in IL-1Ra-deficient mice. (A) CD11c+ cells were isolated from the mLN or spleens of IL-1Ra-deficient mice with or without T. gondii infection at 1 week p.i., and mRNA expression of IL-12p40 from CD11c+ cells of uninfected (white column) or T. gondii-infected (black column) IL-1Ra-deficient mice was evaluated by reverse transcription-PCR as described in Materials and Methods. IL-10 mRNA (B), IL-6 mRNA (C), and IL-1β mRNA (D) expression of CD11c+ cells was similarly measured. The results are normalized with those for GAPDH in the same sample. Values represent the results from three independent experiments with three to five mice in each experimental group. Statistical differences by Student's t test between groups are shown as follows: *, P was <0.05, and ***, P was <0.005.
On the other hand, mRNA expression of IL-10 from CD11c+ cells of T. gondii-infected IL-1Ra-deficient mice also increased in the spleen more than in the mLN (Fig. 2B). Expressions of proinflammatory cytokines, such as IL-1β and IL-6 from CD11c+ cells, also increased in T. gondii-infected IL-1Ra-deficient mice (Fig. 2C and D). Effects of T. gondii infection on the IL-10, IL-1β, and IL-6 mRNA expression of CD11c+ cells in IL-1Ra-deficient mice were similar to those observed in WT mice (see Fig. S1 in the supplemental material). Thus, as in WT mice, anti- or proinflammatory cytokines were produced from DC of regional LN and spleens of IL-1Ra-deficient mice at an early stage of T. gondii infection.
Effect of T. gondii infection on Th cell polarization in IL-1Ra-deficient mice.
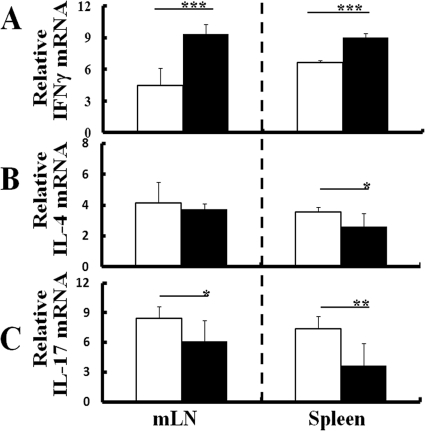
Since the development of arthritis in IL-1Ra-deficient mice was ameliorated by T. gondii infection and the spontaneous development of arthritis in IL-1Ra-deficient mice was proven to be IL-17 dependent (17, 26, 36), the effect of T. gondii infection on Th cell polarization in IL-1Ra-deficient mice was compared with that of WT mice. CD4+ T cells were isolated from the mLN or spleens of WT and IL-1Ra-deficient mice with or without T. gondii infection at 1 week p.i., and Th cell polarization was evaluated by measuring IFN-γ, IL-4, or IL-17 mRNA expression of CD4+ cells as representative Th1, Th2, and Th17 cytokines. CD4+ T cells from T. gondii-infected IL-1Ra-deficient mice expressed significantly higher levels of IFN-γ mRNA in both mLN and spleen than those from uninfected mice (Fig. 3A). Conversely, levels of IL-4 or IL-17 mRNA decreased in infected IL-1Ra-deficient mice (Fig. 3B and C). The effect of T. gondii infection on IFN-γ, IL-4, or IL-17 mRNA expression of CD4+ cells in IL-1Ra-deficient mice was similar to that observed in WT mice (see Fig. S2 in the supplemental material). Thus, T. gondii infection induced marked Th1 cell polarization not only in dLN but also in spleens in IL-1Ra-deficient mice as well as in WT mice at an early phase of infection, and Th1 cell polarization was accompanied by a reduction of Th2 and Th17 cell polarization.
Fig 3.
Effect of T. gondii infection on Th cell polarization in IL-1Ra-deficient mice. CD4+ cells were isolated from either the mLN or spleens of IL-1Ra-deficient mice with or without T. gondii infection at 1 week p.i., and levels of mRNA expression of IFN-γ (A), IL-4 (B), and IL-17 (C) were comparatively measured as described in Materials and Methods. The results from uninfected mice (white columns) or T. gondii-infected mice (black columns) are normalized with GAPDH in the same sample. Values represent results from three independent experiments with three to five mice per group. Statistical differences between groups are shown as follows: *, P was <0.05; **, P was <0.01; and ***, P was <0.005.
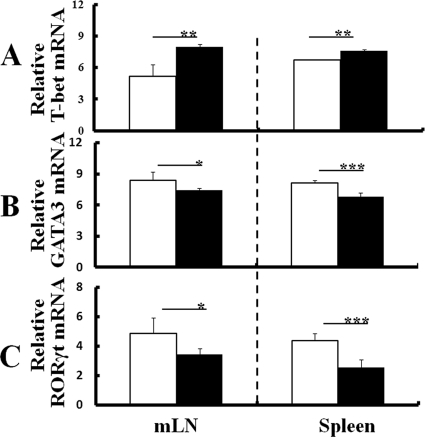
Regulation of transcription factors for Th cell polarization by T. gondii infection.
DC of T. gondii-infected WT and IL-1Ra-deficient mice produced not only IL-12 but also IL-10, IL-1β, and IL-6. Since IL-10 is known as a negative regulator of IFN-γ, it suppresses differentiation into Th1 cells. Also, IL-1β and IL-6 play a role that helps with differentiation into Th17 cells. Therefore, Th cell polarization was further evaluated by measuring mRNA expression of T-bet, GATA-3, and RORγt, the specific transcription factors of Th1, Th2, and Th17 cytokines, in CD4+ T cells isolated from the mLN or spleens of WT and IL-1Ra-deficient mice with or without T. gondii infection at 1 week p.i. CD4+ cells from T. gondii-infected IL-1Ra-deficient mice expressed significantly higher levels of T-bet mRNA in both the mLN and spleen than those from uninfected mice (Fig. 4A). In contrast, levels of GATA-3 or RORγt mRNA expression of CD4+ cells decreased in infected mice compared to those in uninfected mice (Fig. 4B and C). The effect of T. gondii infection on T-bet, GATA-3, or RORγt mRNA expression of CD4+ cells in IL-1Ra-deficient mice was similar to that observed in WT mice (see Fig. S3 in the supplemental material). Thus, although levels of IL-10 mRNA expression of DC increased in IL-1Ra-deficient mice at 1 week p.i., Th1 cell polarization of CD4+ T cells but not Th2 or Th17 cell polarization was confirmed at the transcription factor level in infected mice (Fig. 4A to C). Likewise, although levels of IL-1β and IL-6 mRNA expression of DC increased in IL-1Ra-deficient mice at 1 week p.i., mRNA expression of RORγt did not increase (Fig. 4C), indicating that proinflammatory cytokine production from DC had no effect on the Th cell polarization of infected mice.
Fig 4.
Regulation of transcription factors for Th cell polarization by T. gondii infection. CD4+ cells were isolated from either the mLN or spleens of IL-1Ra-deficient mice with or without T. gondii infection at 1 week p.i., and levels of mRNA expression of T-bet (A), GATA-3 (B), and RORγt (C) were comparatively measured as described in Materials and Methods. The results from uninfected mice (white columns) or T. gondii-infected ice (black columns) are normalized with GAPDH mRNA in the same sample. Values represent results from three independent experiments with three to five mice per group. Statistical differences between groups are shown as follows: *, P was <0.05; **, P was <0.01; and ***, P was <0.005.
Relation of Th cell polarization and the severity of arthritis.
In order to clarify the relation of Th cell polarization and the severity of arthritis, Th cell polarization of CD4+ T cells from IL-1Ra-deficient mice with or without T. gondii infection was analyzed by intracellular cytokine staining. In T. gondii-infected mice that did not develop arthritis until 8 weeks p.i., numbers and mean fluorescent intensities (MFI) of IFN-γ-positive splenic CD4+ cells markedly increased compared to those in uninfected mice with arthritis of the same age (Fig. 5A). On the other hand, numbers of IL-4- and IL-17-positive CD4+ cells from the spleens of infected mice decreased compared to those of uninfected mice (Fig. 5B and C). Differences were not obvious between uninfected and infected mice that developed arthritis (data not shown). The effect of T. gondii infection on CD4+ cells in LN (data not shown) was similar to that observed in spleens (Fig. 5).
Fig 5.
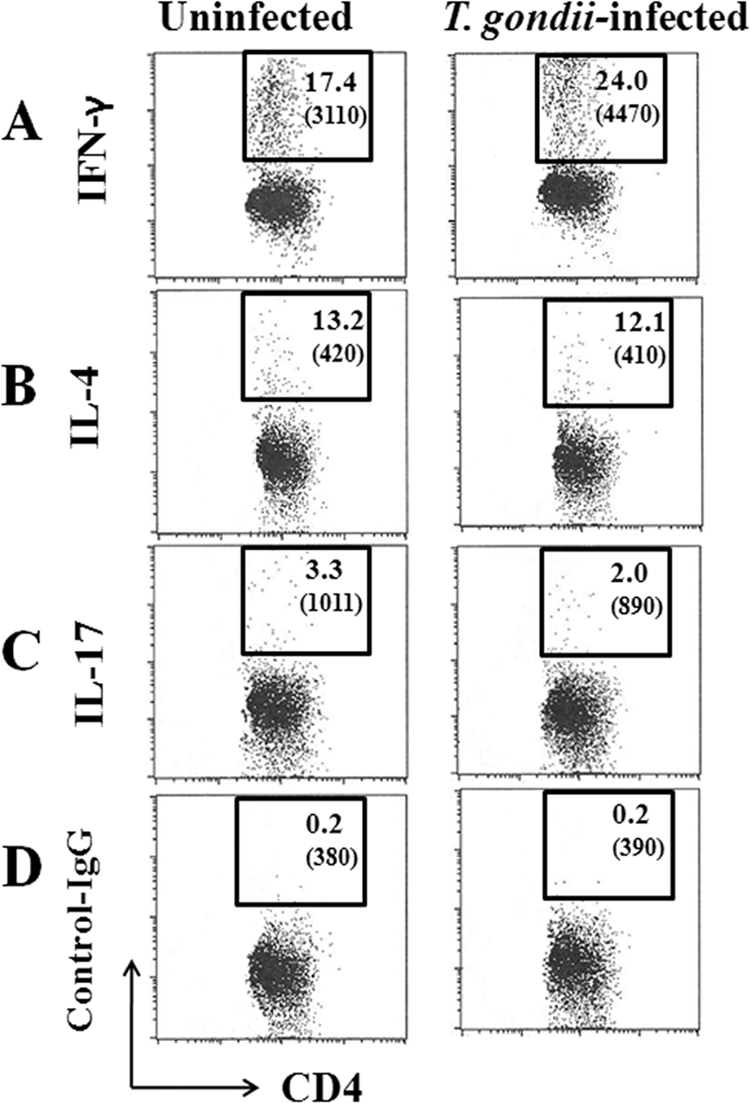
Flow cytometric analyses at the chronic phase of infection. Spleen cells of T. gondii-infected IL-1Ra-deficient mice without the development of arthritis at 8 weeks p.i. were stained with those of age-matched uninfected mice for intracellular IFN-γ (A), IL-4 (B), or IL-17 (C) as described in Materials and Methods. (D) An isotype control was used for staining. Numbers shown in each square are the percentages of cells contained in gated CD4+ cells. The mean fluorescent intensity (MFI) is given in parentheses. Experiments were repeated three times with similar results.
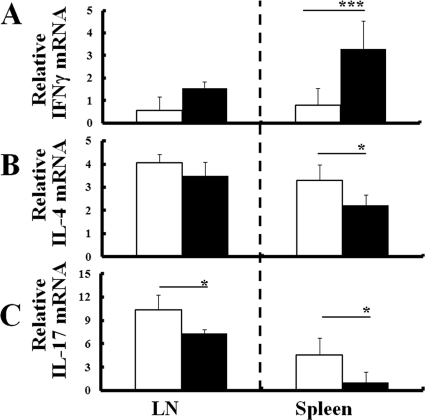
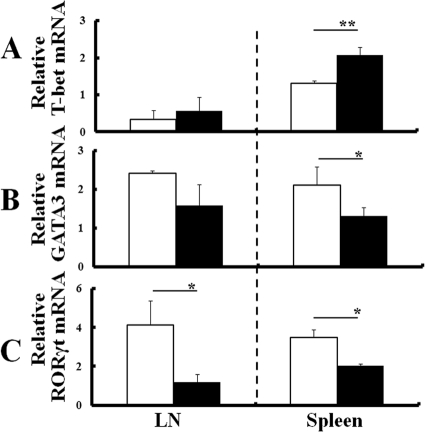
To confirm the relationship of Th cell polarization and arthritis, mRNA expressions of cytokines and transcription factors specific for Th1, Th2, and Th17 cells were measured in CD4+ cells that were isolated from LN or spleens of either uninfected mice with arthritis or infected mice without arthritis at 8 week p.i. CD4+ T cells from the spleens of infected IL-1Ra-deficient mice expressed significantly higher levels of IFN-γ mRNA than those of uninfected mice (Fig. 6A). Conversely, levels of IL-4 or IL-17 mRNA decreased in infected IL-1Ra-deficient mice (Fig. 6B and C). Also, CD4+ cells from infected mice expressed higher levels of T-bet mRNA than those from uninfected mice (Fig. 7A). In contrast, levels of GATA-3 or RORγt mRNA expression decreased in infected mice compared to those in uninfected mice (Fig. 7B and C). Thus, infection-induced Th1 cell polarization with reductions of Th2 and Th17 cell levels was shown in the asymptomatic mice at 8 weeks p.i.
Fig 6.
Th cell polarization in the mice without arthritis at 8 weeks p.i. CD4+ cells were isolated from either LN or spleens of IL-1Ra-deficient mice without the development of arthritis at 8 weeks p.i., and levels of mRNA expression of IFN-γ (A), IL-4 (B), and IL-17 (C) were comparatively measured with those of uninfected mice as described in Materials and Methods. The results from uninfected (white columns) or T. gondii-infected (black columns) mice are normalized with GAPDH mRNA in the same sample. Values represent results from three independent experiments with three mice per group. Statistical differences between groups are shown as follows: *, P was <0.05; and ***, P was <0.005.
Fig 7.
Transcription factors for Th cell polarization in the mice without arthritis at 8 weeks p.i. CD4+ cells were isolated from either LN or spleens of IL-1Ra-deficient mice without the development of arthritis at 8 weeks p.i., and levels of mRNA expression of T-bet (A), GATA-3 (B), and RORγt (C) were comparatively measured with those of uninfected mice as described in Materials and Methods. The results from uninfected mice (white columns) or T. gondii-infected mice (black columns) are normalized with GAPDH mRNA in the same sample. Values represent results from three independent experiments with three mice per group. Statistical differences between groups are shown as follows: *, P was <0.05; and **, P was <0.01.
The Th1 dominancy shown in T. gondii-infected IL-1Ra-deficient mice at 8 weeks p.i. persisted for more than 20 weeks p.i. in mice as long as clinical arthritis did not appear (data not shown). Thus, it has been confirmed that the amelioration of the spontaneous development of arthritis in IL-1Ra-deficient mice by T. gondii infection corresponds to the induction of Th1 cell polarization that accompanies the reduction of Th17 cell polarization.
DISCUSSION
In the present study, we have demonstrated that T. gondii infection ameliorated remarkably the spontaneous development of arthritis in IL-1Ra-deficient BALB/c mice. The early signs of arthritis could be detected at the ankle joints of the hind limbs as early as 4 to 5 weeks of age, and the incidence of arthritis gradually increased to more than 80% at 8 weeks, with almost all IL-1Ra-deficient BALB/c mice affected by 13 weeks of age, whereas the incidence of arthritis was around 30% even at 48 weeks of age in IL-1Ra-deficient C57BL/6 mice (19). Since BALB/c mice are a strain resistant to T. gondii infection and do not die from infection, we took advantage of using IL-1Ra-deficient mice with the BALB/c background and have revealed that T. gondii infection is capable of ameliorating the spontaneous development of arthritis in IL-1Ra-deficient mice.
Among autoimmune diseases, rheumatoid arthritis has been known to be a T cell-driven autoimmune disease (4, 18, 28), and T. gondii infection has effects on Th cell polarization of hosts. Therefore, we examined the effects of T. gondii infection on the activation of DC, since DC are professional APC crucial for initiating T cell-mediated adaptive immunity (3). DC function as directors of Th cell polarization in the dLN for the subsequent adaptive response, and IL-12 selectively promotes the differentiation of Th0 into Th1 cells. The IL-12 production from CD11c+ cells of both WT and IL-1Ra-deficient mice markedly increased in the mLN at 1 week p.i. after p.o. infection, indicating the in vivo activation of DC, which trigger Th1 cell polarization in the dLN at an early stage of T. gondii infection. As we have measured IL-12p40 mRNA expression of CD11c+ cells, one may argue that IL-12 and IL-23 share a common p40 subunit (20). However, increased IL-12p40 mRNA expression measured in CD11c+ cells of the mLN from T. gondii-infected WT and IL-1Ra-deficient mice was confirmed to be IL-12 production but not IL-23 production because of the subsequent IFN-γ production and the lack of IL-23-promoted IL-17 production from CD4+ T cells. Also, levels of surface expression of CD86 and MHC class II molecules on CD11c+ cells in the mLN of T. gondii-infected IL-1Ra-deficient mice were upregulated, confirming DC maturation in the dLN of infected mice.
Additionally, production of pro- and anti-inflammatory cytokines, such as IL-1β, IL-6, and IL-10, from DC rose in T. gondii-infected WT and IL-1Ra-deficient mice. Especially, the production of IL-10 mRNA from DC increased significantly in mLN and spleen, indicating that IL-10 production from DC of T. gondii-infected mice may downregulate the spontaneous development of arthritis that is known to be IL-17 dependent. IL-10, first described as a cytokine that inhibits IFN-γ expression in Th1 cells, plays a role in preventing exaggerated inflammatory and immune responses and protects the host from immune-mediated damage as an anti-inflammatory cytokine (39, 40). In fact, consistently with our report, Gu et al. reported that IL-10 negatively regulates the expression of IL-17 (14), and Heo et al. further revealed that IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients (16). On the other hand, augmentation of expression of proinflammatory cytokines, such as IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α), was reported in arthritic joints of IL-1Ra-deficient mice compared with their expression in WT mice (19). Although proinflammatory cytokine production from DC increased in IL-1Ra-deficient mice at 1 week p.i. in this study, it is considered that the increase of IL-1β and IL-6 reflects acute inflammation caused by T. gondii infection but not regional inflammation in the joints. In addition, though IL-1β might induce the expression and secretion of IL-23 (8), the level of IL-1β mRNA from DC was not statistically different between infected IL-1Ra-deficient and WT mice.
By measuring mRNA expression of representative cytokines (IFN-γ, IL-4, or IL-17) and transcription factors (T-bet, GATA-3, and RORγt) specific for Th1, Th2, and Th17 cells, Th cell polarization was comparatively evaluated in WT and IL-1Ra-deficient mice at 1 week p.i. (i.e., when mice were 3.5 weeks old). T. gondii infection was shown to induce marked Th1 cell polarization not only in the dLN but also in the spleen in IL-1Ra-deficient mice as well as in WT mice. Th2 or Th17 cell polarization was reduced. Since the existence of IL-6 plus transforming growth factor β (TGF-β) together with IL-1β, TNF-α, and IL-23 is known to induce Th cell differentiation into Th17 cells (4, 28), the increase of IL-1β and IL-6 mRNA production from DC in both WT and IL-1Ra-deficient mice after T. gondii infection might trigger Th17 cell differentiation. However, mRNA expression of not only IL-17 but also RORγt decreased in this study, indicating the downregulation to Th17 cell differentiation at the transcription level. The increase of proinflammatory cytokines after infection is clearly an acute-phase response to infection and has no effect on Th cell polarization of the host, i.e., the protective immune responses of infected mice.
In this study, we noticed that IL-17 production was reduced in CD4+ T cells from the mLN and spleens of WT and IL-1Ra-deficient BALB/c mice after T. gondii infection, whereas Guiton et al. reported that IL-17 expression was increased in the ilea of WT C57BL/6 mice and that IL-17 contributed to their ileitis and other inflammatory responses after T. gondii infection (15). It is possible that the IL-17 responses against T. gondii infection are different between C57BL/6 and BALB/c mice because massive necrosis was observed in the ilea of C57BL/6 mice but not in those of BALB/c mice. This had already been reported in acute toxoplasmosis (29). Since it was reported that IL-10 is required for the prevention of necrosis in the small intestine (39), increased IL-10 production and decreased IL-17 expression may explain why T. gondii infection ameliorates inflammation in IL-1Ra-deficient BALB/c mice.
Since the spontaneous development of arthritis in IL-1Ra-deficient mice is IL-17 dependent (17, 26, 36), we conclude that Th1 cell polarization with reduced Th17 cell polarization by T. gondii infection downregulates the severity of arthritis in IL-1Ra-deficient mice. T. gondii infection contributes to the delay of the onset of symptoms, indicating that the infection affects the early stage of arthritis only and that T. gondii infection would not be effective against established arthritis. With regard to this, it was reported that IL-17 is involved in the recruitment of neutrophils to the site of inflammation (27), activation of T cells (35), stimulation of antibody production (34, 41), and enhancement of osteoclastogenesis (37).
We have developed a DNA vaccine encoding a virulent HSP70 molecule specific for tachyzoites (i.e., the pathogenic stage of T. gondii at the acute phase or at acute exacerbation of the chronic phase of infection) targeting epidermal and dermal DC against T. gondii infection (25, 31, 32). The T. gondii HSP70 gene vaccine is proven to induce in vivo DC activation and successive Th1 cell polarization in the dLN, and the vaccine effects persist to the chronic phase of toxoplasmosis (31). DNA vaccination with a gene gun activates an antigen-specific CD4+ T cell response with a coding antigen gene (12). Thus, T. gondii HSP70 gene vaccination with a gene gun will be applied to arthritis in IL-1Ra-deficient mice in a future study.
We herein report a positive effect of T. gondii infection on Th17 cell-mediated spontaneous development of arthritis in IL-1Ra-deficient mice, which may give insight into the development of protozoan-derived prophylactic medicine for T cell-mediated autoimmune diseases. Th1 dominancy with reduced Th17 responses still persisted at the chronic phase of infection in asymptomatic T. gondii-infected mice. This study warrants further association studies between chronically infected human individuals and autoimmune diseases like rheumatoid arthritis.
Supplementary Material
ACKNOWLEDGMENTS
We express our appreciation to the late Akihiko Yano for his inspired direction and supervision.
This work was supported in part by grant-in-aid 21590462 from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://iai.asm.org.
REFERENCES
- 1. Aliberti J, Jankovic D, Sher A. 2004. Turning it on and off: regulation of dendritic cell function in Toxoplasma gondii infection. Immunol. Rev. 201:26–34 [DOI] [PubMed] [Google Scholar]
- 2. Aosai F, et al. 2006. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4. Cell Stress Chaperones 11:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 4. Bettelli E, Oukka M, Kuchroo VK. 2007. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350 [DOI] [PubMed] [Google Scholar]
- 5. Chen M, et al. 2000. AntiHSP70 autoantibody formation by B-1 cells in Toxoplasma gondii-infected mice. Infect. Immun. 68:4893–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen M, et al. 2004. Toxoplasma gondii infection inhibits the development of lupus-like syndrome in autoimmune (New Zealand Black × New Zealand White) F1 mice. Int. Immunol. 16:937–946 [DOI] [PubMed] [Google Scholar]
- 7. Chen M, Aosai F, Norose K, Mun HS, Yano A. 2003. The role of anti-HSP70 autoantibody-forming VH1-J.H1 B-1 cells in Toxoplasma gondii-infected mice. Int. Immunol. 15:39–47 [DOI] [PubMed] [Google Scholar]
- 8. Cho ML, et al. 2006. STAT3 and NF-κB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 176:5652–5661 [DOI] [PubMed] [Google Scholar]
- 9. Colotta F, Ghezzi P, Mantovani A. 1998. Interleukin 1, p 1–18 In Mire-Sluis A, Thore R. (ed), Cytokines. Academic Press, London, United Kingdom [Google Scholar]
- 10. Fang H, et al. 2006. Anaphylactic reaction induced by Toxoplasma gondii-derived heat shock protein 70. Int. Immunol. 18:1487–1497 [DOI] [PubMed] [Google Scholar]
- 11. Fang H, et al. 2008. Toxoplasma gondii-derived heat shock protein 70 induces lethal anaphylactic reaction through activation of cytosolic phospholipase A2 and platelet-activating factor via Toll-like receptor 4/myeloid differentiation factor 88. Microbiol. Immunol. 52:366–374 [DOI] [PubMed] [Google Scholar]
- 12. Garg S, et al. 2003. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat. Immunol. 4:907–912 [DOI] [PubMed] [Google Scholar]
- 13. Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. 1991. Synergistic role of CD4+ and CD8+T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286–292 [PubMed] [Google Scholar]
- 14. Gu Y, et al. 2008. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 38:1807–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guiton R, et al. 2010. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J. Infect. Dis. 202:427–435 [DOI] [PubMed] [Google Scholar]
- 16. Heo YJ, et al. 2010. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol. Lett. 127:150–156 [DOI] [PubMed] [Google Scholar]
- 17. Hirota K, et al. 2007. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horai R, et al. 2004. TNF-alpha is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J. Clin. Invest. 114:1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horai R, et al. 2000. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 191:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter CA. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5:521–531 [DOI] [PubMed] [Google Scholar]
- 21. Hunter CA, Subauste CS, Van Cleave VH, Remington JS. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwakura Y. 2002. Roles of IL-1 in the development of rheumatoid arthritis: consideration from mouse models. Cytokine Growth Factor Rev. 13:341–355 [DOI] [PubMed] [Google Scholar]
- 23. Johnson LL, Sayles PC. 1997. Interleukin-12, dendritic cells, and the initiation of host-protective mechanisms against Toxoplasma gondii. J. Exp. Med. 186:1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang HK, et al. 2004. Toxoplasma gondii-derived heat shock protein 70 stimulates the maturation of human monocyte-derived dendritic cells. Biochem. Biophys. Res. Commun. 322:899–904 [DOI] [PubMed] [Google Scholar]
- 25. Kikumura A, et al. 2010. Protective immunity against lethal anaphylactic reaction in Toxoplasma gondii-infected mice by DNA vaccination with T. gondii-derived heat shock protein 70 gene. Parasitol. Int. 59:105–111 [DOI] [PubMed] [Google Scholar]
- 26. Koenders MI, et al. 2008. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 58:3461–3470 [DOI] [PubMed] [Google Scholar]
- 27. Kolls JK, Linde A. 2004. Interleukin-17 family members and inflammation. Immunity 21:467–476 [DOI] [PubMed] [Google Scholar]
- 28. Laurence A, O'Shea JJ. 2007. TH-17 differentiation: of mice and men. Nat. Immunol. 8:903–905 [DOI] [PubMed] [Google Scholar]
- 29. Liesenfeld O, Kosek J, Remington JS, Suzuki Y. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo W, et al. 1997. Kinetics in parasite abundance in susceptible and resistant mice infected with an avirulent strain of Toxoplasma gondii by using quantitative competitive PCR. J. Parasitol. 83:1070–1074 [PubMed] [Google Scholar]
- 31. Makino M, et al. 2011. Innate immunity in DNA vaccine with Toxoplasma gondii-heat shock protein 70 gene that induces DC activation and Th1 polarization. Vaccine 29:1899–1905 [DOI] [PubMed] [Google Scholar]
- 32. Mohamed RM, et al. 2003. Induction of protective immunity by DNA vaccination with Toxoplasma gondii HSP70, HSP30 and SAG1 genes. Vaccine 21:2852–2861 [DOI] [PubMed] [Google Scholar]
- 33. Mun HS, et al. 2000. Toxoplasma gondii HSP70 as a danger signal in Toxoplasma gondii-infected mice. Cell Stress Chaperones 5:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakae S, et al. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375–387 [DOI] [PubMed] [Google Scholar]
- 35. Nakae S, Nambu A, Sudo K, Iwakura Y. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173–6177 [DOI] [PubMed] [Google Scholar]
- 36. Nakae S, et al. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. U. S. A. 100:5986–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato K, et al. 2006. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203:2673–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki Y, et al. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375–5382 [DOI] [PubMed] [Google Scholar]
- 40. Trinchieri G. 2007. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 204:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang XO, et al. 2008. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205:1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yano A, et al. 1989. Antigen presentation by Toxoplasma gondii-infected cells to CD4+ proliferative T cells and CD8+ cytotoxic cells. J. Parasitol. 75:411–416 [PubMed] [Google Scholar]
- 43. Yano A, et al. 2002. Roles of IFN-γ on stage conversion of an obligate intracellular protozoan parasite, Toxoplasma gondii. Int. Rev. Immunol. 21:405–421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.