Abstract
Intravascular hemolysis is a hallmark event in the immunopathology of malaria that results in increased systemic concentrations of free hemoglobin (Hb). The oxidation of Hb by free radicals causes the release of heme, which amplifies inflammation. To circumvent the detrimental effects of free heme, hosts have developed several homeostatic mechanisms, including the enzyme haptoglobin (Hp), which scavenges cell-free Hb, the monocyte receptor CD163, which binds to Hb-Hp complexes, and heme oxygenase-1 (HO-1), which degrades intracellular free heme. We tested the association between these three main components of the host response to hemolysis and susceptibility to malaria in a Brazilian population. The genetic profiles of the HMOX1 and Hp genes and the plasma levels of a serum inflammatory marker, the soluble form of the CD163 receptor (sCD163), were studied in 264 subjects, including 78 individuals with symptomatic malaria, 106 individuals with asymptomatic malaria, and 80 uninfected individuals. We found that long (GT)n repeats in the microsatellite polymorphism region of the HMOX1 gene, the Hp2 allele, and the Hp2.2 genotype were associated with symptomatic malaria. Moreover, increased plasma concentrations of heme, Hp, HO-1, and sCD163 were associated with susceptibility to malaria. The validation of these results could support the development of targeted therapies and aid in reducing the severity of malaria.
INTRODUCTION
Malaria infection has high morbidity and mortality rates worldwide. During the blood stage of malarial infection, hemoglobin (Hb) is released from red blood cells that have ruptured due to Plasmodium multiplication (27). This unique characteristic of the Plasmodium life cycle leads to increased concentrations of cell-free Hb in the circulation because of intravascular hemolysis and the possible release of the heme prosthetic group from hemoglobin (25). Free heme is highly harmful to cells and tissues, as it can induce oxidative stress, cytotoxicity and inflammation (25), and cell death (30). Patients with severe malaria may exhibit high circulating levels of free heme, which impairs regulatory responses and can cause inflammatory imbalances (1). Under homeostatic conditions, haptoglobin (Hp) can rapidly scavenge cell-free Hb by forming the stable Hb-Hp complex, which is recognized and internalized by the CD163 receptor expressed by monocytes and macrophages in the red pulp of the spleen. Once internalized, the heme is usually degraded by the antioxidant enzyme heme oxygenase-1 (HO-1) (39). A thorough understanding of the factors and pathways that control the accumulation of free heme and the determinants of the unfavorable events that are triggered by this molecule can drive the development of novel therapeutic approaches to treat malaria and other hemolytic diseases.
Haptoglobin is a tetrameric protein (α2β2) that is characterized by α-chain heterogeneity due to an intragenic duplication that resulted in two different alleles, Hp2 and Hp1 (including two subvariants, Hp1F and Hp1S). The diversity in the Hp phenotypes causes different binding affinities for cell-free Hb (Hp1.1 > Hp1.2 > Hp2.2) and CD163 (Hp2.2 > Hp1.2 > Hp1.1) (39). Additionally, polymorphisms in the Hp gene have been associated with different functional capabilities and organic responses, including alterations in immune regulation, oxidative stress, and iron delocalization within monocytes (8, 9, 31, 42–44, 54). Thus, it is necessary to consider the strategies used to study the mechanisms associated with heme regulation by HO-1, Hp, and the Hp receptor, CD163, and their contribution to the susceptibility to malaria.
The haptoglobin receptor CD163 is a member of a group of B cysteine-rich scavenger membrane receptors that is expressed on monocytes and macrophages and has been linked to inflammation. The soluble form of the CD163 receptor (sCD163) is a surrogate for its cellular expression, and sCD163 levels are elevated in many inflammatory processes (18, 29, 33, 45, 46, 50–52, 60, 66). Only one study has shown that sCD163 levels are more elevated in uncomplicated falciparum malaria than in severe malarial anemia and cerebral malaria, and all malaria patients have higher levels of sCD163 than uninfected individuals (41).
In experimental models of malaria, the induction of HO-1 is mostly associated with increased tolerance to Plasmodium infection (26, 53) as a result of the ability of HO-1 to control nonspecific tissue damage and immunopathology by reducing inflammation. However, a few studies have linked HMOX1 (Homo sapiens; P09601) gene polymorphisms to malaria susceptibility in humans (40, 59, 63). Notably, a (GT)n dinucleotide length polymorphism has been associated with varied expression of HO-1 (24). It has been suggested that there is a higher expression of HO-1 mRNA in patients with short (GT)n repeats in the HMOX1 gene than in patients with long (GT)n repeats (24). Although a recent study showed an association between short (GT)n dinucleotide length and cerebral malaria (63), other investigations have not found any correlation between (GT)n dinucleotide length and malaria susceptibility (40). Therefore, there is no definitive evidence that links the length of the (GT)n dinucleotide repeats in the HMOX1 gene with the severity of human malaria.
The main goal of this study was to investigate the Hp, HO-1, and sCD163 pathways that are involved in heme metabolism during malaria-induced intravascular hemolysis by analyzing genotypes and protein plasma levels in patients from the Brazilian Amazon. We tested whether different Hp genotypes and HMOX1 microsatellite polymorphisms are associated with susceptibility to malaria. We have demonstrated that individuals presenting with symptomatic malaria more often have the Hp2.2 genotype, which has previously been associated with a lower Hp binding affinity for cell-free Hb (39). In addition, we show that long (GT)n dinucleotide repeats in the HMOX1 gene are associated with decreased concentrations of plasma HO-1, elevated disease susceptibility, and increased plasma levels of Hp and sCD163 in malaria patients. Furthermore, we address the association between sCD163 and malaria symptomatology. Thus, our findings expand to humans the current concept from experimental models that the susceptibility to malaria is indeed closely linked to specific determinants involved in heme metabolism.
MATERIALS AND METHODS
Ethics.
This study is part of a project that was previously approved by the Ethical Committee of the São Lucas University, Rondônia, Brazil. All of the participants or their legal guardians provided informed consent before entering the study. The clinical investigations were conducted in accordance with the principles expressed in the 1975 Declaration of Helsinki, as revised in 2000.
Subjects.
This study was a retrospective analysis of 264 subjects from the localities of Demarcação (8°10′04.12″S, 62°46′52.33″W) and Buritis (10°12′43″S, 63°49′44″W) in Rondônia State in the Brazilian Amazon. The subjects were studied between 2006 and 2007. The study sample includes 78 subjects with symptomatic malaria, 106 subjects with asymptomatic malaria, and 80 uninfected individuals. These individuals have already been analyzed by our group in other studies (1, 3–7). Active and passive case detections were performed using both microscopy and nested PCR. The symptomatic individuals promptly received antimalarial treatment, and those with asymptomatic infection at the time of enrollment in the study were followed for up to 30 days and subsequently classified as suffering from either symptomatic or asymptomatic malaria.
Genetic experiments.
DNA was extracted from 200 μl of peripheral blood using a standard Qiagen DNA blood minikit (Valencia, CA) according to the manufacturer's protocol. The Hp genotypes were determined by allele-specific PCR as described by Yano et al. (70). The identification of the Hp1F, Hp1S, and Hp2 alleles was based on the analysis of products from three independent PCRs. The PCR products were analyzed by electrophoresing them on 1% agarose gels under nondenaturing conditions. The products were then detected by staining with ethidium bromide and visualized under a UV light.
The 5′-flanking region of the HMOX1 gene, which contains (GT)n repeats, was amplified with the forward primer 5′-AGAGCCTGCAGCTTCTCAGA-3′ and the reverse primer 5′-ACAAAGTCTGGCCATAGGAC-3′ according to the published procedure (37). The PCR products were sequenced on an ABI Prism 3100 automated DNA sequencer using a BigDye 03 Terminator sequencing standards kit (Applied Biosystems, Foster City, CA). The size of each HMOX1 gene (GT)n repeat was calculated using GeneScan Analysis software (PE Applied Biosystems). The number of HMOX1 (GT)n repeats in the DNA strands was determined, and the frequency of repeats in patients was plotted. Assuming a codominant (additive) trait model, the HMOX1 genotypes were defined by the average length of (GT)n repeats. The average length of the HMOX1 gene promoter (GT)n was calculated for each patient.
Plasma measurements.
The plasma levels of interleukin-6 (IL-6), IL-10, and tumor necrosis factor alpha (TNFα) were measured using a cytometric bead array system (BD Biosciences Pharmingen, Franklin Lakes, NJ) according to the manufacturer's protocol. All of the samples were run in a single assay in the main laboratory at the Centro de Pesquisas Gonçalo Moniz, Bahia, Brazil. The plasma levels of HO-1 (Assay Designs, Ann Arbor, MI), Hp (GenWay Biotech, San Diego, CA), and sCD163 (BD Pharmingen, Franklin Lakes, NJ) were measured by enzyme-linked immunosorbent assay. Total heme levels were measured using a chromogenic assay according to the manufacturer's instructions (BioAssay Systems, Hayward, CA). The 413-nm and 576-nm UV-visible spectra for the plasma samples were taken with a Nanodrop apparatus to discriminate total heme from non-hemoglobin-bound heme (free heme), as previously described (68). The plasma measurements of aspartate aminotransferase (AST), alanine aminotransaminase (ALT), total bilirubin, hemoglobin, creatinine, fibrinogen, and C-reactive protein (CRP) were performed at the Federal University of Bahia and Faculdade São Lucas, Brazil.
Score-based laboratory assessment of clinical severity of malaria.
To infer the degree of systemic inflammation and liver damage during malaria, we used two previously published score systems (4): the hepatic inflammatory (HI) score and the hepatic inflammatory parasitic (HIP) score. To determine the scores, the AST, ALT, fibrinogen, CRP, total bilirubin, and parasitemia levels were estimated in 580 people. This sample consisted of 183 noninfected, 195 symptomatic, and 202 asymptomatic individuals from the same area of endemicity in which the current study was performed. Receiver operator characteristic (ROC) curves were calculated for each parameter with the aim of identifying the best cutoff values to use to differentiate between those individuals with symptomatic and those with asymptomatic malaria with the highest sensitivity and specificity and the highest likelihood ratio (4). One point was given for each parameter that was above the established cutoff, with scores ranging from 0 to 6 and from 0 to 5, including or excluding parasitemia, for HIP and HI, respectively. The individuals presenting with higher scores also referred to severe headaches, fatigue and asthenia, hypotension, and hyperthermia more frequently than those with lower scores (4), implying that higher score values are generally associated with an increased clinical severity of malaria.
Statistical analyses.
A chi-squared test was applied to evaluate the association between the following qualitative variables within the patient malaria groups (symptomatic, asymptomatic, and noninfected): Hp genotypes/alleles and short and long (<30 and ≥30 GT repeats, respectively) HMOX1 gene polymorphisms. The plasma levels of Hp, heme, and sCD163 and the HMOX1 gene (GT)n repetitions were compared between groups using a nonparametric Kruskal-Wallis test with Dunn's multiple comparisons. These tests were also used to analyze the difference between Hp genotypes/alleles with Hp levels and the association between sCD163, HMOX1 GT repeat, HO-1, and Hp with the HI/HIP scores. A univariate linear regression analysis was performed to assess the associations between Hp alleles/genotypes or HMOX1 gene polymorphisms and symptomatic malaria. The Mann-Whitney test was used to compare differences in plasma Hp levels, plasma sCD163 levels, and HI/HIP scores between individuals with short or long HMOX1 gene (GT)n repeats. The correlations between Hp levels with parasitemia, ALT, and heme levels were analyzed by Spearman's correlation test. This test was also used to estimate the significance of the correlation between HMOX1 (GT)n repetitions and sCD163, Hp, C-reactive protein, and creatinine levels and HI and HIP scores. ROC curves were used to evaluate the power of sCD163 to discriminate the individuals with symptomatic malaria from those not infected with Plasmodium or those with asymptomatic malaria. Within all comparisons, the differences in which P was <0.05 were considered statistically significant. The statistical analyses were performed using GraphPad Prism (version 5.0b) software (GraphPad Software, San Diego, CA).
RESULTS
Baseline characteristics.
The majority of the individuals studied were female (53.40%) adults (age, 39.95 years; standard deviation [SD], ±14.99) who had lived in the area of endemicity for more than 6 months (73.86% more than 3 years and 57.58% more than 10 years). The patients infected with the malaria parasite (n = 184) were approximately the same age (39.68 years; SD, ±14.83) and included more females (53.26%) and individuals who had lived in the area of endemicity for long periods of time (72.83% more than 3 years and 59.78% more than 10 years). The majority of the individuals infected with a Plasmodium sp. were asymptomatic at the time of the study and during the 30 days of follow-up (n = 106). The individuals with asymptomatic malaria were also older and had lived in the area of endemicity for longer periods than those with symptomatic infections (Table 1). Most of the patients with symptomatic malaria presented with uncomplicated disease, with only 5 cases of severe malaria present. Plasmodium vivax was the main malarial agent (93.40% of symptomatic malaria cases and 91.03% of asymptomatic cases), and Plasmodium falciparum was detected in the rest of the malaria cases.
Table 1.
Baseline characteristics of the individuals enrolled in the study
| Characteristic | Result by subject group |
P value | ||
|---|---|---|---|---|
| Symptomatic malaria (n = 78) | Asymptomatic malaria (n = 106) | Noninfected (n = 80) | ||
| No. (%) male | 39 (50.00) | 47 (44.34) | 37 (46.25) | 0.7468a |
| Median (IQRb) age (yr) | 36 (27.75–50) | 42 (32–49) | 35 (25.25–45) | 0.0307c |
| Median (IQR) no. of previous malaria infection episodes | 6.5 (1–13) | 15 (12–19) | 12.5 (6.25–17) | <0.0001c |
| No. (%) of individuals residing in the area for the following no. of yr: | 0.0011a | |||
| ≤2 | 26 (33.33) | 24 (22.64) | 19 (23.75) | |
| 3 to 10 | 17 (21.80) | 7 (6.60) | 19 (23.75) | |
| >10 | 35 (44.87) | 75 (70.76) | 42 (52.50) | |
Categorized variables were compared using a chi-square test.
IQR, interquartile range.
Ordinal variables were compared using the Kruskal-Wallis test with Dunn's multiple-comparison test.
Haptoglobin genotype.
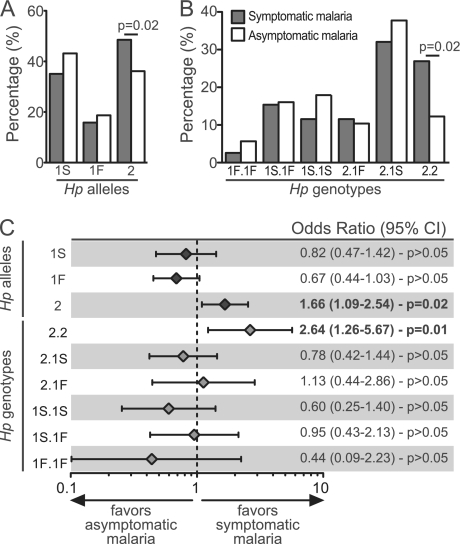
The Hp2.1S genotype was the most frequently detected genotype in all of the different study groups, representing 41.25% (n = 33) of the noninfected individuals, 32.05% (n = 25) of the asymptomatic malaria cases, and 37.74% (n = 40) of those with symptomatic infections (chi-square P = 0.035). The Hp1S allele was observed more frequently in asymptomatic cases and corresponded to 44.81% (n = 95) of the Hp alleles seen in this group. The Hp2 allele was the most commonly observed allele in both the noninfected and symptomatic malaria cases, representing 45.00% (n = 72) and 48.72% (n = 76) of the alleles in each group, respectively (Fig. 1A and B). Remarkably, the Hp2 allele was associated with a higher risk of developing symptomatic malaria than asymptomatic malaria upon Plasmodium infection (odds ratio [OR] = 1.666, P = 0.0228; Fig. 1C). That is, individuals who carry the Hp2 allele have a greater chance of developing symptoms once they are infected by Plasmodium. In addition, the Plasmodium-infected individuals with the homozygous Hp2.2 genotype had an even greater chance of developing malaria symptoms (OR = 2.636, P = 0.0193; Fig. 1C). Thus, these findings show that the Hp2 allele and the Hp2.2 genotype are strongly associated with an increased susceptibility to development of malaria-related symptoms upon Plasmodium infection.
Fig 1.
Haptoglobin genetic profiles influence malaria susceptibility. We studied 80 uninfected healthy subjects, 106 subjects with asymptomatic malaria, and 78 subjects with symptomatic Plasmodium infection. All of the study subjects were from the Brazilian Amazon. (A) Percentage of individuals with symptomatic (gray bars) or asymptomatic (white bars) malaria carrying the different haptoglobin (Hp) alleles, with both homozygous and heterozygous individuals considered for each allele. (B) Percentage of individuals with symptomatic (gray bars) or asymptomatic (white bars) malaria carrying the different Hp genotypes. The differences between the groups illustrated in panels A and B were compared using Fisher's exact test. (C) Univariate linear regression analyses of the different Hp alleles and genotypes were performed to estimate malaria susceptibility. The odds ratios, respective 95% confidence intervals (95% CIs), and P values are shown in each panel.
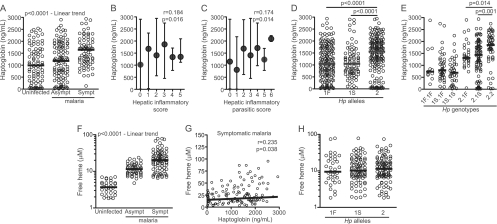
We then tested whether the different Hp genotypes are indeed associated with different systemic concentrations of Hp. Notably, increased plasma levels of Hp were directly associated with the clinical presentation of malaria, with symptomatic individuals exhibiting higher Hp levels than individuals with asymptomatic infection or noninfected individuals (Fig. 2A). By using a previously reported, laboratory-based score to standardize a reproducible evaluation of the severity of P. vivax infection (4), we found that the plasma haptoglobin values were indeed positively correlated with the hepatic inflammatory HI and HIP scores (r = 0.184 and P = 0.016 for HI and r = 0.174 and P = 0.014 for HIP; Fig. 2B and C). Thus, the amount of Hp in the circulation increases during symptomatic infection, and individuals with inflammation-associated liver damage present higher levels of Hp. Notably, those individuals who were homozygous or heterozygous for the Hp2 allele displayed augmented plasma concentrations of Hp compared with carriers of the Hp1F and Hp1S alleles (Fig. 2D). Moreover, of all the Hp2-containing genotypes, individuals with the Hp2.2 genotype exhibited the highest systemic Hp levels (Fig. 2E). Interestingly, the Hp2.2 genotype has been linked to a reduced Hp binding affinity for cell-free Hb (39). Indeed, in the present study, the individuals with symptomatic malaria were more likely to have the Hp2.2 genotype and higher concentrations of free heme in the plasma than those with asymptomatic malaria or uninfected individuals (Fig. 2F). The plasma Hp levels were also positively correlated with the amount of free heme in the plasma (r = 0.2350, P = 0.038; Fig. 2G). These results suggest that individuals with the Hp2 allele or the Hp2.2 genotype produce more Hp protein, probably to compensate for the lower affinity that this Hp has for free Hb in the circulation. Consequently, individuals carrying the Hp2 allele need to produce more Hp protein to have the same amount of free heme as individuals with the other Hp alleles (Fig. 2H). Thus, the Hb binding affinity of Hp seems to be more important than their Hp levels in determining an individual's susceptibility to symptomatic malaria.
Fig 2.
Associations between the systemic levels of haptoglobin and total heme and malaria susceptibility. The plasma concentrations of Hp were measured in the subjects referred to in the legend to Fig. 1. Each symbol represents a single patient, and the lines represent medians. The systemic Hp levels in the different clinical outcome groups were compared (A) and correlated with the degree of hepatic damage and inflammation, as evaluated by the severity scores described in Materials and Methods (B and C). The Hp levels in individuals with different Hp alleles (D) or genotypes (E) were also compared. The systemic levels of free heme were compared in patients with different malaria outcomes (F), correlated with the amounts of Hp in the plasma (G), and then compared among individuals with various Hp alleles (H). The data were compared using the Mann-Whitney test (comparisons between two groups), the Kruskal-Wallis test with Dunn's multiple comparisons, or linear trend analysis (comparisons between more than two groups). In panels B, C, and G, the data were analyzed using Spearman's rank correlation test. P values are shown in each graph.
HMOX1 microsatellite polymorphism.
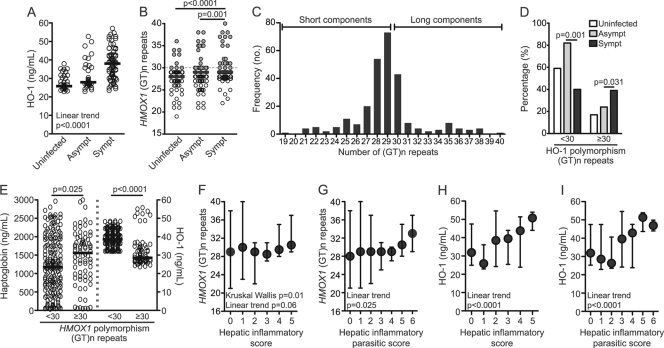
Free heme is a potent inducer of HO-1 (14), and we found that symptomatic patients who presented with high levels of circulating free heme also displayed increased systemic concentrations of HO-1 compared with noninfected individuals or those with asymptomatic infections (Fig. 3A). In addition, the patients with both symptomatic and asymptomatic malaria had longer (GT)n dinucleotide repeats than noninfected volunteers (29.90 ± 3.225, 28.74 ± 2.987, and 28.01 ± 2.868 repeats, respectively; P < 0.001; Fig. 3B). The classification of the HMOX1 polymorphism based on the number of GT repeats varies among studies and depends on the frequency peaks of the repeats found in each study sample (21, 37, 61). In our study, the number of (GT)n repeats ranged from 19 to 40, with three frequency peaks at 28, 29, and 30 repeats (Fig. 3C). The inducibility of the HO-1 gene promoter is known to be negatively correlated with the number of GT repeats (65), and we adapted previous classifications of the HMOX1 gene polymorphisms (69) to stratify the classification into two categories: a short form (<30 GT repeats) and a long form (≥30 GT repeats).
Fig 3.
HMOX1 gene polymorphisms influence susceptibility to malaria. (A) The plasma HO-1 concentrations in noninfected individuals and those with asymptomatic or symptomatic malaria were compared. (B) The numbers of GT repeats in the HMOX1 gene in noninfected individuals and those presenting with asymptomatic or symptomatic malaria were also compared. Gray dots represent individuals carrying ≥30 GT repeats in the HMOX1 gene. (C) Frequency of the different number of (GT)n repeats in the study population. (D) Percentage of noninfected individuals (white bars) and individuals with asymptomatic (gray bars) or symptomatic (black bars) malaria carrying the short or long (GT)n repeats in the HMOX1 gene. (E) Plasma levels of Hp (left) and HO-1 (right) in individuals with short or long (GT)n repeats in the HMOX1 gene (data were compared using a Mann-Whitney test). (F and G) The associations between the number of (GT)n repeats in the HMOX1 gene and the degree of liver damage or disease severity were estimated by the hepatic inflammatory and hepatic inflammatory parasitic scores. (H and I) Plasma HO-1 concentrations in relation to the malaria severity scores. In panels F to I, the symbols represent the median values and the whiskers represent maximum and minimum values. The differences between the groups illustrated in panel D were compared using a chi-square exact test and Fisher's exact test (only the P values from Fisher's exact test are shown). The data from the other panels were compared using the Kruskal-Wallis test with Dunn's multiple comparisons or linear trend analysis. P values are shown in each panel.
The individuals carrying a short form of the HMOX1 gene polymorphism were more likely to present with asymptomatic malaria, whereas those with longer repeats were mostly symptomatic (Fig. 3D). Thus, long HMOX1 GT repeats are associated with an increased susceptibility to develop symptoms upon Plasmodium infection. In agreement with a previous study (24), we found that plasma HO-1 levels were consistently lower in the patients who carry the long form of the HMOX1 gene polymorphism than the patients with the short GT form (Fig. 3E). In contrast, the patients with the long GT form displayed higher levels of Hp (Fig. 3E), arguing that those individuals who have the long form and low concentrations of HO-1 also have higher levels of Hp, possibly as a regulatory mechanism against hemolysis. Although the symptomatic patients presented with higher overall HO-1 levels than uninfected individuals or those with asymptomatic malaria, the patients with longer (GT)n repeats were more likely to have symptomatic malaria and relatively lower levels of HO-1 than the patients with long repeats (Fig. 3A and E). There was no association between the systemic concentrations of HO-1 and the Hp genotypes (data not shown).
The individuals with ≥30 GT repeats had greater susceptibility for developing symptomatic malaria than did the individuals with <30 repeats (OR = 3.35, confidence interval [CI] = 1.91 to 5.88, P = 0.0002). Intriguingly, we found an association between the severity scores and the number of GT repeats (Fig. 3F and G) and the HO-1 plasma values (Fig. 3H and I). Thus, the patients with the long form of the HMOX1 gene polymorphism displayed higher severity scores than did those carrying shorter HMOX1 GT forms. Despite carrying the long form of the HMOX1 polymorphism more frequently, the symptomatic malaria patients who presented with increased inflammatory damage had higher systemic concentrations of the HO-1 protein. This finding suggests that higher HO-1 levels are a counterregulatory response to inflammation, despite the fact that this genetic background is associated with a lower HMOX1 induction. Thus, these observations suggest that, like the Hp genotype, the long forms of the HMOX1 gene polymorphism can increase susceptibility to malaria, but with a different level of complexity.
Systemic levels of the Hp receptor sCD163.
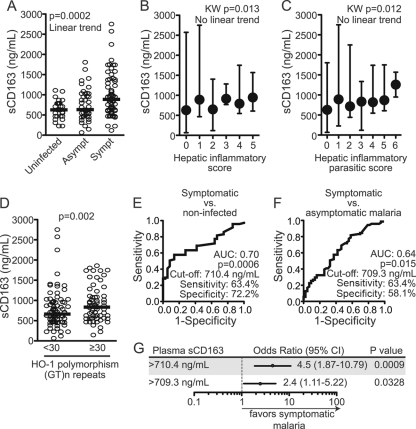
The binding of Hp to the CD163 receptor on the surface of monocytes or macrophages leads to the removal of the Hp complexes formed by cell-free Hb and haptoglobin (39). The soluble form of the CD163 receptor, sCD163, is a surrogate marker of systemic inflammation, and increased levels of this marker are correlated with a poor prognosis in a number of pathological conditions. In this study, the individuals with symptomatic malaria had higher systemic sCD163 levels than did those with symptomless infection or noninfected individuals (Fig. 4A). In addition, the sCD163 levels were positively correlated with plasma Hp levels (r = 0.2477, P = 0.0028), and there were no associations between sCD163 concentrations and the Hp genotypes of the patients (data not shown). The sCD163 levels were also correlated with the plasma concentrations of TNF (r = 0.2095, P = 0.0101), IL-6 (r = 0.1991, P = 0.0146), C-reactive protein (r = 0.2618, P = 0.0012), and creatinine (r = 0.2693, P = 0.0009). The laboratory scores that suggest malaria severity (HI and HIP) were positively correlated with sCD163 (HI, r = 0.1831 and P = 0.0249; HIP, r = 0.1726 and P = 0.0353; Fig. 4B and C). These findings indicate that sCD163 is indeed associated with systemic inflammation and malaria symptomatology.
Fig 4.
Soluble CD163 and susceptibility to malaria. (A) The systemic concentration of sCD163 was quantified in all subjects and correlated with different malaria outcomes. (B and C) The systemic levels of sCD163 were tested for associations with the hepatic inflammatory scores using Kruskal-Wallis tests with linear trend posttest analysis. (D) The plasma sCD163 levels in individuals with short and long (GT)n repeats in the HMOX1 gene were compared. The data were compared using a Mann-Whitney test. ROC curve analyses were performed to depict the power of sCD163 to discriminate individuals with symptomatic malaria from those not infected with Plasmodium (E) or from those with asymptomatic malaria (F). AUC, area under the curve. The cutoff values for sCD163 levels were established by C statistics and are shown in each graph. (G) A univariate logistic regression analysis was performed to test associations between sCD163 concentrations above the established cutoff values, and the sCD163 concentrations were used to determine the chance of developing malaria-related symptoms by comparison with the sCD163 concentrations for the uninfected individuals (first line; sCD163 cutoff, 710.4 ng/ml) or individuals with asymptomatic malaria (second line; sCD163 cutoff, 709.3 ng/ml). The odds ratios, respective 95% confidence intervals (95% CIs), and P values are shown in each panel.
Interestingly, the systemic sCD163 concentrations were positively correlated with the number of (GT)n dinucleotide repetitions in the HMOX1 gene polymorphisms (r = 0.1646, P = 0.0441), and individuals with ≥30 GT repeats had higher levels of sCD163 than did those with <30 GT repeats (Fig. 4D). Although we found a positive association between HMOX1 gene polymorphisms and systemic sCD163 levels, we could not detect a correlation between this soluble marker and the plasma concentrations of HO-1 or free heme levels (data not shown). This finding supports the idea that sCD163 plays a role in the onset of malaria symptoms, and this effect may not be directly associated with the genetic profiles of the Hp and HMOX1 genes. Indeed, plasma sCD163 levels could discriminate those patients with symptomatic malaria from noninfected individuals (Fig. 4E) and those with asymptomatic malaria (Fig. 4F). Univariate logistic regression analysis confirmed the association between high sCD163 levels and a susceptibility to development of malaria symptoms (Fig. 4G). Interestingly, the individuals who were carriers of the Hp2.2 and long HMOX1 (GT)n repeats and exhibited high systemic concentrations of sCD163 were more likely to have symptomatic malaria than the individuals without any of the three potential risk factors, e.g., Hp1.1 or Hp2.1 carriers with short HMOX1 (GT)n repeats and low systemic concentrations of sCD163 (chi-square P = 0.0055). These data indicate that a combined contribution of these three factors involved in the detoxification of free heme (Hp, sCD163, and HO-1) contributes to the determination of malaria susceptibility.
DISCUSSION
To our knowledge, the present study is the first to simultaneously assess genetic alterations and the plasma concentrations of different key components of the detoxification of free heme in malaria patients. In addition, we are the first to report an association between the Hp and HMOX1 genes and sCD163 levels and susceptibility to disease in the context of Plasmodium vivax infection. From a clinical standpoint, the infections caused by P. vivax and P. falciparum are unequal and happen as the result of different host immune and inflammatory responses. The malaria caused by P. falciparum is more frequently associated with acute life-threatening complications, whereas vivax malaria is usually mild and nonlethal. These differences may be associated with the differential induction of the host's defense mechanisms for circumventing the deleterious effects of heme. Our study does not address these differences, and additional epidemiological and mechanistic studies are necessary to answer this question. Our results demonstrate that individuals with the Hp2.2 phenotype have a higher risk of developing symptomatic (as opposed to asymptomatic) malaria upon Plasmodium infection. The presence of the Hp2.2 genotype has been associated with an increase in redox-active iron and oxidative stress compared with the presence of the Hp1.1 genotype (9, 47). Moreover, the Hb-Hp2.2 complex, but not other Hp1 complexes, can be internalized by monocytes and stimulate the release of proinflammatory cytokines (57). Indeed, the Hp2.2 phenotype has been associated with susceptibility to several inflammatory conditions, including malaria (11, 20, 22, 28, 36, 55, 58). Haptoglobin is considered an acute-phase protein that increases 2- to 4-fold during the response to acute inflammation (35). We observed that heme and Hp levels were higher in those individuals with symptomatic malaria than those with symptomless infection or those not infected with Plasmodium. Interestingly, the subjects with the Hp2.2 genotype presented with augmented systemic concentrations of Hp compared with those carrying the Hp1 allele. Although acute and severe hemolysis will always lead to a reduction in Hp clearance, as seen in severe malaria (32), the response to chronic or low-level hemolysis, which is commonly seen in mild vivax malaria, is difficult to predict. Evidence suggests that, unlike the Hb–Hp1-1 complex, the Hb-Hp2.2 complex can stimulate the release of IL-10 and IL-6 (31) and that IL-6 expression increases the synthesis of Hp. It is also possible that the higher Hp production in individuals with the Hp2 allele acts as a compensatory mechanism for the lower affinity of this Hp for cell-free Hb (39).
We describe herein that subjects with the long form (≥30 GT repeats) of the HMOX1 gene polymorphism have greater susceptibility to developing symptomatic malaria than individuals with the short form (<30 repeats), suggesting that the HMOX1 gene polymorphism is involved in susceptibility to Plasmodium infection. The individuals who carried longer (GT)n dinucleotide repeats and had symptomatic infections also had higher HI and HIP inflammatory scores, suggesting an association between the HMOX1 gene and the control of inflammation. Sambo et al. (59) found that shorter GT repeats in the HMOX1 gene are associated with patients with cerebral malaria as opposed to patients with uncomplicated malaria or a noninfected control group. However, this difference was not seen when comparing the group of cerebral malaria patients with patients exhibiting other severe forms of malaria. The malaria patients in this study were mostly infected by P. vivax and more frequently exhibited the noncomplicated forms of the disease. Furthermore, another study also reported an association between short (GT)n dinucleotide repetitions in the HMOX1 gene and human cerebral malaria caused by P. falciparum, suggesting that higher expression of HO-1 is detrimental for malaria (63). Nevertheless, increased concentrations of HO-1 have been strongly associated with protection against malaria in mice. This protection mainly occurs through the production of carbon monoxide (CO) gas, which binds to cell-free Hb with very high affinity. This interaction results in the formation of carboxyhemoglobin, which prevents heme release and an increase in intravascular free heme (26, 53).
In general, we found that the individuals with symptomatic malaria had higher plasma HO-1 concentrations than did individuals with symptomless infection or noninfected subjects. HO-1 is an intracellular enzyme, and the source of this molecule in the plasma is unclear. A reasonable explanation would be the release of HO-1 after cellular lysis during inflammation. Additionally, elevated plasma HO-1 levels have been reported with other diseases, such as vasculitis in Henoch-Schonlein purpura (19), hemophagocytic syndrome from hematological disorders (38, 49), type 2 diabetes (13), and prostate cancer (16). We speculate that HO-1 could play an anti-inflammatory role by degrading heme, which would dictate the severity of malaria. However, this effect remains to be established. Some studies using experimental models of malaria suggest that deleting the HMOX1 gene or pharmacologically inhibiting HO-1 activity in mice accounts for the pathogenesis of malaria, as these mice will not have the enzyme responsible for the detoxification of deleterious free heme (25). In contrast, our results show that individuals with symptomatic malaria have higher plasma HO-1 levels than do those with asymptomatic infection. In symptomatic individuals, the increased amounts of free heme and the cytokine storm that is associated with inflammation could be inducing increased levels of HO-1 as a counterregulatory response, especially considering the fact that HMOX1 gene expression is highly inducible by heme (64). Nevertheless, we have also found a group of symptomatic subjects who were more likely to be carriers of long (GT)n dinucleotide repeats in the HMOX1 gene microsatellite and had lower systemic levels of HO-1. In particular, those individuals with the long form of the HMOX1 polymorphism are generally those who have lower expression of the enzyme as a result of the genetic factor. In our study, the individuals with symptomatic malaria who presented with low expression of HO-1 were also the ones who carried the long form of the HMOX1 (GT)n polymorphism. In these cases, the genetic factor is probably preventing the counterregulatory increase in HO-1. Furthermore, malaria symptomatology may be associated with either increased or decreased expression of HO-1. High HO-1 levels may result from a counterregulatory response to infection and the cytokine storm and can lead to the increased synthesis of iron (a heme catabolism product), which can also be harmful to humans (63). Low HO-1 levels can result from a genetic component that results in a higher concentration of deleterious free heme in the circulation and is associated with symptomatic malaria (25, 26). Our results argue that both high and low levels of HO-1 may be associated with a greater chance of developing symptoms after Plasmodium infection. Our group is currently performing mechanistic studies to better understand the effects of HO-1 in human malaria.
This study revealed that plasma sCD163 levels gradually increased in correlation with the severity of the malaria infection. sCD163 has been identified to be an anti-inflammatory mediator that inhibits human T-lymphocyte activation and proliferation, and the binding of Hb-Hp complexes to sCD163 has been shown to suppress the supply of heme iron that is available to hemolytic bacteria (34, 67). Interestingly, several inflammatory processes are associated with elevated levels of sCD163 (18, 29, 33, 45, 46, 50–52, 60, 66), including falciparum malaria (41). Therefore, because symptomatic malaria is associated with a higher inflammatory response, the increased sCD163 concentrations in the symptomatic group probably serve as a counterregulatory mechanism against inflammation. Consistent with the inflammatory response seen during malaria infection, our results found a positive correlation between the levels of sCD163, TNF-α, and acute-phase proteins such as C-reactive protein and Hp. Indeed, sCD163 levels have already been positively correlated with TNF-α levels in falciparum malaria (41) and C-reactive protein in diabetes (50). TNF-α is able to induce the hepatic synthesis of Hp and regulates the expression of CD163 in monocytes and macrophages (41). IL-6 and IL-10 stimulate the expression of membrane-bound CD163 and have been positively correlated with sCD163 levels (17, 62). In this study, sCD163 levels were correlated with IL-6 levels; however, sCD163 levels did not correlate with IL-10 levels. Furthermore, sCD163 was positively correlated with HI and HIP scores, confirming the relationship between this molecule and inflammation in malaria.
Studies that simultaneously evaluate the different steps of the heme detoxification process may help to explain some of the controversies surrounding the effects of alterations in specific elements of this pathway. We have demonstrated that the individuals carrying the Hp2.2 genotype and the longer HMOX1 gene (GT)n dinucleotide repeats who also presented with high systemic concentrations of sCD163 have a greater susceptibility to developing clinical malaria than do those without these three potential risk factors. As shown in other studies (10–12, 15, 22, 23, 40, 41, 48, 56, 63) as well as in our work, genetic alterations in the HMOX1 and Hp genes and changes in sCD163 levels are all important elements during Plasmodium infection. A summary of the determinants involved in heme metabolism and our major findings is depicted in Fig. 5. The majority of the malaria cases in our study were caused by P. vivax, which limits our ability to compare our results with most of the findings in the current literature, which has focused on falciparum malaria. Research aimed at understanding the key factors involved in the immunopathogenesis of susceptibility to vivax malaria has been relatively neglected and made a low priority. There are clear similarities in the diseases caused by P. falciparum and P. vivax. Both infections cause hemolysis, for example. However, there are important and well-described differences between P. vivax and P. falciparum that result in different parasitemia thresholds for triggering severe malaria (2). The different clinical outcomes of these diseases make it important to expand the studies investigating the factors that are associated with the susceptibility to infection and disease severity in P. vivax malaria. Thus, studies assessing larger populations of P. vivax-infected individuals are needed to clarify the roles that sCD163, Hp, and HO-1 play in heme metabolism during human malaria infections. The validation of the results presented in the current study may provide new resources for the development of future targeted therapies that could aid in reducing malaria severity.
Fig 5.
Heme metabolism and malaria outcomes. The diagram illustrates a summary of the major mechanisms that are triggered by hemolysis during malaria. (A) Under homeostatic conditions, the free Hb that is released by dead red blood cells is rapidly scavenged by haptoglobin, and this molecular complex is removed from the circulation by the haptoglobin-Hb receptor CD163 on the surface of monocytes and macrophages. The Hb is processed inside these cells in an event that releases heme, which is further metabolized by the antioxidant enzyme HO-1. During an acute malarial attack, there is an accumulation of circulating free hemoglobin that is not compensated for by the amount of Hp available in the blood. The excess of free Hb is then oxidized by free radicals, releasing free heme. Free heme is very toxic to the cells and induces inflammation, macrophage activation, and oxidative stress. The host homeostatic responses triggered by heme include the induction of Hp, CD163, and HO-1. (B) We found that the intensity of the malaria-related symptoms is associated with the levels of circulating free heme. The individuals who developed symptoms after Plasmodium infection exhibited higher levels of Hp, soluble CD163, and HO-1. Nevertheless, this counterregulatory response is not sufficient to reduce the amount of free heme in the plasma, which also might explain the higher inflammatory scores estimated in symptomatic patients. These susceptible individuals carried the Hp2 allele or the Hp2.2 genotype more frequently than did other individuals. In addition, they more frequently carried long GT repeats in the HMOX1 polymorphism, which are paradoxically associated with a relatively lower plasma HO-1 concentration. The individuals with clinical immunity against malaria who remained asymptomatic upon Plasmodium infection tended to carry the Hp1S allele and have short GT repeats in the HMOX1 polymorphism more frequently than the symptomatic individuals. These protected individuals still had modest elevations in the levels of free heme and haptoglobin, with no differences in the concentrations of sCD163 and HO-1 compared with noninfected individuals.
ACKNOWLEDGMENTS
We thank Sebastião Souza-Neto, Antonio Reis-Filho, Jorge Clarêncio, Imbroinise Rafaelle-Netto, Jaqueline França-Costa, Jorge Tolentino, and Adorielze Leite for their technical and logistical support. We also thank Aldina Barral, Erney Camargo, and Luis Camargo for their critical discussions and revision of the data.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Andrade BB, et al. 2010. Heme impairs prostaglandin E2 and TGF-beta production by human mononuclear cells via Cu/Zn superoxide dismutase: insight into the pathogenesis of severe malaria. J. Immunol. 185:1196–1204 [DOI] [PubMed] [Google Scholar]
- 2. Andrade BB, Barral-Netto M. 2011. Biomarkers for susceptibility to infection and disease severity in human malaria. Mem. Inst. Oswaldo Cruz 106(Suppl 1):70–78 [DOI] [PubMed] [Google Scholar]
- 3. Andrade BB, et al. 2010. Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malar. J. 9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade BB, et al. 2010. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar. J. 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrade BB, et al. 2010. Plasma superoxide dismutase-1 as a surrogate marker of vivax malaria severity. PLoS Negl. Trop. Dis. 4:e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrade BB, et al. 2009. Anti-Anopheles darlingi saliva antibodies as marker of Plasmodium vivax infection and clinical immunity in the Brazilian Amazon. Malar. J. 8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrade BB, et al. 2011. Hepatitis B infection is associated with asymptomatic malaria in the Brazilian Amazon. PLoS One 6:e19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arredouani M, et al. 2003. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 108:144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. 2005. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ. Res. 96:435–441 [DOI] [PubMed] [Google Scholar]
- 10. Atkinson SH, et al. 2007. The haptoglobin 2-2 genotype is associated with a reduced incidence of Plasmodium falciparum malaria in children on the coast of Kenya. Clin. Infect. Dis. 44:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkinson SH, et al. 2006. Seasonal childhood anaemia in West Africa is associated with the haptoglobin 2-2 genotype. PLoS Med. 3:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aucan C, Walley AJ, Greenwood BM, Hill AV. 2002. Haptoglobin genotypes are not associated with resistance to severe malaria in The Gambia. Trans. R. Soc. Trop. Med. Hyg. 96:327–328 [DOI] [PubMed] [Google Scholar]
- 13. Bao W, et al. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS One 5:e12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beschorner R, et al. 2000. Long-term expression of heme oxygenase-1 (HO-1, HSP-32) following focal cerebral infarctions and traumatic brain injury in humans. Acta Neuropathol. 100:377–384 [DOI] [PubMed] [Google Scholar]
- 15. Bienzle U, et al. 2005. Limited influence of haptoglobin genotypes on severe malaria in Ghanaian children. Trop. Med. Int. Health 10:668–671 [DOI] [PubMed] [Google Scholar]
- 16. Blann AD, Balakrishnan B, Ryan P, Lip GY. Increased levels of plasma haemoxygenase-1 in prostate cancer. Prostate Cancer Prostatic Dis. 14:114–117 [DOI] [PubMed] [Google Scholar]
- 17. Buechler C, et al. 2000. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 67:97–103 [PubMed] [Google Scholar]
- 18. Burdo TH, et al. 2011. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 204:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen T, et al. 2011. Elevated serum heme oxygenase-1 and insulin-like growth factor-1 levels in patients with Henoch-Schonlein purpura. Rheumatol. Int. 31:321–326 [DOI] [PubMed] [Google Scholar]
- 20. Chen YC, et al. 2011. Haptoglobin polymorphism as a risk factor for chronic kidney disease: a case-control study. Am. J. Nephrol. 33:510–514 [DOI] [PubMed] [Google Scholar]
- 21. Chin HJ, et al. 2009. The heme oxygenase-1 genotype is a risk factor to renal impairment of IgA nephropathy at diagnosis, which is a strong predictor of mortality. J. Korean Med. Sci. 24(Suppl):S30–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox SE, et al. 2007. Haplotype association between haptoglobin (Hp2) and Hp promoter SNP (A-61C) may explain previous controversy of haptoglobin and malaria protection. PLoS One 2:e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elagib AA, Kider AO, Akerstrom B, Elbashir MI. 1998. Association of the haptoglobin phenotype (1-1) with falciparum malaria in Sudan. Trans. R. Soc. Trop. Med. Hyg. 92:309–311 [DOI] [PubMed] [Google Scholar]
- 24. Exner M, Minar E, Wagner O, Schillinger M. 2004. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 37:1097–1104 [DOI] [PubMed] [Google Scholar]
- 25. Ferreira A, Balla J, Jeney V, Balla G, Soares MP. 2008. A central role for free heme in the pathogenesis of severe malaria: the missing link? J. Mol. Med. (Berl.) 86:1097–1111 [DOI] [PubMed] [Google Scholar]
- 26. Ferreira A, et al. 2011. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145:398–409 [DOI] [PubMed] [Google Scholar]
- 27. Francis SE, Sullivan DJ, Jr, Goldberg DE. 1997. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51:97–123 [DOI] [PubMed] [Google Scholar]
- 28. Friis H, et al. 2003. Iron, haptoglobin phenotype, and HIV-1 viral load: a cross-sectional study among pregnant Zimbabwean women. J. Acquir. Immune Defic. Syndr. 33:74–81 [DOI] [PubMed] [Google Scholar]
- 29. Funding M, et al. 2005. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol. Scand. 83:234–239 [DOI] [PubMed] [Google Scholar]
- 30. Gozzelino R, Soares MP. 2011. Heme sensitization to TNF-mediated programmed cell death. Adv. Exp. Med. Biol. 691:211–219 [DOI] [PubMed] [Google Scholar]
- 31. Guetta J, Strauss M, Levy NS, Fahoum L, Levy AP. 2007. Haptoglobin genotype modulates the balance of Th1/Th2 cytokines produced by macrophages exposed to free hemoglobin. Atherosclerosis 191:48–53 [DOI] [PubMed] [Google Scholar]
- 32. Gyan B, et al. 2002. Elevated levels of nitric oxide and low levels of haptoglobin are associated with severe malarial anaemia in African children. Acta Trop. 83:133–140 [DOI] [PubMed] [Google Scholar]
- 33. Hiraoka A, et al. 2005. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J. Gastroenterol. 40:52–56 [DOI] [PubMed] [Google Scholar]
- 34. Hogger P, Erpenstein U, Rohdewald P, Sorg C. 1998. Biochemical characterization of a glucocorticoid-induced membrane protein (RM3/1) in human monocytes and its application as model system for ranking glucocorticoid potency. Pharm. Res. 15:296–302 [DOI] [PubMed] [Google Scholar]
- 35. Imrie H, et al. 2007. Low prevalence of an acute phase response in asymptomatic children from a malaria-endemic area of Papua New Guinea. Am. J. Trop. Med. Hyg. 76:280–284 [PubMed] [Google Scholar]
- 36. Kasvosve I, et al. 2000. Haptoglobin polymorphism and mortality in patients with tuberculosis. Int. J. Tuberc. Lung Dis. 4:771–775 [PubMed] [Google Scholar]
- 37. Kimpara T, et al. 1997. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum. Genet. 100:145–147 [DOI] [PubMed] [Google Scholar]
- 38. Kirino Y, et al. 2005. Increased serum HO-1 in hemophagocytic syndrome and adult-onset Still's disease: use in the differential diagnosis of hyperferritinemia. Arthritis Res. Ther. 7:R616–R624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kristiansen M, et al. 2001. Identification of the haemoglobin scavenger receptor. Nature 409:198–201 [DOI] [PubMed] [Google Scholar]
- 40. Kuesap J, Hirayama K, Kikuchi M, Ruangweerayut R, Na-Bangchang K. 2010. Study on association between genetic polymorphisms of haem oxygenase-1, tumour necrosis factor, cadmium exposure and malaria pathogenicity and severity. Malar. J. 9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kusi KA, et al. 2008. Levels of soluble CD163 and severity of malaria in children in Ghana. Clin. Vaccine Immunol. 15:1456–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langlois MR, Delanghe JR. 1996. Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 42:1589–1600 [PubMed] [Google Scholar]
- 43. Langlois MR, Delanghe JR, De Buyzere ML, Bernard DR, Ouyang J. 1997. Effect of haptoglobin on the metabolism of vitamin C. Am. J. Clin. Nutr. 66:606–610 [DOI] [PubMed] [Google Scholar]
- 44. Langlois MR, et al. 2000. The haptoglobin 2-2 phenotype affects serum markers of iron status in healthy males. Clin. Chem. 46:1619–1625 [PubMed] [Google Scholar]
- 45. Levy AP, et al. 2007. Downregulation of the hemoglobin scavenger receptor in individuals with diabetes and the Hp 2-2 genotype: implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ. Res. 101:106–110 [DOI] [PubMed] [Google Scholar]
- 46. Matsushita N, et al. 2002. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin. Exp. Immunol. 130:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Melamed-Frank M, et al. 2001. Structure-function analysis of the antioxidant properties of haptoglobin. Blood 98:3693–3698 [DOI] [PubMed] [Google Scholar]
- 48. Minang JT, et al. 2004. Haptoglobin phenotypes and malaria infection in pregnant women at delivery in western Cameroon. Acta Trop. 90:107–114 [DOI] [PubMed] [Google Scholar]
- 49. Miyazaki T, et al. Serum HO-1 is useful to make differential diagnosis of secondary hemophagocytic syndrome from other similar hematological conditions. Int. J. Hematol. 91:229–237 [DOI] [PubMed] [Google Scholar]
- 50. Moller HJ, Frikke-Schmidt R, Moestrup SK, Nordestgaard BG, Tybjaerg-Hansen A. 2011. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin. Chem. 57:291–297 [DOI] [PubMed] [Google Scholar]
- 51. Moreno JA, et al. 2010. Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arterioscler. Thromb. Vasc. Biol. 30:1253–1262 [DOI] [PubMed] [Google Scholar]
- 52. Nakayama W, et al. 2012. Serum levels of soluble CD163 in patients with systemic sclerosis. Rheumatol. Int. 32:403–407 [DOI] [PubMed] [Google Scholar]
- 53. Pamplona A, et al. 2007. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 13:703–710 [DOI] [PubMed] [Google Scholar]
- 54. Philippidis P, et al. 2004. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 94:119–126 [DOI] [PubMed] [Google Scholar]
- 55. Quaye IK, Ababio G, Amoah AG. 2006. Haptoglobin 2-2 phenotype is a risk factor for type 2 diabetes in Ghana. J. Atheroscler. Thromb. 13:90–94 [DOI] [PubMed] [Google Scholar]
- 56. Quaye IK, et al. 2000. Haptoglobin 1-1 is associated with susceptibility to severe Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94:216–219 [DOI] [PubMed] [Google Scholar]
- 57. Rogerson S. 2006. What is the relationship between haptoglobin, malaria, and anaemia? PLoS Med. 3:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ryndel M, et al. 2010. The haptoglobin 2-2 genotype is associated with carotid atherosclerosis in 64-year old women with established diabetes. Clin. Chim. Acta 411:500–504 [DOI] [PubMed] [Google Scholar]
- 59. Sambo MR, et al. 2010. Transforming growth factor beta 2 and heme oxygenase 1 genes are risk factors for the cerebral malaria syndrome in Angolan children. PLoS One 5:e11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schaer DJ, et al. 2005. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur. J. Haematol. 74:6–10 [DOI] [PubMed] [Google Scholar]
- 61. Sheu CC, et al. 2009. Heme oxygenase-1 microsatellite polymorphism and haplotypes are associated with the development of acute respiratory distress syndrome. Intensive Care Med. 35:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sulahian TH, et al. 2000. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12:1312–1321 [DOI] [PubMed] [Google Scholar]
- 63. Takeda M, et al. 2005. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to cerebral malaria in Myanmar. Jpn. J. Infect. Dis. 58:268–271 [PubMed] [Google Scholar]
- 64. Tenhunen R, Marver HS, Schmid R. 1970. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J. Lab. Clin. Med. 75:410–421 [PubMed] [Google Scholar]
- 65. Wagener FA, et al. 2008. HMOX1 promoter polymorphism modulates the relationship between disease activity and joint damage in rheumatoid arthritis. Arthritis Rheum. 58:3388–3393 [DOI] [PubMed] [Google Scholar]
- 66. Walter RB, Bachli EB, Schaer DJ, Ruegg R, Schoedon G. 2003. Expression of the hemoglobin scavenger receptor (CD163/HbSR) as immunophenotypic marker of monocytic lineage in acute myeloid leukemia. Blood 101:3755–3756 [DOI] [PubMed] [Google Scholar]
- 67. Weaver LK, et al. 2006. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J. Leukoc. Biol. 80:26–35 [DOI] [PubMed] [Google Scholar]
- 68. Winterbourn CC. 1985. Handbook of methods for oxygen radical research, vol 1 CRC Press, Boca Raton, FL [Google Scholar]
- 69. Yamada N, et al. 2000. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 66:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yano A, Yamamoto Y, Miyaishi S, Ishizu H. 1998. Haptoglobin genotyping by allele-specific polymerase chain reaction amplification. Acta Med. Okayama 52:173–181 [DOI] [PubMed] [Google Scholar]