Abstract
We previously demonstrated that one or more products of the genes in the pil and com gene clusters of the opportunistic human respiratory pathogen nontypeable Haemophilus influenzae (NTHI) are required for type IV pilus (Tfp) biogenesis and function. Here, we have now demonstrated that the pilABCD and comABCDEF gene clusters are operons and that the product of each gene is essential for normal pilus function. Mutants with nonpolar deletions in each of the 10 pil and com genes had an adherence defect when primary human airway cells were used as the target. These mutants were also diminished in their ability to form a biofilm in vitro and, additionally, were deficient in natural transformation. Collectively, our data demonstrate that the product of each gene within these operons is required for the normal biogenesis and/or function of NTHI Tfp. Based on the similarity of PilA to other type IV pilins, we further predicted that the product of the pilA gene would be the major pilin subunit. Toward that end, we also demonstrated by immunogold labeling and mass spectrometry that PilA is indeed the majority type IV pilin protein expressed by NTHI. These new observations set the stage for experiments designed to dissect the function of each of the proteins encoded by genes within the pil and com gene clusters. The ability to characterize individual proteins with vital roles in NTHI colonization or pathogenesis has the potential to reduce the burden of NTHI-induced diseases through development of a Tfp-derived vaccine or a pilus-directed therapeutic.
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHI) is a commensal of the human airway, yet it can cause multiple upper and lower respiratory tract diseases when host immune defenses are compromised (2). The type IV pilus (Tfp) of NTHI plays an important role in essential biological functions, including adherence to epithelial cells, which is a critical first step in the pathogenesis of any NTHI-induced disease. The Tfp is also involved in the following functions: competence, twitching motility, biofilm formation, and long-term colonization of the mammalian nasopharynx (3, 16). Expression of Tfp is common for many human pathogens, including Pseudomonas aeruginosa, Neisseria gonorrhoeae, Neisseria meningitidis, and Vibrio cholerae; however, whereas the products of genes associated with expression of these filamentous structures have been well characterized in several bacterial species (1), to date the gene products involved in the biogenesis of NTHI Tfp and their function are not yet well characterized.
Natural transformation, the process of horizontal exchange of genetic information in bacteria, is widely studied in organisms belonging to the genera Haemophilus, Bacillus, Neisseria, and Streptococcus. In most Gram-negative bacteria, such as Neisseria spp., the type IV pilus or pseudopilus system is required for DNA uptake (11, 15, 19). In H. influenzae strain Rd, genes within the competence regulon have been characterized by several groups. Using cassette mutagenesis, Dougherty and Smith constructed mutants deficient in ampD in the strain Rd background, some mutations of which extended through the pilA gene (HI0299 in strain Rd) (10). These mutants were not transformable. Tomb and coworkers characterized a second gene cluster named com, whose mutants were also transformation deficient (30, 31). In strain Rd, Redfield and coworkers demonstrated that the pil and com gene clusters were upregulated during competence development and, further, that a 22-bp competence regulatory element, designated CRE, is present upstream of both gene clusters (28).
In the extensively characterized clinical isolate NTHI strain 86-028NP (3, 5, 12, 13, 16, 20, 23), we have shown that the pilABCD and comABCDEF gene clusters play a role in canonical Tfp-associated phenotypes. We observed formation of a structure that was type IV pilus-like when strain 86-028NP cells were grown on a defined medium at alkaline pH. A mutant lacking the gene that encodes the putative major pilin subunit, PilA, was constructed by interruption of the pilA gene with an omega-Kan element (26); it did not make an observable Tfp. A mutant deficient in the gene that encodes the putative NTHI Tfp secretin ComE was also constructed. Under conditions where type IV pili were known to be expressed, the latter mutant made structures that appeared to be bulbous-tipped and thickened Tfp surrounded by outer membrane (3).
PilA has been proposed to be the major pilin subunit. PilB has homology to hexameric type II secretion and Tfp assembly ATPases. PilC is predicted to be a cytoplasmic membrane protein of the GspF family, and PilD has homology to type IV prepilin peptidases. As noted above, ComE is thought to be the secretin. ComA, ComB, ComC, and ComD are proposed to be important for biogenesis or stability of the secretin. The function of ComF is unknown, although this gene is annotated as an amidophosphorylribosyltransferase.
In order to fully characterize the genes and their products, we first demonstrated that the pil and com gene clusters were operons. Since the gene clusters were operons, it was likely that the previously described strains contained polar mutations (3, 16). Thus, to more precisely characterize the role of each gene product, here we constructed nonpolar mutants in each gene within the pil and com operons and then evaluated each for the relative ability to adhere to primary human respiratory epithelial cells, form a biofilm in vitro, and be naturally transformed. Our data demonstrated that all products of the pil and com loci were necessary for optimal function of Tfp in adherence to epithelial cells, biofilm formation, and transformation. Additionally, we demonstrated that PilA is the major pilin subunit of NTHI Tfp by mass spectrometry and transmission electron microscopy of immunogold-labeled bacterial cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains and plasmids are listed in Table S1 in the supplemental material. NTHI strain 86-028NP is a low-passage-number clinical isolate originally recovered from a child with chronic otitis media (18). This isolate has been extensively characterized to date in multiple in vitro systems, as well as in animal models (4, 18, 21, 24, 29), and its genome has been sequenced (12). NTHI parent and mutant strains were grown on chocolate agar or on chemically defined agar modified to pH 9.0 (7) for 18 to 20 h at 37°C in a humidified atmosphere of 5% CO2 before suspension in either 1× DPBS (Dulbecco's phosphate-buffered saline; Invitrogen, Carlsbad, CA) or brain heart infusion (BHI; Difco, Detroit, MI) broth supplemented with NAD (Sigma, St. Louis, MO) and heme (Sigma) to a final concentration of 2 μg ml−1 each (sBHI). Escherichia coli strains were grown in Luria-Bertani broth (LB) or on LB agar at 37°C. Antibiotics (Sigma) were added to E. coli cultures, where appropriate, at the following concentrations in μg ml−1: ampicillin, 100; kanamycin, 20; and spectinomycin, 50, unless otherwise specified. Antibiotics were added to NTHI cultures, where appropriate, at the following concentrations in μg ml−1: kanamycin, 20; spectinomycin, 200; and streptomycin, 1000. Competent NTHI cells were generated using a modified MIV protocol as previously described (3).
Construction of strains with nonpolar mutations and complementation of the mutations.
A streptomycin-resistant derivative of strain 86-028NP was constructed by amplification of the streptomycin resistance allele of the rpsL gene from NTHI strain 2019 and transformation of the amplicon into strain 86-028NP using the MIV methodology, followed by selection for streptomycin-resistant clones. One clone was saved as 86-028NPrpsL. Strains containing nonpolar mutations in the pil and com genes were constructed in this genetic background by utilizing a modification of the FLP recombinase technology of Datsenko and Wanner (9) as previously described (32). Genes were deleted such that a gene(s) was replaced with a small open reading frame (ORF) containing the translational start of the gene, an FLP scar generated when the cassette was removed by recombination, and the last 21 nucleotides (nt) of the deleted gene. The last 21 nt of the deleted gene were included so that the ribosomal binding site or other sequences needed for the proper translation of the downstream gene would be present in the construct.
The pil and com mutations were individually complemented in each strain with derivatives of pSPEC1 (3) that contained the targeted pil or com gene under the control of its native promoter. With the exception of the pilB-complementing plasmid, the plasmids used for complementation were constructed from pPIL1 and pCOM1, constructs that were previously described (3). To generate the complementing plasmids, inverse PCR and/or digestion with a unique restriction enzyme followed by repair was employed to generate derivatives that expressed only the gene of interest. Generation of the comB-complementing plasmid is described in detail; other constructs were generated using a similar strategy. Inverse PCR was performed twice. The first PCR utilized pCOM1 as the template. Primers with NcoI restriction sites were used to create an in-frame deletion of comA. The second reaction used a self-ligated amplicon from the first reaction as the template. The PCR was performed using primers with MluI restriction sites to create an in-frame deletion of comCDEF. The plasmid containing the pilB gene was constructed as follows. The region containing the pil promoter and pilB from the ΔpilA mutant was PCR amplified, and the resulting product was cloned into the FspI site pSPEC1. After confirmation of each construct by sequencing, mutants were transformed with the appropriate plasmid by electroporation. All PCRs were performed with Phusion Hot Start DNA polymerase (Thermo Fisher Scientific, Pittsburgh, PA), and primer sequences are listed in Table S2 in the supplemental material. All restriction enzymes were purchased from New England BioLabs (NEB; Beverly, MA).
Construction of pRSPILA and generation of antisera.
To generate antisera reactive with native NTHI Tfp, we amplified the pilA gene without the first 117 bp of the gene using primers that contained NdeI and BglII restriction sites on their 5′ and 3′ ends, respectively. The resulting amplicon was blunt end ligated into pCR-Blunt II-TOPO (Invitrogen) and transformed into E. coli TOP10 cells (Invitrogen). A plasmid that contained the correct insert was isolated from a single clone and digested with NdeI and BglII, and the resulting fragment was ligated to pET15b (Novagen, Madison, WI) that had been digested with the same enzymes. Ligation products were transformed into E. coli TOP10 cells (Invitrogen), plasmids were isolated, and a plasmid with the correct restriction pattern was sequenced and saved as pRSPILA and then transformed into Origami(DE3) cells (Novagen).
Cells were grown to an optical density at 600 nm (OD600) of 0.4 in 500 ml of LB broth containing ampicillin at 50 μg ml−1. IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma) was added to a final concentration of 0.4 mM to induce expression of pilA, and the culture was shaken overnight at 225 rpm at 19°C. Cells were pelleted, lysed in Bug Buster (Novagen), and centrifuged at 16,000 × g for 20 min at 4°C to separate soluble and insoluble fractions. The soluble fraction was incubated with nickel-nitrilotriacetic acid (Ni-NTA) resin (Novagen) for 1 h and washed twice with 1× wash buffer (50 mM NaH2PO4, 0.3 M NaCl, 20 mM imidazole, pH 8.0), and proteins were eluted with 1× elution buffer (50 mM NaH2PO4, 0.3 M NaCl, 250 mM imidazole, pH 8.0). The purified recombinant soluble PilA protein (rsPilA) was dialyzed against PBS at pH 7.1 and used for the production of polyclonal rabbit antibody by Proteintech (Chicago, IL).
Construction of pSXY.
The sxy gene, which encodes a positive regulator of the competence regulon in Haemophilus spp. (28), as well as ∼125 bp of flanking DNA upstream and downstream of the gene, was amplified from genomic DNA purified from strain 86-028NP. The amplicon was TA cloned into pGEM-T Easy (Promega, Madison, WI) and transformed into E. coli DH5α. A plasmid with the correct sequence was digested with EcoRI to release the insert. The resulting EcoRI fragment was cloned into pGZRS-39A that had been digested with EcoRI. The resulting plasmid, pSXY, was transformed into both NTHI strain 86-028NPrpsL and 86-028NPrpsLΔpilA.
Determination that the pil and the com gene clusters are operons.
RNA was purified from competent strain 86-028NP cells and analyzed by reverse transcription-PCR (RT-PCR). Competent cells were prepared and then harvested at 3,200 × g for 10 min at 4°C, and the supernatant was discarded. The pellet was resuspended in 20 ml of TRIzol (Invitrogen), and samples were processed according to the manufacturer's protocol. Samples were DNase treated as follows. In a 50-μl total volume, 200 μg of total RNA was mixed with 5 μl 10× DNase I buffer (NEB), 7.5 U DNase I (NEB), and 0.5 μl RNaseOut (Invitrogen) and incubated at 37°C for 1 h. Following incubation at 37°C, samples were treated with phenol-chloroform, precipitated in isopropanol, and rehydrated in 50 μl water. RNA was purified using the RNeasy minikit (Qiagen, Santa Clara, CA). RT-PCR was performed using the QuantiTect SYBR green kit (Qiagen). Briefly, 25-μl reaction mixtures contained 12.5 μl of 2× QuantiTect SYBR green master mix, 0.4 μM (each) primer, 0.5 μl QuantiTect RT mix, and 1 ng of RNA. Reaction mixtures were cycled under the following conditions: 50°C for 30 min, 95°C for 15 min, and 45 cycles of 94°C for 15 s, 60°C for 30 s, and 72°C for 1 min. Samples containing no reverse transcriptase were included as negative controls. PCR products were electrophoresed and visualized on a Flashgel (Lonza, Basel, Switzerland).
Assessment of the relative ability of strains to adhere to primary human respiratory epithelial cells.
One day prior to assay, normal human bronchial epithelial (NHBE; Lonza) cells were seeded into Costar 96-well plates (Costar, Corning, NY) at a concentration of 3 × 104 cells per well in 50 μl of bronchial epithelial growth medium (BEGM) (Lonza). After overnight growth, medium was replaced with 50 μl of fresh BEGM. Bacterial strains to be tested for adherence to NHBE cells were streaked onto chocolate agar containing appropriate antibiotics and incubated overnight at 37°C with 5% CO2. Bacteria were then scraped from plates and suspended in DPBS to an OD490 of 0.65 and further diluted 1:6 in sBHI to a final volume of 6 ml. Cultures were incubated at 37°C with 5% CO2 until an OD490 of 0.65 was reached, at approximately 3 h, and then used in the adherence assay. Two hours prior to inoculating epithelial cells with bacteria, the medium was replaced with 200 μl of antibiotic-free BEGM that contained 10% heat-inactivated fetal bovine serum (Lonza) in order to inhibit nonspecific bacterial binding. One hundred fifty microliters of medium was removed from each well, and 2 μl of bacterial suspension was added to each well (at a multiplicity of infection [MOI] of 100:1). Plates were then incubated for 60 min at 37°C with 5% CO2. NHBE cells were then washed three times with 200 μl of 1× DPBS to remove nonadherent bacteria, after which 50 μl of trypsin-EDTA solution (Lonza) was added per well. After 5 min of incubation at 37°C, cell suspensions were removed from the wells, diluted in DPBS, and plated on chocolate agar to quantify adherent bacteria. Data were expressed as the percentage of adherent CFU relative to the parent strain and based on triplicate wells. Each strain was assayed a minimum of three times on separate days.
Assessment of relative ability of strains to form a biofilm in vitro.
Cultures were prepared as described above for the adherence assay. Upon reaching an OD490 of 0.65, cultures were diluted 1:2,500 in prewarmed sBHI and 200 μl of cell suspension was pipetted into each well of an 8-well glass chamber slide (Nunc, Rochester, NY) and incubated at 37°C with 5% CO2. After 16 h, medium was replaced with prewarmed sBHI and the chamber slide was placed back into the incubator for an additional 8 h. Following incubation, biofilms were gently washed 3 times with 200 μl sterile saline and then stained using a Live/Dead BacLight kit (Invitrogen) per the manufacturer's instructions. Poststaining, biofilms were washed three times with sterile saline and fixed with 10% phosphate-buffered formalin for a minimum of 1 h. Fixed biofilms were washed 3 times with sterile saline, chambers and gaskets were removed, and coverslips were placed on slides. Biofilms were imaged by confocal laser scanning microscopy using a ×63 objective on an LSM 710 confocal microscope (Carl Zeiss, Thornwood, NY) (17). Two sets of Z-stack images were taken per well, and at least 3 biological replicates were performed per strain analyzed. Images were analyzed using COMSTAT (14).
Determination of the ability of mutants and complemented mutants to be transformed.
A derivative of pGEM-T EZ that contained a genomic fragment from strain Rd KW20 that included the cya gene as well as ∼700 bp of sequence 5′ and 3′ of cya was generated. The cya gene was insertionally inactivated by cloning a kanamycin cassette between the KpnI and BamHI sites in the cya gene to form pRSM2948. Alternatively, a spectinomycin cassette was cloned into the BamHI site of cya to form pRSM3241 (32). Transformability was determined via a modified MIV method using DNA from pRSM2948 for transformations of each mutant and complemented strain (3). DNA from pRSM3241 was used for transformation experiments in strains that carried pSXY.
Demonstration that PilA is the pilin subunit of NTHI Tfp.
Strains were streaked onto chocolate agar, or chocolate agar containing kanamycin when appropriate, and incubated at 37°C with 5% CO2, overnight. Cells were suspended in 10 ml sBHI, with or without kanamycin as appropriate, and incubated at 37°C and 180 rpm until an OD600 of approximately 0.3 was reached. One hundred microliters of each strain was plated onto both chocolate agar and chemically defined medium agar at pH 9.0 and grown overnight at 37°C with 5% CO2. For whole-cell extracts, cells from each strain were suspended in 2 ml DPBS, pH 7.4, in the presence of complete protease inhibitor (Roche, Rockford, IL). For sheared pilus preparations, cells were suspended in 5 ml of DPBS, pH 7.4, in the presence of complete protease inhibitor; vortexed for 2 min; and centrifuged at 3,200 × g for 15 min, and protein in the supernatant was concentrated by precipitation in 10% saturated ammonium sulfate. Protein content of all samples was determined using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Samples were boiled in SDS-PAGE sample buffer, and protein (5 μg of protein for whole cells or 2 μg of protein for shear preparations) was subjected to SDS-PAGE. Following separation by SDS-PAGE, proteins were either subjected to silver staining (Pierce) or Coomassie blue staining or transferred to nitrocellulose for Western blot analysis using anti-rsPilA antibody. The blot was blocked with Tris-buffered saline containing 0.05% Tween 20 (TTBS) and 2% bovine serum albumin (Sigma) for 1 h, incubated with a 1:1,000,000 dilution of anti-rsPilA antibody for 1 h, washed with TTBS, and incubated with a 1:2,000 dilution of an alkaline phosphatase-conjugated goat anti-rabbit IgG serum (Invitrogen) for 1 h. Following three washes with TTBS and one wash in alkaline phosphatase substrate buffer, the immunoblot was developed with 5-bromo-4-chloro-3-indolylphosphate (BCIP)–nitroblue tetrazolium (NBT) (Sigma). Presumptive PilA bands were excised from Coomassie blue-stained gel lanes that contained protein from 86-028NPrpsL(pSXY) along with the same region of the gel that contained protein from 86-028NPrpsLΔpilA(pSXY) and submitted to The Ohio State University Mass Spectrometry and Proteomics Facility for identification.
Electron microscopy.
Strains 86-028NPrpsL(pGZRS39A), 86-028NPrpsL(pSXY), and 86-028NPrpsLΔpilA(pSXY) were streaked onto chemically defined agar (pH 9.0) and incubated for 48 h at 37°C with 5% CO2. Bacteria were scraped from agar plates, suspended in 10 μl of sterile saline, and floated onto Formvar-coated 300-mesh nickel grids (Electron Microscopy Sciences, Hatfield, PA). Grids were washed 3 times by being submerged in 1 ml of sterile saline and floated onto sterile saline that contained a 1:25 dilution of rabbit anti-rsPilA antibody for 1 h. Grids were washed as previously described and floated onto saline that contained a 1:100 dilution of goat anti-rabbit IgG conjugated to 10-nm gold particles (EY Laboratories, San Mateo, CA) for 1 h. Grids were washed as previously described and then floated on saline containing 2% paraformaldehyde (Electron Microscopy Sciences) for 30 min. Grids were washed as previously described and then negatively stained with an equal volume of 2.0% (wt/vol) ammonium acetate (Sigma) and 2.0% (wt/vol) ammonium molybdate (Sigma) in water that had been filtered through Whatman no. 1 filter paper (Whatman, Maidstone, England). After 5 min, grids were blotted and allowed to air dry overnight prior to viewing with a Tecnai G2 Spirit transmission electron microscope (FEI, Hillsboro, OR) at The Ohio State University Campus Microscopy and Imaging Facility.
Statistical analysis.
Statistical analyses were performed using analysis of variance (ANOVA). Post hoc analysis was performed using Tukey's test, and a P value of <0.05 was considered to be statistically significant. Analyses were performed using GraphPad Prism.
RESULTS
The pil and com operons.
The pil gene cluster of NTHI strain 86-028NP contains four genes designated pilA, pilB, pilC, and pilD (Fig. 1A), and the com gene cluster contains six genes designated comA, comB, comC, comD, come, and comF (Fig. 1B). We previously reported that a polar mutant deficient in the expression of pilA was also deficient in expression of both Tfp and twitching motility (3). Subsequently, we showed that this mutant is deficient in biofilm formation both in vitro and in a chinchilla model of experimental otitis media. In addition, the mutant had an adherence defect in vitro and was unable to maintain long-term colonization of the chinchilla nasopharynx (16). Information gained from the study of these mutants, as well as mutants constructed in strain Rd (10, 30, 31), was important but limited, as these mutations were likely polar. In order to demonstrate that the pil and com gene clusters were in fact cotranscribed, we performed RT-PCR across the pilAB, pilBC, pilCD, comAB, comBC, comCD, comDE, and comEF junctions (Fig. 1). Each junction region was amplified, which indicated that there was transcription through each intergenic region. Although there is a possibility that the intergenic transcription observed does not result in the full-length transcription of each downstream gene, our data together with the short distance between, or actual overlap of, adjacent genes suggest that the 2 gene clusters are operons. We therefore constructed nonpolar mutants deficient in each gene in both gene clusters and also constructed strains in which each mutation was complemented.
Fig 1.
Data demonstrated that the NTHI pil and com gene clusters were indeed operons. (A) Organization of the NTHI strain 86-028NP pil operon and RT-PCR across the junctions between the pil genes. (B) Organization of the NTHI strain 86-028NP com operon and RT-PCR across the junctions between the com genes.
Relative ability to form a biofilm in vitro.
We have previously shown that strain 86-028NP forms a very robust biofilm in vitro, with an architecture characterized by distinct towers and water channels (3). When the parent strains 86-028NP and 86-028NPrpsL were allowed to form a biofilm in a chamber slide for 24 h, the biofilms had mean thicknesses of 15.6 or 16.6 μm and biomasses of 5.0 μm3 (μm2)−1, respectively. Biomass, as calculated by COMSTAT, is the total volume of the biomass divided by the area of the substratum (14). All biofilms formed by pil and com mutants exhibited a significant (P < 0.05) reduction in thickness compared to those biofilms formed by the parental isolates (Fig. 2A). Additionally, all pil mutant biofilms and the comC mutant biofilm demonstrated significantly (P < 0.05) reduced biomass compared to parent biofilms. While there was no statistically significant difference between the biomasses of the comA, comB, comD, comE, and comF mutant biofilms and those of the biofilms of the parent strains, there was a trend of reduced mutant biofilm biomass compared to the parent strain biofilm biomass (Fig. 2B). Interestingly, biofilms formed by the pil mutants were consistently thinner and had reduced biomass compared to com mutant biofilms. Biofilms formed by the complemented pilA and comE mutants were restored to a biomass and a thickness equivalent to those of parent strains.
Fig 2.

NTHI pil and com mutant biofilms had reduced thickness and decreased biomass. COMSTAT analysis of Z-stack composite images of NTHI biofilms formed after 24 h in vitro. (A) Average biofilm thickness. (B) Biofilm biomass. Error bars represent standard errors of the means of 3 or more biological replicates. *, statistical significance at P < 0.05.
Relative ability to adhere to primary human respiratory epithelial cells.
When confluent normal human bronchial epithelial (NHBE) cells were incubated with either the parental isolate or any of the mutant 86-028NPrpsL strains for 60 min and the adherent bacteria were subsequently enumerated, we found that the mutants deficient in each pil gene or those deficient in comA, comC, comD, comE, or comF were all significantly (P < 0.05) less adherent to normal human bronchial epithelial cells than were the parent strains (Fig. 3). While the adherence defect of the comB mutant was not statistically significant, there was a trend of reduced adherence. Complemented pilB, pilD, and comE mutants were restored in their ability to adhere to NHBE cells compared to the parent strains. Binding of strain 86-028NPrpsL and the pilA mutant to empty culture wells was tested. Both strains bound to empty culture wells at levels 2 orders of magnitude lower than that observed with 86-028NPrpsL adhering to NHBE cell-containing wells (data not shown). This demonstrated that the observed difference in adherent bacteria was due to a reduction in binding to NHBE cells rather than to plastic.
Fig 3.

NTHI pil and com mutants had adherence defects on normal human bronchial epithelial cells. Relative adherence of NTHI parent strains, mutants, and complemented mutant strains to normal human bronchial epithelial cells. Error bars represent standard errors of the means of 3 or more biological replicates. *, statistical significance at P < 0.05.
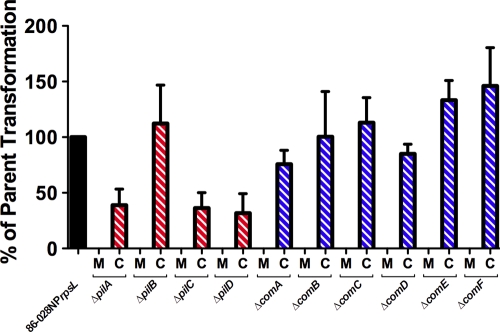
Relative transformability.
Transformation frequency in each of the nonpolar pil locus and com locus mutants was below our limit of detection (Fig. 4). Complementation of each mutation partially restored transformability compared to parental levels. Additional time and an increase in DNA did not compensate for the deficiency in transformation (data not shown). These data demonstrated that each of the pil operon and com operon gene products was required for transformation.
Fig 4.
Mutations in NTHI pil and com genes result in a loss of transformability. Transformation of the pil and com mutants (M) was below our level of detection. Complementation (C) of each mutation restored transformation, although not necessarily to parent levels. Therefore, the expression of each product of the pil and com loci was required for natural transformation. Error bars represent standard errors of the means of 3 or more biological replicates.
Demonstration that PilA is the major pilin subunit of NTHI Tfp.
Although sequence homology suggested that the PilA protein was the major pilin subunit, direct demonstration of this has been hampered by the low level of expression of pili during growth on rich media, as well as under competence-inducing conditions (data not shown).
The Redfield and Barcak groups identified a gene that encodes a transcriptional regulator termed sxy (27) or tfoX (34), respectively. Mutants lacking this protein are nontransformable, whereas certain mutants with base changes 5′ to the sxy gene are constitutively transformable (6, 27, 34).
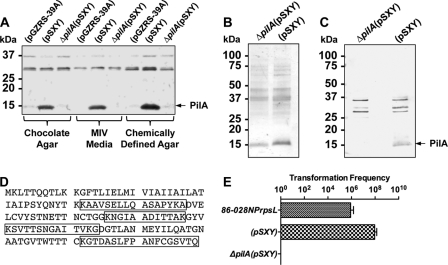
In order to upregulate pil expression, we expressed sxy from a multicopy plasmid, designated pSXY. Figure 5A shows a Western blot of whole-cell extracts from the overexpressing sxy strain that reacted with antibody against recombinant soluble PilA (anti-rsPilA), whereas the vector control and the pilA mutant whole-cell extracts did not. Silver stains of sheared-cell supernatants prepared from bacterial cells that were overexpressing sxy demonstrated a band at the expected mass of PilA in the parental extract (Fig. 5B). Western blot analysis of sheared-cell supernatants from the overexpressing sxy strain reacted with anti-rsPilA antibody whereas sheared-cell supernatants from the pilA mutant did not, demonstrating that the ∼14-kDa band was PilA (Fig. 5C). The putative PilA band and the same region from the sxy-overexpressing pilA mutant lane on the Coomassie blue-stained gel were excised and submitted to The Ohio State University Mass Spectrometry Facility for protein identification. Peptides corresponding to PilA were identified in samples of the sxy-overexpressing strain and not samples of the sxy-overexpressing pilA mutant. A band with a molecular mass similar to that of PilA in the pilA mutant lane of Fig. 5B is most likely outer membrane protein P6 as determined by size and mass spectrometry analysis (data not shown). Taken together, these data indicated that Tfp was being produced in strain 86-028NPrpsL(pSXY) and not in strain 86-028NPrpsLΔpilA(pSXY). Additionally, the transformation frequency of strains 86-028NPrpsL(pSXY) and 86-028NPrpsLΔpilA(pSXY) was determined and compared to that observed with strain 86-028NPrpsL (Fig. 5E). Overexpression of sxy resulted in a hypercompetent phenotype consistent with the upregulation of expression of PilA under these conditions.
Fig 5.
PilA is the major subunit of NTHI Tfp. (A) Western blot of whole-cell extracts from strains 86-028NPrpsL(pGZRS-39A), 86-028NPrpsL(pSXY), and 86-028NPrpsLΔpilA(pSXY) grown on chocolate agar, MIV medium, or a chemically defined agar. (B) Silver-stained gel of strain 86-028NPrpsLΔpilA(pSXY) and 86-028NPrpsL(pSXY) sheared-cell supernatants. (C) Western blot of strain 86-028NPrpsLΔpilA(pSXY) and 86-028NPrpsL(pSXY) sheared-cell supernatants using anti-rsPilA antibody. (D) Sequence of PilA with mass spectrometry-identified peptides from strain 86-028NPrpsL(pSXY) highlighted. (E) Transformation efficiency of strains 86-028NPrpsL, 86-028NPrpsL(pSXY), and 86-028NPrpsLΔpilA(pSXY).
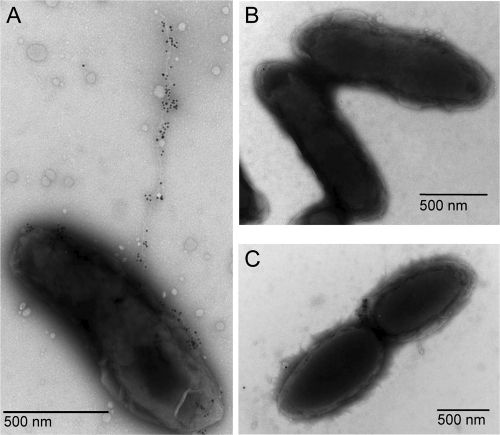
In our initial description of the Tfp in NTHI, we demonstrated pili by electron microscopy when the parent was grown on a chemically defined agar at pH 9.0 (3). When strain 86-028NPrpsL(pSXY) was grown under the same conditions and then immunogold labeled using the anti-rsPilA antibody, labeled pili were readily detected (Fig. 6A), compared to an absence of pili on either the pilA mutant overexpressing sxy (Fig. 6B) or the empty-vector control (Fig. 6C).
Fig 6.
Immunolabeling of NTHI Tfp with rabbit anti-rsPilA antibody. (A) 86-028NPrpsL(pSXY), an sxy overexpression strain. (B) 86-028NPrpsL(pGZRS-39A), empty-vector control strain. (C) 86-028NPrpsLΔpilA(pSXY), pilA mutant overexpressing sxy.
DISCUSSION
Nontypeable Haemophilus influenzae strains express a multifunctional type IV pilus that is critical to several essential biological functions, including both adherence and long-term colonization of the mammalian nasopharynx (16). Expression of a functional Tfp by this heterogeneous group of microorganisms is accomplished via the utilization of a uniquely small set of genes that is approximately half the number employed by other bacterial species that similarly produce Tfp (22). This observation suggested to us that it would be important to more closely examine the products of each of the genes within both the pil and com loci in order to better understand their role(s) in each of the multiple functions attributable to expression of a Tfp.
Here, via generation of a series of mutants, we demonstrated that nonpolar mutations in any of the genes within the pil and com operons, and targeted for investigation here, did not result in an in vitro growth defect.
Further analysis of each of the 10 nonpolar mutants defined a role for each of these gene products in competence/transformability. Competence was dependent not only on each of the six genes within the com operon but upon each of the four genes within the pil locus as well, which suggested that the filamentous appendage itself plays a role in uptake of foreign DNA from the environment, as has been suggested by others (33). Transformation in certain complemented pil mutants was not restored to wild-type levels. Complementation of such genes carried on multicopy plasmids may alter the stoichiometry necessary for optimal uptake of DNA; thus, certain complemented mutant strains may not exhibit parental levels of transformation.
To further characterize the role of each of the studied gene products in functions essential to pathogenesis, we assessed the ability of each of the mutants generated here both to adhere to primary human airway epithelial cells and to form a biofilm in vitro as these functions represent the first step in the disease process and a phenotype necessary for chronic and/or recurrent disease, respectively. We found that mutations in any gene within the pil or com locus resulted in compromised ability to adhere to primary human respiratory epithelial cells. Collectively, these data suggested that, as with other Tfp-expressing bacteria, assembly, export, and function of Tfp are complex and dependent on multiple gene products. Importantly, complementation of genes that encode the putative prepilin peptidase (pilD), assembly ATPase (pilB), and secretin (comE) restored the adherence phenotype to those demonstrated by the parental isolates. These mutants were chosen to be complemented as their products are considered core Tfp biogenesis proteins and appeared to be specifically required for Tfp production/function.
We have previously shown that PilA is essential to biofilm formation by NTHI both in vitro and in vivo (16). Here, we expand greatly on this initial observation to demonstrate the essential role of multiple other gene products within the Tfp regulon in the formation of a robust biofilm characteristic for NTHI strain 86-028NP. The inability to express any given gene product of either the pil or the com locus resulted in the formation of biofilms of altered architecture with a reduced thickness and biomass that resulted in a more condensed and lawn-like appearance of the biofilm with the notable absence of water channels. This change in biofilm biomass and thickness could be attributed to reduced interbacterial interaction mediated by Tfp; loss of twitching motility, which has been shown to play a role in P. aeruginosa biofilm development (25); or a change in early biofilm development during microcolony formation, where Tfp is known to play a role (8). Interestingly, there are no PilT homologs that have been identified in NTHI, similar to the toxin-coregulated pilus system in Vibrio cholerae, even though Tfp of V. cholerae and, as demonstrated here, Tfp of NTHI play a role in biofilm formation (8).
It is clear from both previous work and from those data presented here that the Tfp of NTHI strain 86-028NP, and by inference other NTHI isolates that also express Tfp, serves multiple essential biological functions. Importantly, we have demonstrated indirectly earlier (via use of a reporter construct that luminesced under the control of the pilA promoter), that NTHI appeared to express Tfp when inducing the formation of a biofilm within the middle ear of a chinchilla during the course of experimental otitis media (16). This expression in vivo, in conjunction with limited PilA sequence diversity among NTHI strains (3), lends strong support to our efforts to develop a PilA-derived vaccine candidate for the prevention of NTHI-induced diseases of the respiratory tract. In fact, to date, preclinical studies have shown that immunization with a recombinant soluble form of PilA resulted in both significantly earlier resolution of existing disease and significant protection from ascending experimental otitis media due to NTHI in a polymicrobial model (23, 24). Whereas the mechanisms that underlie the significant protection shown have not yet been fully elucidated, antibodies directed against rsPilA (both local and systemic) played a clear role in the protection afforded. Interestingly, we have been able to show that both invasive and noninvasive regimens for delivery of PilA-derived vaccine candidates have been highly efficacious preclinically, thus suggesting their potential ease of deliverability. The ability of PilA-derived vaccine candidates to afford protection, or induce resolution of existing NTHI-induced diseases of other niches within the mammalian airway, in addition to that efficacy already demonstrated in the middle ear, is an area of active investigation.
The merits of a pilus target vaccine to combat NTHI disease only underscore the need to understand not only the biological processes in which NTHI Tfps are involved but the biogenesis of these important structures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Huachun Zhong, Beth Baker, and Feng Ye for their technical assistance. We also thank Jennifer Neelans for manuscript preparation.
This work was funded by NIDCD/NIH R01DC007464 to R.S.M. and R01DC003915 to L.O.B.
Footnotes
Published ahead of print 10 February 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Ayers M, Howell PL, Burrows LL. 2010. Architecture of the type II secretion and type IV pilus machineries. Future Microbiol. 5: 1203– 1218 [DOI] [PubMed] [Google Scholar]
- 2. Bakaletz LO. 2010. Immunopathogenesis of polymicrobial otitis media. J. Leukoc. Biol. 87: 213– 222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakaletz LO, et al. 2005. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect. Immun. 73: 1635– 1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakaletz LO, Leake ER, Billy JM, Kaumaya PT. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15: 955– 961 [DOI] [PubMed] [Google Scholar]
- 5. Bakaletz LO, et al. 1988. Frequency of fimbriation of nontypeable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect. Immun. 56: 331– 335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cameron AD, Volar M, Bannister LA, Redfield RJ. 2008. RNA secondary structure regulates the translation of sxy and competence development in Haemophilus influenzae. Nucleic Acids Res. 36: 10– 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman HN, Daines DA, Jarisch J, Smith AL. 2003. Chemically defined media for growth of Haemophilus influenzae strains. J. Clin. Microbiol. 41: 4408– 4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craig L, Pique ME, Tainer JA. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2: 363– 378 [DOI] [PubMed] [Google Scholar]
- 9. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97: 6640– 6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dougherty BA, Smith HO. 1999. Identification of Haemophilus influenzae Rd transformation genes using cassette mutagenesis. Microbiology 145: 401– 409 [DOI] [PubMed] [Google Scholar]
- 11. Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 59: 376– 385 [DOI] [PubMed] [Google Scholar]
- 12. Harrison A, et al. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187: 4627– 4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrison A, et al. 2007. The OxyR regulon in nontypeable Haemophilus influenzae. J. Bacteriol. 189: 1004– 1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heydorn A, et al. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146: 2395– 2407 [DOI] [PubMed] [Google Scholar]
- 15. Johnsborg O, Eldholm V, Havarstein LS. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 158: 767– 778 [DOI] [PubMed] [Google Scholar]
- 16. Jurcisek JA, et al. 2007. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol. Microbiol. 65: 1288– 1299 [DOI] [PubMed] [Google Scholar]
- 17. Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. 2011. In vitro biofilm formation in an 8-well chamber slide. J. Vis. Exp. 47: pii: 2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kennedy BJ, Novotny LA, Jurcisek JA, Lobet Y, Bakaletz LO. 2000. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect. Immun. 68: 2756– 2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kruger NJ, Stingl K. 2011. Two steps away from novelty—principles of bacterial DNA uptake. Mol. Microbiol. 80: 860– 867 [DOI] [PubMed] [Google Scholar]
- 20. Mason KM, Bruggeman ME, Munson RS, Bakaletz LO. 2006. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 62: 1357– 1372 [DOI] [PubMed] [Google Scholar]
- 21. Mason KM, Munson RS, Jr, Bakaletz LO. 2005. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect. Immun. 73: 599– 608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattick JS. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56: 289– 314 [DOI] [PubMed] [Google Scholar]
- 23. Novotny LA, et al. 2009. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 28: 279– 289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Novotny LA, Clements JD, Bakaletz LO. 2011. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal Immunol. 4: 456– 467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30: 295– 304 [DOI] [PubMed] [Google Scholar]
- 26. Perez-Casal J, Caparon MG, Scott JR. 1991. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J. Bacteriol. 173: 2617– 2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redfield RJ. 1991. sxy-1, a Haemophilus influenzae mutation causing greatly enhanced spontaneous competence. J. Bacteriol. 173: 5612– 5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Redfield RJ, et al. 2005. A novel CRP-dependent regulon controls expression of competence genes in Haemophilus influenzae. J. Mol. Biol. 347: 735– 747 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki K, Bakaletz LO. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect. Immun. 62: 1710– 1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomb JF, Barcak GJ, Chandler MS, Redfield RJ, Smith HO. 1989. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J. Bacteriol. 171: 3796– 3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomb JF, el-Hajj H, Smith HO. 1991. Nucleotide sequence of a cluster of genes involved in the transformation of Haemophilus influenzae Rd. Gene 104: 1– 10 [DOI] [PubMed] [Google Scholar]
- 32. Tracy E, Ye F, Baker BD, Munson RS., Jr 2008. Construction of non-polar mutants in Haemophilus influenzae using FLP recombinase technology. BMC Mol. Biol. 9: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Schaik EJ, et al. 2005. DNA binding: a novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol. 187: 1455– 1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zulty JJ, Barcak GJ. 1995. Identification of a DNA transformation gene required for com101A+ expression and supertransformer phenotype in Haemophilus influenzae. Proc. Natl. Acad. Sci. U. S. A. 92: 3616– 3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.