Abstract
Tuberculous meningitis leads to a devastating outcome, and early diagnosis and rapid chemotherapy are vital to reduce morbidity and mortality. Since Mycobacterium tuberculosis is a kind of cytozoic pathogen and its numbers are very few in cerebrospinal fluid, detecting M. tuberculosis in cerebrospinal fluid from tuberculous meningitis patients is still a challenge for clinicians. Ziehl-Neelsen stain, the current feasible microbiological method for the diagnosis of tuberculosis, often needs a large amount of cerebrospinal fluid specimen but shows a low detection rate of M. tuberculosis. Here, we developed a modified Ziehl-Neelsen stain, involving cytospin slides with Triton processing, in which only 0.5 ml of cerebrospinal fluid specimens was required. This method not only improved the detection rate of extracellular M. tuberculosis significantly but also identified intracellular M. tuberculosis in the neutrophils, monocytes, and lymphocytes clearly. Thus, our modified method is more effective and sensitive than the conventional Ziehl-Neelsen stain, providing clinicians a convenient yet powerful tool for rapidly diagnosing tuberculous meningitis.
INTRODUCTION
Tuberculous meningitis (TBM) is the most severe form of tuberculosis and causes substantial morbidity and mortality (18). The early diagnosis of and prompt initiation of chemotherapy for TBM are crucial to a successful outcome. However, the early and accurate detection of Mycobacterium tuberculosis in the cerebrospinal fluid (CSF) of TBM patients still remains a challenge for clinicians, mainly due to the lack of rapid, efficient, and practical detection methods (30).
Currently, mycobacterial culture is the gold standard for detecting M. tuberculosis, but it is time-consuming and requires specialized safety procedures in laboratories (19, 26). Serological methods are convenient but lack sensitivity and specificity (4, 7). Although the PCR technique is rapid, it is costly for routine use in developing countries where most tuberculosis cases occur (5, 17, 21, 24). Conventional smear microscopy with the Ziehl-Neelsen (ZN) stain is a rapid and practical method for detecting acid-fast bacilli (AFB), especially in low-income countries, due to its rapidity, low cost, and high positive predictive value for tuberculosis (14). However, the Ziehl-Neelsen method is severely handicapped by its low detection rate, ranging from 0 to 20% for CSF specimens (31–33). One of the main reasons behind this is that M. tuberculosis can hardly be stained by acid-fast dyes once it enters the cells. Another important reason is that the Ziehl-Neelsen method requires a large volume of CSF for TBM diagnosis, as it is incapable of detecting bacilli that are fewer than 10,000 in number per slide or per ml of specimen (32, 33). Therefore, it is important to develop an alternative, cost-effective method for detecting intracellular M. tuberculosis. Additionally, knowing which cell type is infected by M. tuberculosis in the CSF of TBM patients could help us to unravel new antituberculotic candidates (10).
To reveal the presence of intracellular M. tuberculosis and improve the detection of extracellular M. tuberculosis from a small volume of CSF specimens, we developed a highly efficient Ziehl-Neelsen stain involving the use of only 0.5-ml CSF specimens from TBM cases. The formed elements in the CSF, including the bacilli and cells, were compactly collected onto the slides by cytospinning followed by staining with acid-fast dyes containing the detergent Triton X-100. Using this modified staining method, AFB can be clearly revealed within the immune cells, and the detection rate of extracellular AFB was significantly improved as well.
MATERIALS AND METHODS
Study subjects.
The study protocol was approved by the Institutional Review Board of Xijing Hospital, the Fourth Military Medical University, Xian, China, and written informed consent was obtained from all patients or their legal surrogates. Twenty-nine patients whose CSF specimens were culture positive for Mycobacterium by the MGIT 960 mycobacteria culture system were diagnosed with TBM and enrolled in this study. For the identification of M. tuberculosis and differentiation of non-M. tuberculosis bacteria from positive cultures, the Ziehl-Neelsen stain and a commercial TB real-time PCR kit (Qiagen GmBH, Hilden, Germany) were used.
Conventional Ziehl-Neelsen stain.
Two-ml CSF specimens were centrifuged at 3,000 × g for 15 min, and the sediment was smeared on slides as previously described (27). All smears were stained by the conventional Ziehl-Neelsen method for the presence of AFB as depicted earlier (14) and observed under a light microscope.
Modified Ziehl-Neelsen stain.
Cytospin was used to collect the formed elements of CSF specimens. In brief, 0.5-ml CSF specimens were loaded into the chamber in which poly-l-lysine-coated slides were inserted and centrifuged at 1,000 × g for 5 to 10 min. After the media were aspirated, the cells were fixed with 4% paraformaldehyde (pH 7.4) for 10 min at room temperature. The cells on cytospin slides then were permeabilized with 0.3% TritonX-100 for 30 min, followed by conventional Ziehl-Neelsen stain in which acid-fast dye contained 0.3% Triton X-100. The cells were counterstained with methyl blue for 5 min.
Combination of AO stain and immunofluorescence.
The cytospin slides were prepared as described above and then permeabilized with 0.3% Triton X-100 for 30 min. Auramine-O (AO) (Sigma, St. Louis, MO) staining was performed as described by the manufacturer. After AO stain, the cytospin slides were incubated with the following primary antibodies in phosphate-buffered saline (PBS) containing 2% normal donkey serum at 4°C overnight: mouse anti-CD11b (Millipore, Bilerica, MA), mouse anti-ED1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-CD3, and mouse anti-CD20 (MaiXin Biotechnology, China). After a wash with PBS, the cells were treated with biotinylated anti-mouse or anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) for 3 h and then with Cy3-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. The cells were counterstained with Hoechst 33342 (Sigma). Immunofluorescent signals were observed under a laser confocal microscope (LSM 510; Carl Zeiss Microscopy).
Data analysis.
All of the smear and cytospin slides stained by the conventional or modified method were observed under oil immersion at a magnification of ×1,000. A total of 300 visual fields on each slide were observed, among which AFB-positive fields were counted by three experienced observers independently. All of the data were displayed as means ± standard errors of the means (SEM) and analyzed using one-way analysis of variance (ANOVA). Differences were considered statistically significant when P < 0.05.
RESULTS
A total of 48 CSF samples were collected from 29 TBM patients (Table 1). When analyzed by the samples, 35 samples (35/48; 72.92%) were culture positive and 8 (8/48; 16.67%) were Ziehl-Neelsen smear positive, while all of the samples (48/48; 100%) were positive by our modified Ziehl-Neelsen stain. The sensitivity of the Ziehl-Neelsen stain was 22.9% (95% confidence intervals [CI], 8.9 to 36.7) (n = 8/35) and 16.7% (95% CI, 11.29 to 22.05) (n = 8/48) compared to those of culture and the modified Ziehl-Neelsen stain, respectively. The sensitivity of culture was 72.92% (95% CI, 66.51 to 79.33) (n = 35/48) compared to that of the modified Ziehl-Neelsen stain. The specificity of all techniques was 100% (n = 48/48). When analyzed by patients, the sensitivities of culture and the modified Ziehl-Neelsen stain were identical (100%, 29/29), while that of the Ziehl-Neelsen stain was 27.6% (95% CI, 9.29 to 35.89) (n = 8/29) compared to those of culture and the modified Ziehl-Neelsen stain. The specificity of all techniques was 100% (n = 29/29). These data suggest a higher sensitivity of our modified Ziehl-Neelsen stain than those of mycobacterial culture and the conventional Ziehl-Neelsen stain.
Table 1.
Sensitivity of all techniques analyzed by sample (n = 48) and patient (n = 29)a
| Analysis type (n) | % Sensitivity (no. of positive samples) of each technique |
||
|---|---|---|---|
| Culture | ZN stain | Modified ZN stain | |
| Patient (29) | 100 (29) | 27.6 (8) | 100 (29) |
| Sample (48) | 72.9 (35) | 16.7 (8) | 100 (48) |
Forty-eight CSF samples were collected from 29 TBM patients. ZN, Ziehl-Neelsen. The specificity of all techniques in all cases was 100%.
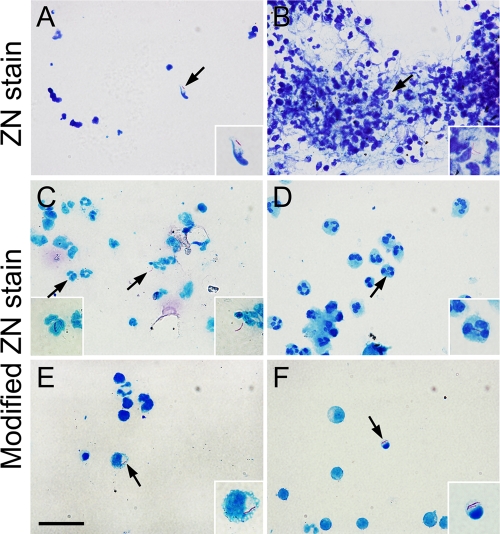
In the conventional method, a relatively large CSF volume (2 ml) was used for smear slides. Ziehl-Neelsen stain showed that the AFB were sparsely distributed and the integrity of immune cells was violated (Fig. 1A). In some regions of smear slides, aggregated cells often were observed in AFB-positive fields (Fig. 1B). We noted that in the conventional method, all of the AFB observed were distributed extracellularly and no AFB were found within the cells. In the modified method, 0.5-ml CSF samples were used for cytospin slides. Ziehl-Neelsen stain with Triton processing showed that the immune cells were distributed evenly on the slides and maintained the integrity of cellular morphology (Fig. 1C to F). Moreover, using this method, we clearly observed AFB within the immune cells, including neutrophils, monocytes, and lymphocytes (Fig. 1D to F). Importantly, we also found that the number of extracellular AFB was significantly increased in the modified method. In addition, we noted that cells containing AFB displayed an abnormal morphology relative to those free of AFB. For example, a larger percentage of neutrophils with more lobes showed an obvious right shift (Fig. 1D, arrow) and monocytes displayed a larger cell body and foam-like cytoplasm (Fig. 1E, arrow), while lymphocytes showed a smaller cell body and nuclear pyknosis (Fig. 1F, arrow). These data suggest that upon and following phagocytosis by the immune cells, intracellular M. tuberculosis also exerts an effect on the immune cells.
Fig 1.
Comparison of the conventional and modified Ziehl-Neelsen (ZN) stain for CSF samples from tuberculous meningitis patients. (A and B) The conventional stain shows damaged cellular structure (A) and cell aggregations (B). No intracellular acid-fast bacilli (AFB) are observed with the conventional method. (C) The modified method can concentrate AFB in the CSF. Intracellular AFB are frequently observed in neutrophils (D), monocytes (E), and lymphocytes (F). Arrows show acid-fast-dye-positive AFB. Insets show higher magnification views of AFB or cells indicated by arrows. Scale bars, 20 (A to F) and 5 μm (insets).
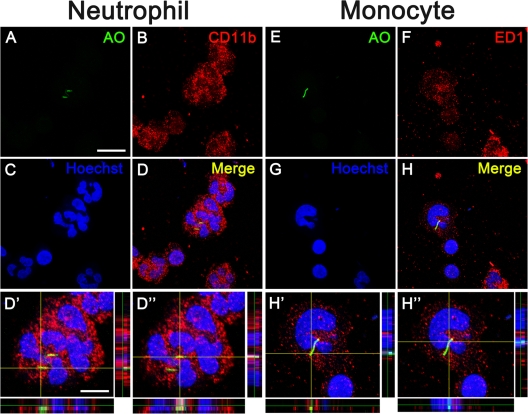
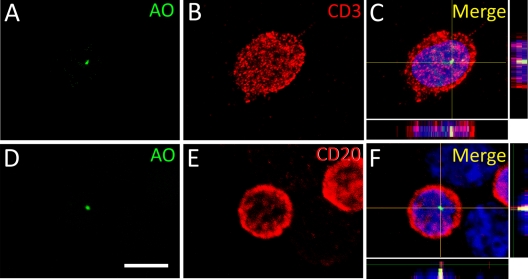
To confirm the intracellular localization of AFB and exclude the possibility that the bacilli inadvertently adhere to the cell surface during cytospinning, we performed the double labeling of AO, a fluorescent dye used for staining tubercle bacilli (28, 31), the activated neutrophil marker CD11b, monocyte marker ED1, and T lymphocyte marker CD3 or B lymphocyte marker CD20. Our results showed that AO+ bacilli were indeed located in the cytoplasm of neutrophils, monocytes, or lymphocytes, but not on the surface of these cells (Fig. 2 and 3).
Fig 2.
Intracellular distribution of AFB in neutrophils and monocytes on the modified Ziehl-Neelsen stain. Double labeling of AO (A and E, green) with CD11b (B, red) and ED1 (F, red) shows the intracellular location of AFB in neutrophils (A to D) and monocytes (E to H). AO, CD11b, and ED1 label AFB, neutrophils, and monocytes, respectively. The nuclei are stained by Hoechst 33342 (blue). Panels D′, D″, H′, and H″ show higher magnification views of panels D and H in z axis projections. Scale bars, 20 (A to H) and 5 μm (D′, D″, H′, and H″).
Fig 3.
Intracellular distribution of AFB in lymphocytes on the modified Ziehl-Neelsen stain. Double labeling of AO (A and D, green) with CD3 (B, red) and CD20 (E, red) shows the intracellular location of AFB in lymphocytes (C and F). The nuclei are stained by Hoechst 33342 (blue). Scale bar, 5 μm.
For the quantitative analysis of extracellular AFB, the conventional method identified extracellular AFB in 8 of 48 CSF specimens (16.7%), while the modified method identified all of the specimens (100%; 48/48). For the quantification of intracellular AFB, the modified method detected intracellular AFB in 45 of 48 CSF specimens (93.8%), while none was detected by the conventional method (Table 2). Furthermore, through the observation of 300 fields on each slide from 48 CSF samples, we found that the number of extracellular and intracellular AFB-positive fields with the conventional method was 20.0 ± 63.1 and 0 fields, respectively, and 73.5 ± 58.0 (P < 0.001) and 24.3 ± 22.0 (P < 0.001) fields with the modified method, respectively (Fig. 4).
Table 2.
Positive rate of intracellular and extracellular ABF in the CSF samples (n = 48) detected by ZN stain and modified ZN stain
| ABF group (n) | % Positive rate (no. of positive samples) of each technique |
|
|---|---|---|
| ZN stain | Modified ZN stain | |
| Extracellular (48) | 16.7 (8) | 100 (48) |
| Intracellular (48) | 0 (0) | 93.8 (45) |
Fig 4.
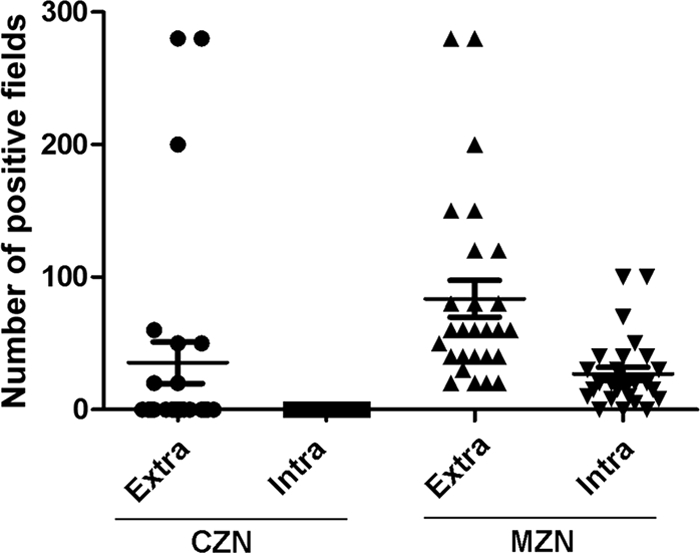
Comparison of AFB-positive fields by the conventional (CZN) and modified (MZN) Ziehl-Neelsen stain. Three hundred fields on each slide from 48 CSF specimens were observed. Compared to the ZN stain, which reveals no intracellular AFB-positive fields, the modified ZN stain definitely identifies AFB within the immune cells. Moreover, the modified stain reveals more extracellular AFB-positive fields than the conventional ZN stain.
DISCUSSION
In the present study, we developed a modified Ziehl-Neelsen method on cytospin slides with Triton processing. Only using small CSF samples, our modified method yielded a 93.8% detection rate of intracellular AFB, while the conventional method failed to detect any intracellular AFB. Also, this method can improve the detection rate of extracellular AFB from 16.7% of that of the conventional Ziehl-Neelsen stain to 100%. Moreover, our method had a higher sensitivity than the mycobacterium culture method and is easier to implement than AO staining, in which expensive fluorescence microscopy is required.
We attributed the higher detection rate of M. tuberculosis by the modified method to two reasons. First, cytospinning was employed, by which AFB within cells were concentrated compactly in a small circular area on the slide with low-speed centrifugation. In contrast to the conventional method, in which the cells are mostly destroyed, our modified method preserves the cellular integrity and consequently prevents the loss of intracellular AFB from inside the cells. In addition, coating the slides with poly-l-lysine further prevents cells and bacilli in the CSF from falling off during staining. Second, we employed Triton X-100, which permeates the cellular membrane and facilitates the entry of acid-fast dye. Thus, the modified method combining cytospin and Triton permeation significantly improves the efficiency of the Ziehl-Neelsen stain. Furthermore, the cytospin method concentrates extracellular AFB in the CSF. Consequently, the CSF specimens from all 48 specimens were positive for extracellular AFB by the modified stain, whereas the conventional method had a low detection rate (16.7%; 8/48). Triton processing also improves the permeability of the unique bacterial wall of AFB (15), which resists staining by acid-fast dyes. Thus, the modified method also improved the detection rate of extracellular AFB.
Accumulating evidence shows that the presence of intracellular M. tuberculosis is a crucial indicator of the body's immune response to tuberculosis (26, 28). Monocytes, neutrophils, and lymphocytes are three major immune cell types in the CSF of TBM, and they play different roles in the pathogenesis of TBM. Monocytes are regarded as the main immune cells for host defense against M. tuberculosis. However, a group of studies have shown that the antigen-presenting function of macrophages is significantly impaired following infection with M. tuberculosis (9, 12, 20, 22). Thus, it is proposed that other immune cells can be recruited to enhance the immune response against M. tuberculosis infection (16). In the present study, we revealed that M. tuberculosis was present in both neutrophils and lymphocytes, indicating that these two types of cells are indeed involved in the host's immune response against M. tuberculosis. Neutrophils have both bactericidal and immunomodulatory functions (3, 13, 25). When infected with M. tuberculosis, neutrophils can directly kill invading M. tuberculosis via the generation of reactive oxygen species and the release of preformed oxidants and proteolytic enzymes, and/or indirectly eliminate M. tuberculosis by releasing an array of cytokines and chemokines to attract other inflammatory cells. On the other hand, infected neutrophils also provide a permissive site for the active replication of M. tuberculosis (6, 8), which in turn induces the apoptosis of neutrophils (1, 2). Macrophages are capable of phagocytizing apoptotic neutrophils, resulting in the eventual elimination of intracellular M. tuberculosis (29). Unexpectedly, our results also showed an intracellular distribution of M. tuberculosis in CD3+ T lymphocytes. Previous studies have shown that dendritic cells, a derivative from the lymphocyte precursor, can phagocytize M. tuberculosis and exert their antigen-presenting functions (11, 23). These findings together prompt us to propose that lymphocytes play a similar role in TBM, compensating for the decreased antigen-presenting function of macrophages. Thus, our present study suggests that in addition to monocytes, neutrophils and lymphocytes also participate in the host defense against M. tuberculosis infection.
Conclusions.
Given the acute need for a simple, efficient, and practical method for detecting M. tuberculosis within the cells and from small CSF samples, our modified Ziehl-Neelsen staining method can efficiently reveal the presence of AFB in the immune cells of small CSF samples from TBM patients and improve the detection rate of extracellular AFB as well. Therefore, this method will be of tremendous value in improving both the diagnosis and treatment of tuberculosis.
ACKNOWLEDGMENTS
We are grateful to Bo-Quan Jin (Department of Immunology, the Fourth Military Medical University) and Zhi-Kai Xu (Department of Microbiology, the Fourth Military Medical University) for valuable comments on the manuscript and Bo Cui (associate editor for Journal of Biomedical Research, Nanjing University) for his laborious work on English correction.
This work was supported by a grant from the Discipline-Boosting Program of Xijing Hospital (no. XJZT10Z03).
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Alemán M, GarcíA A, Saab MA. 2002. Mycobacterium tuberculosis-induced activation accelerates apoptosis in peripheral blood neutrophils from patients with active tuberculosis. Am. J. Respir. Cell Mol. Biol. 27:583–592 [DOI] [PubMed] [Google Scholar]
- 2. Alemán M, et al. 2004. Mycobacterium tuberculosis triggers apoptosis in peripheral neutrophils involving toll-like receptor 2 and p38 mitogen protein kinase in tuberculosis patients. Infect. Immun. 72:5150–5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appelberg R, Silva MT. 1989. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin. Exp. Immunol. 78:478–483 [PMC free article] [PubMed] [Google Scholar]
- 4. Attallah AM, et al. 2003. Rapid and simple detection of a Mycobacterium tuberculosis circulating antigen in serum using dot-ELISA for field diagnosis of pulmonary tuberculosis. J. Immunoassay Immunochem. 24:73–87 [DOI] [PubMed] [Google Scholar]
- 5. Dora JM, et al. 2008. Polymerase chain reaction as a useful and simple tool for rapid diagnosis of tuberculous meningitis in a Brazilian tertiary care hospital. Braz. J. Infect. Dis. 12:245–247 [DOI] [PubMed] [Google Scholar]
- 6. Dorhoi A, Reece ST, Kaufmann SH. 2011. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol. Rev. 240:235–251 [DOI] [PubMed] [Google Scholar]
- 7. El-Masry S, El-Kady I, Zaghloul MH, Al-Badrawey MK. 2008. Rapid and simple detection of a mycobacterium circulating antigen in serum of pulmonary tuberculosis patients by using a monoclonal antibody and Fast-Dot-ELISA. Clin. Biochem. 41:145–151 [DOI] [PubMed] [Google Scholar]
- 8. Eum SY, et al. 2010. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fulton SA, et al. 2004. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 72:2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaspar MM, et al. 2008. Developments on drug delivery systems for the treatment of mycobacterial infections. Curr. Top. Med. Chem. 8:579–591 [DOI] [PubMed] [Google Scholar]
- 11. Hedlund S, et al. 2010. Dendritic cell activation by sensing Mycobacterium tuberculosis-induced apoptotic neutrophils via DC-SIGN. Hum. Immunol. 71:535–540 [DOI] [PubMed] [Google Scholar]
- 12. Jo EK. 2008. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr. Opin. Infect. Dis. 21:279–286 [DOI] [PubMed] [Google Scholar]
- 13. Kasahara K, et al. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127–137 [DOI] [PubMed] [Google Scholar]
- 14. Koch ML, Cote RA. 1965. Comparison of fluorescence microscopy with Ziehl-Neelsen stain for demonstration of acid-fast bacilli in smear preparations and tissue sections. Am. Rev. Respir. Dis. 91:283–284 [DOI] [PubMed] [Google Scholar]
- 15. Koley D, Bard AJ. 2010. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. U. S. A. 107:16783–16787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leveton C, et al. 1989. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect. Immun. 57:390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorino G, et al. 1999. Polymerase chain reaction, with sequencing, as a diagnostic tool in culture-negative bacterial meningitis. Clin. Microbiol. Infect. 5:92–96 [DOI] [PubMed] [Google Scholar]
- 18. Marais S, et al. 2010. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect. Dis. 10:803–812 [DOI] [PubMed] [Google Scholar]
- 19. Mishra A, et al. 2005. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: correlation with conventional techniques. J. Clin. Microbiol. 43:5670–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noss EH, et al. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910–918 [DOI] [PubMed] [Google Scholar]
- 21. Pai M, et al. 2003. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect. Dis. 3:633–643 [DOI] [PubMed] [Google Scholar]
- 22. Pai RK, et al. 2004. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603–6614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pal R, et al. 2010. Generation of self-renewing immature dendritic cells from mouse spleen that can take up mycobacteria and present antigens to T cells. APMIS 118:729–738 [DOI] [PubMed] [Google Scholar]
- 24. Pal RB, Desai MM. 2007. Polymerase chain reaction for the rapid diagnosis of tuberculous meningitis. J. Indian Med. Assoc. 105:21–24 [PubMed] [Google Scholar]
- 25. Petrofsky M, Bermudez LE. 1999. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1 beta and have a putative role in early host response. Clin. Immunol. 91:354–358 [DOI] [PubMed] [Google Scholar]
- 26. Radhakrishnan VV, Sehgal S, Mathai A. 1990. Correlation between culture of Mycobacterium tuberculosis and detection of mycobacterial antigens in cerebrospinal fluid of patients with tuberculous meningitis. J. Med. Microbiol. 33:223–226 [DOI] [PubMed] [Google Scholar]
- 27. Ratnam S, March SB. 1986. Effect of relative centrifugal force and centrifugation time on sedimentation of mycobacteria in clinical specimens. J. Clin. Microbiol. 23:582–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shenai S, et al. 2011. Evaluation of light emitting diode-based fluorescence microscopy for the detection of mycobacteria in a tuberculosis-endemic region. Int. J. Tuberc. Lung Dis. 15:483–488 [DOI] [PubMed] [Google Scholar]
- 29. Tan BH, et al. 2006. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 177:1864–1871 [DOI] [PubMed] [Google Scholar]
- 30. Thwaites GE, Tran TH. 2005. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 4:160–170 [DOI] [PubMed] [Google Scholar]
- 31. Trusov A, et al. 2009. Comparison of Lumin LED fluorescent attachment, fluorescent microscopy and Ziehl-Neelsen for AFB diagnosis. Int. J. Tuberc. Lung Dis. 13:836–841 [PubMed] [Google Scholar]
- 32. Ulrichs T, et al. 2005. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J. Pathol. 205:633–640 [DOI] [PubMed] [Google Scholar]
- 33. Yeager H, Jr, Lacy J, Smith LR, LeMaistre CA. 1967. Quantitative studies of mycobacterial populations in sputum and saliva. Am. Rev. Respir. Dis. 95:998–1004 [DOI] [PubMed] [Google Scholar]