Abstract
Enterobacteriaceae are important pathogens of both nosocomial and community-acquired infections. In particular, strains with broad-spectrum beta-lactamases increasingly cause problems in health care settings. Rapid and reliable typing systems are key tools to identify transmission, so that targeted infection control measures can be taken. In this study, we evaluated the performance of Raman spectroscopic analysis (RA) for the typing of multiresistant Escherichia coli and Klebsiella pneumoniae isolates using the SpectraCell RA bacterial strain analyzer (River Diagnostics). Analysis of 96 unrelated isolates revealed that RA generated highly reproducible spectra and exhibited a discriminatory power that is comparable to pulsed-field gel electrophoresis. Furthermore, adequate results were obtained for three collections of clinical isolates. RA was able to discriminate outbreak-related isolates from isolates that were not involved in an outbreak or transmission. Furthermore, it was found that the RA approach recognized clones, irrespective of the extended-spectrum β-lactamase type. It can be concluded that RA is a suitable typing technique for E. coli and K. pneumoniae isolates. Combining high reproducibility, speed, and ease-of-use, this technique may play an important role in monitoring the epidemiology of these important nosocomial species.
INTRODUCTION
Enterobacteriaceae are important pathogens causing both nosocomial and community-acquired infections (19). The emergence of multi-antibiotic resistance in Enterobacteriaceae is of great concern (7, 18). Resistance to broad-spectrum cephalosporins is typically associated with the acquisition of mobile genetic elements such as plasmids and transposons. Such plasmids contain genes that encode for extended-spectrum β-lactamases (ESBLs) but may also contain other resistance genes as well. For most Enterobacteriaceae, resistance to carbapenems has been uncommon. However, Klebsiella pneumoniae has recently acquired a novel mechanism conferring resistance to carbapenems, known as K. pneumoniae carbapenemase (KPC) β-lactamase (19).
In recent years, the overall number of infections by ESBL-producing Enterobacteriaceae has been increasing (21). Approximately 20% of K. pneumoniae infections in intensive care units in the United States are now caused by isolates that are resistant to broad-spectrum cephalosporins (15). For Escherichia coli, the number of urinary tract infections caused by the virulent CTX-M-15-producing isolates is increasing (8).
The epidemiology of resistance is complex since it is believed that it combines the spread of certain bacterial strains with the independent spread of plasmids. On the other hand, studies show that transmission is associated with the spread of a single successful clone (13, 16, 17, 23). The sources that might lead to transmission are numerous since not only patients with infections but also colonized patients and the environment may serve as reservoirs (1).
In general, standard infection control measures can reduce up to 30 to 40% of nosocomial infections by prevention of transmission (6, 24). These measures may include hand hygiene and respiratory or cough hygiene. When an outbreak is suspected, additional actions such as the isolation of patients should be taken. The success of prevention is higher when additional actions can be taken at the beginning of an outbreak. This requires the early detection of a possible transmission and thus a fast and reliable typing system that can provide information in a real-time setting. Continuous monitoring for the presence of certain (virulent) clones in the hospital or on high-risk wards would facilitate the early detection of transmission.
In recent years, Raman spectroscopic analysis (RA) has been validated for the bacterial typing of different species (12, 25, 26). RA is a label-free, optical technology based on the inelastic scattering of light by molecules. The change in wavelength is molecule-specific and can be displayed in a Raman spectrum. These can be seen as spectroscopic fingerprints and reflect the overall molecular composition of a sample. Since different microorganisms will differ in their molecular makeup, this will be reflected in their spectra, enabling the accurate epidemiological characterization of those microorganisms.
In the present study, a RA-based protocol was developed for the epidemiological typing of E. coli and K. pneumoniae isolates. Using multiple collections of ESBL- or KPC-producing isolates, the value of RA in surveillance and detection of hospital outbreaks was explored.
MATERIALS AND METHODS
Strain collections.
A total of 241 isolates were used in four different subcollections. Isolates were stored at −80°C in glycerol containing brain heart infusion broth (Becton Dickinson, Franklin Lakes, NJ) until further use.
Collection I comprised of 96 isolates and was used for the technical evaluation of RA (reproducibility of RA measurements and assessment of its discriminatory power). This collection consisted of 48 ESBL-positive E. coli isolates and 38 ESBL-positive K. pneumoniae isolates that were obtained during a 3-month surveillance study in Indonesia (10a). Another 10 K. pneumoniae isolates were selected from the collection of clinical isolates of the Department of Medical Microbiology and Infectious Diseases of the Erasmus Medical Center (Rotterdam, Netherlands). All isolates in collection I were obtained from different patients and were classified as genetically unrelated based on pulsed-field gel electrophoresis (PFGE) (10a).
Collection II was used for retrospective outbreak analysis. It contained 38 K. pneumoniae isolates from a previously described outbreak on a surgical ward in Lelystad, Netherlands (5). Based on the PFGE results, 20 SHV-5-producing isolates were involved in the outbreak, whereas 18 isolates from the same hospital and the same time period, showed unique PFGE patterns. These unique isolates were obtained from the same ICU (n = 9) or from other wards in the same hospital (n = 9).
Collection III contained 24 KPC-2-producing K. pneumoniae isolates that were selected from a large surveillance study performed at the Microbiology Department of the National School of Public Health (Athens, Greece) (3). This collection also contained one VIM-1-producing K. pneumoniae isolate. This isolate was not included in the surveillance study but was isolated in a previous period.
The isolates were collected from hospitals all over Greece in an 18-month period. Isolates displaying a similarity of 85% or more in their PFGE profile were considered to belong to the same PFGE type. Isolates were also typed by multilocus sequence typing (MLST) (4). The presence of the blaKPC gene was confirmed by PCR and sequencing (3). This collection was used to evaluate the accuracy of RA to identify possible epidemic spread of a K. pneumoniae strain over a prolonged period and in a larger geographical area.
Collection IV was used to evaluate the accuracy to identify different E. coli clones carrying different ESBL genes isolated at the Erasmus MC in Rotterdam. This collection contained 82 E. coli strains obtained from different patients in 2008. Characterization of the β-lactamase genes was performed by different PCR based methods, and the amplicons were subsequently sequenced (11, 14, 28). All isolates had been previously typed using the repetitive-sequence-based PCR (rep-PCR) DiversiLab microbial typing system (bioMérieux, France) as described by Lau et al. (9).
Raman spectroscopy.
Isolates were grown on Trypticase soy agar (Becton Dickinson, Franklin Lakes, NJ). Culturing and sample preparation were performed as described previously (27). Raman spectra were collected using a SpectraCell RA bacterial strain analyzer (River Diagnostics, Netherlands) according to the manufacturer's instructions.
Data analysis.
Spectrum pretreatment and cluster analysis were performed using the SpectraCell RA software (River Diagnostics). Histogram plots and correlation matrices were created using MATLAB version 7.1 (The MathWorks, USA).
Similarity between spectra.
In the SpectraCell RA software, the similarity between two measured samples is expressed as the squared Pearson correlation coefficient (R2 value).
Reproducibility of RA and discrimination between isolates.
To be able to use RA for bacterial typing, the similarity between spectra of unrelated isolates should be lower than the similarity between spectra obtained from replicate cultures. The distribution of similarities can be visualized in a graph. The overlap between both curves indicates the discriminatory power of RA. The smaller the overlap, the better RA is able to discriminate isolates.
Determination of the similarity threshold and cutoff.
Two different similarity values were used to indicate relatedness between isolates. The similarity threshold (breakpoint at a lower R2 value) is chosen such that 99% of all replicate spectra have an R2 value above this threshold (i.e., 99% of the red curve is positioned above this value). Two isolates with an R2 value below the similarity threshold are considered different by RA and are assigned different RA types. This implies that for 1% of the replicates a misidentification as unrelated is allowed.
The cutoff (breakpoint at a higher R2 value) is set such that 97% of all genetically unrelated isolates show R2 values below this threshold (i.e., 97% of the blue curve is positioned below the cutoff). Two isolates with an R2 value above the cutoff are considered indistinguishable by RA and are assigned the same RA type. This implies that for 3% of the unrelated isolates a misidentification as indistinguishable is allowed. If an R2 value between two isolates is found in the area between the similarity threshold and the cutoff, these isolates are considered to be potentially related.
Correlation matrix.
To analyze spectral relationships between different isolates, a correlation matrix was created. This matrix displays the similarity of each pair of spectra using a color index. The diagonal indicates R2 values of 1, since this represents the similarity of each isolate with itself. The values above the diagonal are the reverse graphic image of the values below this diagonal. In each matrix, red clusters indicate isolates that are indistinguishable based on the previously set cutoff. The gray areas indicate samples that are classified as unrelated based on the previously set similarity threshold. The samples that are potentially related are indicated by yellow to orange.
Spectra were sorted based on similarity. Each horizontal line in the matrix represents all R2 values of an isolate with all other isolates in the matrix. Correlation coefficients were calculated between each group of R2 values. By sorting these correlation coefficients based on height, the isolates with high similarity are grouped together.
RESULTS
Reproducibility of RA and discrimination between unrelated isolates.
The repeatability of RA was determined for E. coli and K. pneumoniae separately using the isolates of collection I. All isolates were measured in triplicate, generating 288 Raman spectra.
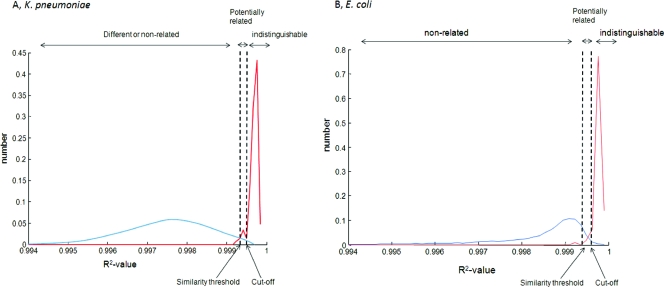
Figure 1 displays the R2 distributions between replicates (in red) and between genotypic unrelated isolates (in blue). For both species, the distribution of R2 values between replicates is narrow and the overlap with the unrelated distribution is low. The similarity threshold was 0.9993 for both species. The calculated cutoffs were 0.9994 for K. pneumoniae and 0.9995 for E. coli.
Fig 1.
Graphical representation of the similarity distributions for K. pneumoniae (A) and E. coli (B). For each species, the distribution of R2 values between replicate measurements (red curve) and the distribution of R2 values between genetically unrelated isolates (blue curves) are displayed. The similarity threshold and cutoff are indicated by the black doted lines. These lines determine the classification of isolates in three categories of relatedness; unrelated, potentially related, and indistinguishable.
For the replicate measurements of the K. pneumoniae isolates, it was found that 95% was correctly indicated as indistinguishable, while for E. coli this percentage was 97%. None of the replicate measurements were incorrectly classified as unrelated. For analysis of the other isolate collections, the cutoffs and thresholds determined here will be used.
To evaluate the discriminatory power of RA, the ability to distinguish unrelated isolates was tested. The RA results of collection I show that for E. coli two isolates were identified as potentially related, while two K. pneumoniae isolates were found to be indistinguishable. All isolates of this collection were previously found to be unrelated according to the gold standard, PFGE. Compared to this gold standard, this means that for both species 46 isolates were classified correctly as unique (96% true negatives), while 2 isolates gave a false-positive result (4%).
Retrospective K. pneumoniae outbreak analysis.
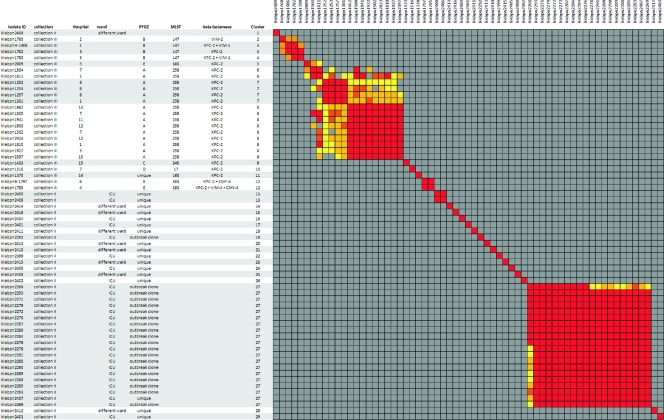
During a previously described outbreak in the Netherlands, multiple patients suffered from an infection or colonization with a K. pneumoniae strain carrying blaSHV-5 (5). Several isolates obtained during this outbreak were included for retrospective analysis with RA (collection II). The correlation matrix based on RA for these isolates is displayed in Fig. 2. Based on PFGE, it was concluded that 20 of these 38 isolates were assigned the same PFGE profile and thus involved in the outbreak (isolates indicated as an “outbreak clone”).
Fig 2.
Similarity matrix of the K. pneumoniae isolates from collection II and collection III. Red clusters indicate isolates that are indistinguishable based on the cutoff. The gray areas indicate isolates that are unrelated based on the similarity threshold. The isolates that are potentially related are indicated by yellow to orange. The indicated cluster numbers represent the clusters with indistinguishable isolates based on the cutoff.
For the 20 isolates with PFGE type A, RA assigned 19 isolates as identical (RA cluster 27). This indicates that RA was able to adequately recognize the outbreak. The remaining isolate with the same PFGE profile was assigned a unique RA type and thus excluded from the outbreak.
The isolates that were not involved in the outbreak according to PFGE (18 isolates indicated as “unique”) were divided into 17 RA types. Fifteen isolates were assigned a unique RA type; two isolates shared the same RA type. One unique isolate was assigned the RA outbreak type.
KPC-2-producing K. pneumoniae infections in Greek hospitals.
In Fig. 2 the RA correlation matrix of collection III is shown. In this figure the isolate numbers, hospital, PFGE type, and MLST are indicated. For this collection the results obtained using MLST are in complete concordance with the PFGE type (e.g., PFGE profiles with a similarity of 85% or more). Overall, the RA results show a slightly higher discriminatory power compared to PFGE and MLST. No RA related clusters were found that contained multiple PFGE types.
For the three PFGE types that hold multiple isolates (PFGE types A, B, and E) multiple RA clusters were found. It was also found that the RA clusters that contained isolates with an identical PFGE or MLST type were potentially related. The RA types 9, 10, and 11 were isolate specific and corresponded to distinct PFGE and MLST types. Furthermore, it was observed that for all three methods clusters were found that contained two or more isolates that differed in β-lactamase content.
Prevalence of ESBL-positive E. coli strains in a tertiary care center in the Netherlands.
In 2008, 82 ESBL-positive E. coli isolates were found at the Erasmus MC (collection IV). Most of the isolates were obtained from urine samples (52%) or wound swabs (13%).
Characterization of the β-lactamase genes revealed that nine different β-lactamase genes were found. For 77 (90%) of the isolates, the presence of a CTX-M gene was determined. Resistance genes blaCTX-M-15 (47%), blaCTX-M-61 (25%), and blaCTX-M-14 (14%) were the most frequently observed genes.
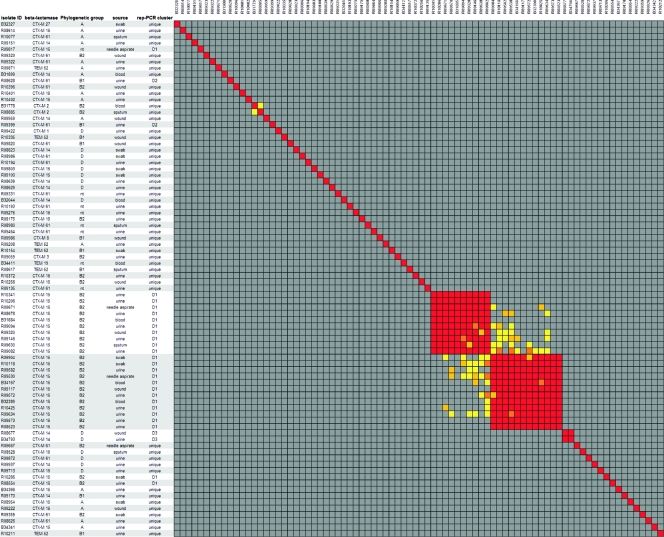
The results of the RA analysis and rep-PCR analysis are summarized in Fig. 3. In this figure, the indication of rep-PCR clusters (D1-D2-D3) is based on a similarity between profiles of at least 95%.
Fig 3.
Similarity matrix of the E. coli isolates from collection IV. Red clusters indicate isolates that are indistinguishable based on the cutoff. The gray areas indicate isolates that are unrelated based on the similarity threshold. The isolates that are potentially related are indicated by yellow to orange. The indicated cluster numbers represent the clusters with indistinguishable isolates based on the cutoff.
The 82 isolates were divided into 61 RA clusters and 56 rep-PCR clusters. RA found 58 isolates to be unrelated, while rep-PCR assigned 53 isolates a unique type. This demonstrates a large variance and thus lack of clonality between E. coli isolates. Furthermore, it indicates that clustering of isolates is independent of the present of a certain β-lactamase gene.
Among the CTX-M-15-containing isolates, several indistinguishable isolates were found. The 41 isolates harboring this ESBL gene were divided into 21 different RA types and 17 different rep-PCR types. In total, 22 isolates were found in two large RA clusters: RA cluster 44 (n = 10) and RA cluster 45 (n = 12). These two clusters were related, as indicated in Fig. 3 by the yellow/orange blocks connecting both clusters. Furthermore, all 22 isolates belonged to the same rep-PCR type.
DISCUSSION
In this study, a total of 130 E. coli and 111 K. pneumoniae isolates were used to evaluate the performance of RA as a bacterial typing tool. This procedure resulted in highly reproducible spectra for the two species. Based on the triplicate measurements of the isolates of collection I, two species-specific similarity thresholds and cutoffs were determined and applied to the clinical isolate sets. Based on these values, it was found that 96% of the isolates were in concordance with the gold standard, PFGE. No epidemiological link was found between the two E. coli isolates and the two K. pneumoniae isolates that gave a false-positive result.
Besides the ability to discriminate between isolates, it is also important that a typing system is capable of recognizing outbreak related isolates as clonally related. Therefore, a retrospective evaluation was performed on SHV-5-positive K. pneumoniae isolates obtained during a well-characterized outbreak (5). For this collection, a good concordance was found with PFGE. RA recognized 19 of 20 isolates of the outbreak clone (sensitivity of 95%) and classified 15 of 18 unrelated isolates as unique (specificity of 83%).
When we analyzed a collection of KPC-2-positive K. pneumoniae strains, RA showed a higher discriminatory power than PFGE (based on 85% similarity) and MLST. However, the RA clusters containing multiple isolates sharing the same PFGE type were found to be potentially related. This implies that, although differences were found, RA is still able to identify the epidemic spread of a certain PFGE clone over a prolonged period of time (18 months).
The final isolate collection used harbored 82 ESBL-positive E. coli isolates which were obtained in 2008 at the Erasmus MC. Characterization of the bla genes revealed that half of the isolates are blaCTX-M-15 positive. This finding demonstrates the emergence of this ESBL type in the Netherlands and confirms previous studies that reported the emergence of this gene worldwide (1, 2, 10, 20).
RA seems to lack association with antibiotic resistance profiles, since a high percentage of unique types were found within a group of strains harboring the same ESBL type. This was also observed in some cases in the K. pneumoniae collection from Greece. In both collections, this finding was confirmed by the other typing methods used.
On the other hand, RA revealed two related clusters, harboring a total of 22 isolates. All of these isolates possessed CTX-M-15 combined with high resistance rates to non-β-lactam antibiotics. Furthermore, these isolates belonged to phylogenetic type B2 and had the same rep-PCR type. This shows the ability of RA to identify the presence of a predominant clone, suggesting a common source.
It can be concluded that adequate results were obtained for the clinical K. pneumoniae and E. coli isolates studied in more detail, using a defined similarity threshold and cutoff. RA showed a discriminatory power that is comparable to that of PFGE and was able to identify a defined outbreak and the presence of certain clones. The importance of monitoring the epidemiology of E. coli and K. pneumoniae is evident in the light of the rapid evolution of antimicrobial resistance in these species. Combining high reproducibility, speed, and ease-of-use, RA may play an important role in detecting the pandemic spread of the most relevant clonal strains.
ACKNOWLEDGMENTS
We thank Alkiviadis Vatopoulos, Department of Microbiology, National School of Public Health (Athens, Greece), for his helpful comments and review of the manuscript.
We also thank P. Gruteke, Onze Lieve Vrouwe Gasthuis (Amsterdam, Netherlands), for providing the collection II isolates.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Cantón R, et al. 2008. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14(Suppl 1):144–153 [DOI] [PubMed] [Google Scholar]
- 2. Coque TM, et al. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giakkoupi P, et al. 2009. KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Euro Surveill. 14:268–272 [DOI] [PubMed] [Google Scholar]
- 4. Giakkoupi P, et al. 2011. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009–10). J. Antimicrob. Chemother. 66:1510–1513 [DOI] [PubMed] [Google Scholar]
- 5. Gruteke P, et al. 2003. Patterns of resistance associated with integrons, the extended-spectrum beta-lactamase SHV-5 gene, and a multidrug efflux pump of Klebsiella pneumoniae causing a nosocomial outbreak. J. Clin. Microbiol. 41:1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harbarth S, Sax H, Gastmeier P. 2003. The preventable proportion of nosocomial infections: an overview of published reports. J. Hosp. Infect. 54:258–266 [DOI] [PubMed] [Google Scholar]
- 7. Henshke-Bar-Meir R, et al. 2006. Assessment of the clinical significance of production of extended-spectrum beta-lactamases (ESBL) by Enterobacteriaceae. Infection 34:66–74 [DOI] [PubMed] [Google Scholar]
- 8. Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21:26–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lau SH, et al. 2010. Rapid identification of uropathogenic Escherichia coli of the O25:H4-ST131 clonal lineage using the DiversiLab repetitive sequence-based PCR system. Clin. Microbiol. Infect. 16:232–237 [DOI] [PubMed] [Google Scholar]
- 10. Lau SH, et al. 2008. UK epidemic Escherichia coli strains A-E, with CTX-M-15 beta-lactamase, all belong to the international O25:H4-ST131 clone. J. Antimicrob. Chemother. 62:1241–1244 [DOI] [PubMed] [Google Scholar]
- 10a. Lestari ES, Severin JA. 2009. Antimicrobial resistance in Indonesia: prevalence, determinants, and genetic basis, p 220–234 Ph.D. thesis. Erasmus University, Rotterdam, Netherlands [Google Scholar]
- 11. Mabilat C, Goussard S. 1993. PCR detection and identification of genes for extended-spectrum β-lactamases, p 553–559 In Persing DH, Smith TF, Tenover FC, White TJ. (ed), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Maquelin K, Dijkshoorn L, van der Reijden TJ, Puppels GJ. 2006. Rapid epidemiological analysis of Acinetobacter strains by Raman spectroscopy. J. Microbiol. Methods 64:126–131 [DOI] [PubMed] [Google Scholar]
- 13. Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 14. Nuesch-Inderbinen MT, Hachler H, Kayser FH. 1996. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with the E test. Eur. J. Clin. Microbiol. Infect. Dis. 15:398–402 [DOI] [PubMed] [Google Scholar]
- 15. Paterson DL. 2006. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 34:S20–S28; S64–S73 [DOI] [PubMed] [Google Scholar]
- 16. Peirano G, Costello M, Pitout JD. Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX-M-15 in community hospitals. Int. J. Antimicrob. Agents 36:19–23 [DOI] [PubMed] [Google Scholar]
- 17. Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 35:316–321 [DOI] [PubMed] [Google Scholar]
- 18. Pitout J D. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 70:313–333 [DOI] [PubMed] [Google Scholar]
- 19. Pitout JD. 2008. Multiresistant Enterobacteriaceae: new threat of an old problem. Expert Rev. Anti-Infect. Ther. 6:657–669 [DOI] [PubMed] [Google Scholar]
- 20. Pitout JD, et al. 2009. Using a commercial DiversiLab semiautomated repetitive sequence-based PCR typing technique for identification of Escherichia coli clone ST131 producing CTX-M-15. J. Clin. Microbiol. 47:1212–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitout JD, Nordmann P, Laupland KB, Poirel L. 2005. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J. Antimicrob. Chemother. 56:52–59 [DOI] [PubMed] [Google Scholar]
- 22. Reference deleted.
- 23. Warren RE, Harvey G, Carr R, Ward D, Doroshenko A. 2008. Control of infections due to extended-spectrum beta-lactamase-producing organisms in hospitals and the community. Clin. Microbiol. Infect. 14(Suppl 1):124–133 [DOI] [PubMed] [Google Scholar]
- 24. Weist K, Pollege K, Schulz I, Ruden H, Gastmeier P. 2002. How many nosocomial infections are associated with cross-transmission? A prospective cohort study in a surgical intensive care unit. Infect. Control Hosp. Epidemiol. 23:127–132 [DOI] [PubMed] [Google Scholar]
- 25. Willemse-Erix DF, et al. Towards Raman-based epidemiological typing of Pseudomonas aeruginosa. J. Biophotonics 3:506–511 [DOI] [PubMed] [Google Scholar]
- 26. Willemse-Erix DF, et al. 2009. Optical fingerprinting in bacterial epidemiology: Raman spectroscopy as a real-time typing method. J. Clin. Microbiol. 47:652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willemse-Erix HF, et al. Proof of principle for successful characterization of methicillin-resistant coagulase-negative staphylococci isolated from skin by use of Raman spectroscopy and pulsed-field gel electrophoresis. J. Clin. Microbiol. 48:736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J. Antimicrob. Chemother. 57:154–155 [DOI] [PubMed] [Google Scholar]