Abstract
Spondylodiscitis caused by Campylobacter species is a rare disease which is most often caused by Campylobacter fetus. We report a case of culture-negative spondylodiscitis and a psoas abscess due to Campylobacter jejuni in a 68-year-old woman, as revealed by 16S rRNA gene and Campylobacter-specific PCRs from biopsied tissue.
CASE REPORT
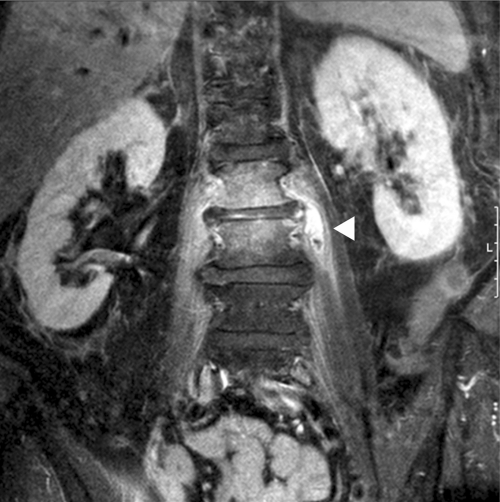
In April 2011, a 68-year-old Caucasian woman was admitted to the hospital after the general practitioner had found her in a somnolent state with hypoglycemia. Confusion persisted despite administration of intravenous (i.v.) glucose. The woman had been suffering from acute-onset left-sided lumboischialgia (L1) for about 2 weeks, and she had received 4 mg dexamethasone with 100 mg diclofenac intramuscularly. Persisting lumbar pain had led to the consultation of an orthopedist who had given her a facet joint infiltration 10 days later. On admission, the patient was in a state of confusion and unable to stand because of severe lower back and left hip pain. Her body temperature was 36.8°C, her pulse was 78 beats/min, and her blood pressure was 170/85 mmHg. There were no focal neurological deficits, and a cranial computed tomography (CT) was normal. Laboratory investigation revealed pathological values for C-reactive protein (90.3 mg/liter; normal values, ≤5 mg/liter) and fibrinogen (786 mg/dl; normal, 200 to 400 mg/dl) levels, an elevated leukocyte count (20,400/μl, predominantly neutrophil granulocytes), a low hemoglobin concentration (113 g/liter; normal, 123 to 153 g/liter), and an elevated platelet count (572,000/μl). A serum electrophoresis result was normal. The patient's past medical history included diabetes with polyneuropathy, hypertension, restless leg syndrome, hyperthyroidism (at admission, euthyroid), left- and right-sided coxarthrosis, and right-sided cervicobrachialgia. She had not traveled abroad. After 5 days, confusion almost disappeared. A CT of the lumbar spine was performed, showing irregularities of the base plate of the second lumbar vertebral body. Magnetic resonance imaging (MRI) revealed extensive spondylodiscitis with a left-sided abscess in the psoas muscle (Figure 1). The patient underwent a CT-guided fine-needle biopsy of the lumbar spine. At this time, the C-reactive protein level was 87.1 mg/liter and the leukocyte count showed 11,700 cells/μl.
Fig 1.
Magnetic resonance imaging of the patient's spine. Signs of spondylodiscitis between lumbar bodies 2 and 3, with a reduction of lumbar disc height and diffuse enhancement caused by inflammation, are visible. Abscess formation (arrow) in the left psoas muscle with surrounding inflammation is shown. Coronary view, fat-suppressed T1 image after gadolinium application.
Aerobic and anaerobic bacterial culture of the biopsy material yielded no growth after 2 days of incubation, and 16S rRNA gene PCR was performed from stored material. Sequencing of the amplicon and a database comparison (NCBI BLAST, Ribosomal Database Project, and green genes database) revealed 100% identity with both Campylobacter coli and Campylobacter jejuni. As repeated 16S PCR analysis failed to discriminate between the two species, additional specific PCRs for C. jejuni and C. coli (4) were performed. The mapA gene of C. jejuni was successfully amplified from the biopsy material, whereas the ceuE gene of C. coli was not detected by PCR. Sequencing of the amplicon from the mapA-specific PCR revealed 99% identity (523 nucleotides [nt]/526 nt) with C. jejuni (GenBank accession number X80135.1). Bacterial cultures under aerobic, anaerobic, and additional microaerophilic conditions remained sterile after a prolonged incubation time of 10 days, and repeated blood cultures and stool cultures for Campylobacter spp. were negative. Campylobacteriosis serology was performed, and a Western blot analysis (recomLine Campylobacter IgG and IgA; Mikrogen, Neuried, Germany) of the patient's serum was positive, showing strong bands for PEB4, OMP18, and P39 in the IgG blot. IgA blotting revealed the presence of a P39 band. Mycobacterial cultures did not yield growth after 3 weeks. The patient was treated with a combination of ciprofloxacin (400 mg twice a day [b.i.d.] i.v. for 1 week) and meropenem (1 g three times a day [t.i.d.] for 4 weeks). At the end of antimicrobial treatment, laboratory investigations showed a C-reactive protein level of 1.6 mg/liter and normal leukocyte and platelet counts. Unfortunately, the patient did not return for follow-up examinations.
The species most often isolated from rare Campylobacter-related spondylodiscitis is Campylobacter fetus (1, 3, 8, 9, 12, 13), possibly because C. fetus tends to cause invasive disease more frequently than C. jejuni and C. coli. To the best of our knowledge, only one case each of spondylodiscitis due to C. jejuni (5) and C. coli (6) has been reported. As 16S rRNA gene PCR analysis could not discriminate between C. jejuni and C. coli in our case, we conducted specific PCRs for each species (4). The mapA gene of C. jejuni was successfully amplified from the biopsy material, thus proving C. jejuni as the cause of the spondylodiscitis in the case of the 68-year-old woman presented here. The range of the 16S rRNA gene PCR also encompasses mycobacteria and Brucella spp., organisms which are also known to cause spondylodiscitis. These bacteria were not detected molecularly or by culture in the case presented. Moreover, the acute extraintestinal campylobacteriosis was also reflected by positive IgA serology. The patient did not recall diarrheic symptoms or fever. However, there was leukocytosis and confusion on admission, as well as occasional loose stools, which were attributed to the numerous drugs the patient received for her medical conditions. Although the patient did not receive antibiotic treatment prior to the tissue biopsy, bacterial cultures remained sterile for an extended incubation period. Specific Campylobacter cultures under microaerophilic conditions were set up from cool stored remains of the biopsy material only after the 16S PCR was positive, which was possibly too late. We assume that the spondylodiscitis and the psoas abscess were due to a previous bacteremia following a short asymptomatic (or oligosymptomatic) gastrointestinal infection. Campylobacter bacteremia has been found to have an incidence of 0.1% to 1% in relation to enteritis (5), and patients aged 65 or higher had an incidence which was 3 times higher than that in younger age groups (10). In line with other reports about Campylobacter spondylodiscitis, our patient was over 60 years of age (1, 3, 6, 12, 13) and had an underlying medical condition (8, 12). However, cases without explicit predisposing conditions have also been reported in the elderly (3, 6, 13), and Campylobacter bacteremia studies showed inconsistent results regarding patient age and underlying diseases (5). As also seen in our case, spondylodiscitis due to Campylobacter spp. is often accompanied by nearby abscesses, such as in the psoas muscle (3, 6), near the vertebral column (1, 6), or near the dura mater (12, 13). One case of concomitant pyogenic meningoencephalitis has also been described (9).
Erythromycin and fluoroquinolones are often recommended for the treatment of systemic campylobacteriosis (7). No susceptibility testing could be performed in the case presented, as no viable isolate could be cultured. The patient was primarily treated with meropenem because a recent retrospective analysis of Campylobacter stool isolates from our hospital had revealed high rates of intermediate susceptibility to erythromycin (51%) and resistance to ciprofloxacin (52%) and doxycycline (38%) (11). The ciprofloxacin and tetracycline resistance rates which had been detected in the survey approximated the resistance data of Campylobacter spp. from animal reservoirs in Germany (2). The antibacterial treatment reported by others consisted of cefotaxime followed by ciprofloxacin (6, 12), ofloxacin plus rifampin, which was later changed to amoxicillin (3), or doxycycline and erythromycin (13) and was combined with surgery in almost all patients. There are no international recommendations for the treatment of lumbar spondylodiscitis (3). The outcome of Campylobacter spondylodiscitis after treatment was favorable in most cases, except two cases caused by C. fetus in the elderly in which death occurred (1, 12).
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Bachmeyer C, Grateau G, Sereni D, Cremer GA. 1992. Campylobacter fetus spondylodiscitis. Rev. Rhum. Mal. Osteoartic. 59:77–79 (In French.) [PubMed] [Google Scholar]
- 2. Bundesinstitut für Riskobewertung 2010. Wissenschaftliche Bewertung der Ergebnisse des Resistenzmonitorings nach dem Zoonosen-Stichprobenplan 2009. Stellungnahme Nr. 047/2010 des BfR, vom 1, November 2010. (In German.) [Google Scholar]
- 3. Chaillon A, et al. 2010. Campylobacter fetus subspecies fetus spondylodiscitis. J. Med. Microbiol. 59:1505–1508 [DOI] [PubMed] [Google Scholar]
- 4. Denis M, et al. 1999. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol. 29:406–410 [DOI] [PubMed] [Google Scholar]
- 5. Feodoroff B, Lauhio A, Ellström P, Rautelin H. 2011. A nationwide study of Campylobacter jejuni and Campylobacter coli bacteremia in Finland over a 10-year period, 1998-2007, with special reference to clinical characteristics and antimicrobial susceptibility. Clin. Infect. Dis. 53:e99–e106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemaire X, et al. 2010. Spondylodiscitis and an aortic aneurysm due to Campylobacter coli. Ann. Clin. Microbiol. Antimicrob. 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luber P, Wagner J, Hahn H, Bartelt E. 2003. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli strains isolated in 1991 and 2001-2002 from poultry and humans in Berlin, Germany. Antimicrob. Agents Chemother. 47:3825–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathieu E, Koeger AC, Rozenberg S, Bourgeois P. 1991. Campylobacter spondylodiscitis and deficiency of cellular immunity. J. Rheumatol. 18:1929–1931 [PubMed] [Google Scholar]
- 9. Ozeki T, Nokura K, Koga H, Yamamoto H. 2002. A case of meningoencephalitis and spondylodiscitis caused by Campylobacter fetus subsp. fetus infection. Rinsho Shinkeigaku 42:38–41 (In Japanese.) [PubMed] [Google Scholar]
- 10. Skirrow MB, Jones DM, Sutcliffe E, Benjamin J. 1993. Campylobacter bacteraemia in England and Wales, 1981-91. Epidemiol. Infect. 10:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valenza G, Frosch M, Abele-Horn M. 2010. Antimicrobial susceptibility of clinical Campylobacter isolates collected at a German university hospital during the period 2006-2008. Scand. J. Infect. Dis. 42:57–60 [DOI] [PubMed] [Google Scholar]
- 12. Wong JS, Anderson TP, Chambers ST, On SL, Murdoch DR. 2009. Campylobacter fetus-associated epidural abscess and bacteremia. J. Clin. Microbiol. 47:857–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamashita K, Aoki Y, Hiroshima K. 1999. Pyogenic vertebral osteomyelitis caused by Campylobacter fetus subspecies fetus. A case report. Spine 24:582–584 [DOI] [PubMed] [Google Scholar]