Abstract
The presence of heteroresistance was represented by 23% of 235 fluoroquinolone (FQ)-resistant Mycobacterium tuberculosis isolates in Beijing, China, from 2008 to 2010. The main mechanism of FQ heteroresistance is due to the segregation of a single M. tuberculosis strain in patients; the majority of isolates with multidrug-resistant tuberculosis contained a mixture of bacterial subpopulations consisting of various mutant types, suggesting that the improper use of FQ is the major cause of FQ resistance.
TEXT
Drug-resistant Mycobacterium tuberculosis is prevalent worldwide and has become a major threat to tuberculosis (TB) control programs and treatment of TB in many countries. As shown in the latest data, multidrug-resistant tuberculosis (MDR-TB), which is resistant to isoniazid (INH) and rifampin (RIF), was found in 3.3% of new cases and 21% of recurrent TB cases globally. Additionally, extensively drug-resistant tuberculosis (XDR-TB) occurred in 58 countries worldwide, suggesting that first-line drugs and at least two types of second-line drugs cannot be used for TB treatment (19). The majority of these drug-resistant strains acquired drug resistance due to improper drug use; thus, identification of drug-resistant strains can aid in the use of more-effective treatments and reduce the occurrence of drug resistance.
In some patients, M. tuberculosis strains are composed of a mixture of susceptible and resistant subpopulations isolated from clinical specimens, so-called heteroresistant strains (14). Heteroresistance is considered a preliminary stage to full resistance and provides bacteria with an opportunity to explore the possibility of growth in the presence of antibiotics (12). This phenomenon is not rare in M. tuberculosis and has been identified in strains with resistance to isoniazid, rifampin, ethambutol (EMB), and streptomycin (1, 3, 9). The clinical significance of heteroresistance is unclear, but it is still an obstacle to both molecular drug resistance testing and successful therapy (7).
Fluoroquinolone (FQ) is an important antituberculosis drug, especially for MDR-TB patients. In the new classification of anti-TB drugs proposed by the World Health Organization, FQ is categorized in the third of five groups of anti-TB drugs based on efficacy. FQ is also a major component in regimens for the treatment of MDR-TB (20). However, the occurrence of FQ resistance is usually high among patients with MDR-TB; for example, it has been observed in 25 to 48% of MDR-TB cases in certain regions of China (6, 21). While heteroresistance to FQ has been observed occasionally by the molecular detection of FQ-resistant isolates in previous studies (2, 4), a more systemic analysis is needed.
To estimate the occurrence of this phenomenon in China, the frequency and composition of heteroresistant isolates in 235 clinical isolates were analyzed. These 235 isolates were obtained from patients with pulmonary tuberculosis in Beijing, China, between 2008 and 2010. These isolates showed phenotypic FQ resistance and resistance to other major anti-TB drugs, including both first-line and second-line drugs. Drug testing was performed on Lowenstein-Jensen (LJ) medium by the absolute concentration method (11). FQ resistance was identified by isolates growing on LJ slants with 5 μg/ml ofloxacin and 2 μg/ml levofloxacin. The higher criteria of FQ resistance applied in this study limited the range of FQ-resistant isolates caused mainly by mutation of the drug-resistant gene rather than the efflux pump effect, which leads to low-level drug resistance (17).
Resistance to FQ typically results from mutations in the gyrA and gyrB DNA gyrase genes. The predominant mutations observed in clinical isolates occurred on codons 90, 91, and 94, which are located in the quinolone resistance-determining region (QRDR) of gyrA (5, 8, 10, 15). Thus, genotypic FQ resistance testing was performed by QRDR sequence analysis of gyrA in this study. Primary cultures of each isolate were collected from Lowenstein-Jensen slants. The extraction of genomic DNA and detection of mutations responsible for resistance to FQ were performed by PCR amplification and sequencing of the gyrA gene as previously described (18).
In addition to the 58 isolates identified as wild-type QRDR, the majority (75.3% [177/235]) contained a mutant QRDR type. There were 123 isolates with a clear analysis pattern (regarded as homoresistant) with a single mutation occurring on codon 88, 90, 91, or 94 of gyrA. The first three predominant mutant genotypes (i.e., D94G, A90V, and D94N) were detected from 43.9% (54/123), 28.5% (35/123), and 9.0% (11/123) of the isolates, respectively. The remaining 54 isolates (23%) (n = 235) were suspected to be heteroresistant isolates, as sequencing revealed a mixture of multiple QRDR-type strains. All detected isolates carried the S95T polymorphism, which is not thought to be involved in FQ resistance.
The QRDR region of suspected heteroresistant isolates was amplified and cloned into the pGEM-T Easy system (Promega, Madison, WI), and the templates were sequenced using T7 and SP6 commercial primers. QRDR genotypes from 12 clones of each isolate were analyzed in parallel. For those isolates containing more than two QRDR genotypes in gyrA, further verification was performed by QRDR genotype analysis of 30 single clones of each isolate. These single clones were acquired from subcultures of each isolate on Lowenstein-Jensen slants, and the compositions of QRDR genotypes from these isolates were determined by sequencing analysis.
These 54 isolates were demonstrated to be heteroresistant to FQ and divided into two groups based on the different genotypes within each isolate. One group of isolates (28 isolates) was composed of wild-type and mutant QRDR-type subpopulations (group I), of which 21 isolates possessed strains with both wild-type and mutant QRDR genotypes. Additionally, six isolates contained wild-type and two mutant QRDR genotypes with different missense mutations on codon 94. Only one isolate was found to be a mixture of wild-type and three mutant QRDR genotypes. Group II contained 26 isolates with a mixture of different QRDR types, of which 20 isolates were found to contain two mutant QRDR genotypes. A mixture of three or more mutant genotypes occurred in the remaining isolates.
Among these heteroresistant isolates, substitutions in codons 90 and 94 were the predominant mutations related to FQ resistance. D94G, D94N, and A90V were the three most prevalent mutant genotypes, but their frequencies were inconsistent with those in homoresistant isolates. D94G emerged with the highest frequency among the three groups of isolates: group I, group II, and homoresistant isolates. However, the prevalent mutant genotypes among these groups (D94G D94Y D94N, D94N D94G A90V, and D94G A90V D94N, respectively) were slightly different. It was noticed that A90V emerged occasionally in group I, as detected in the three isolates (Table 1). However, A90V became prevalent between both group II and the homoresistant group. Changes in these predominant mutants reflect the development of drug resistance in the course of TB treatment with FQ.
Table 1.
Molecular characteristics and drug susceptibility patterns of heteroresistant isolates
| Group | QRDR typea | MIRU-VNTR typeb | Combined resistance profile determined by DSTc | No. of isolates with drug susceptibility pattern |
|---|---|---|---|---|
| Group I isolates | ||||
| W; D94A | 1 | INH, RIF, STR | 1 | |
| W; D94A | M | INH, RIF, STR, PAS | 1 | |
| W; D94A | M | INH, RIF | 1 | |
| W; D94G | 1 | INH, RIF, STR, KAN, AMI, PAS | 1 | |
| W; D94G | 1 | INH, STR | 1 | |
| W; D94G | M | RIF | 1 | |
| W; D94G | 1 | 2 | ||
| W; D94G | 1 | RIF, AMI | 1 | |
| W; D94G | M | INH, RIF, STR, KAN, AMI, PAS | 2 | |
| W; D94G | 1 | STR | 1 | |
| W; D94G | 1 | INH, RIF, PAS | 1 | |
| W; D94G | M | INH, RIF, STR, KAN, AMI | 1 | |
| W; D94N | 1 | RIF, STR, KAN, AMI, PAS | 1 | |
| W; D94N | 1 | INH, RIF, STR, KAN, AMI, CAP, PAS | 1 | |
| W; D94N | 1 | INH, RIF, STR | 1 | |
| W; D94Y | 1 | 1 | ||
| W; D94Y | M | 1 | ||
| W; D94Y | 1 | STR, AMI | 1 | |
| W; D94Y | 1 | INH, RIF, KAN, CAP | 1 | |
| W; A90V; D94N | 1 | 1 | ||
| W; A90V; D94Y | 1 | 1 | ||
| W; D94G; D94Y | 1 | INH, STR | 1 | |
| W; D94G; D94Y | 1 | EMB | 1 | |
| W; D94N; D94G | 1 | INH, RIF, STR, KAN, AMI, PAS | 1 | |
| W; S91P; D94A | 1 | INH, RIF, EMB, STR | 1 | |
| W; A90V; S91P; D94G | 1 | INH, RIF, STR, KAN, AMI | 1 | |
| Group II isolates | ||||
| A90V; D94A | M | INH, RIF, STR, KAN, AMI | 1 | |
| A90V; D94G | 1 | 1 | ||
| A90V; D94G | 1 | PAS | 1 | |
| A90V; D94G | 1 | INH, RIF, STR | 1 | |
| A90V; D94H | 1 | INH, RIF, STR, KAN, AMI, PAS | 1 | |
| A90V; D94N | 1 | INH, STR, AMI | 1 | |
| A90V; D94N | 1 | INH, RIF, STR | 1 | |
| D94G; D94Y | 1 | INH, RIF, STR, KAN, AMI | 1 | |
| D94H; D94G | 1 | INH, RIF, STR | 1 | |
| D94N; D94A | 1 | INH, RIF, KAN, AMI | 1 | |
| D94N; D94G | 1 | INH, RIF, STR | 1 | |
| D94N; D94G | 1 | INH, RIF | 1 | |
| D94N; D94G | 1 | 1 | ||
| D94N; D94G | 1 | INH, RIF, STR, KAN, AMI, CAP | 1 | |
| D94N; D94Y | 1 | INH, RIF, STR, KAN, AMI, CAP | 1 | |
| S91P; D94N | 1 | INH, RIF, STR | 1 | |
| A90V; A90V+D94G | M | INH, STR, KAN, PAS | 1 | |
| A90V; D94N; D94Y | 1 | INH, RIF, STR | 1 | |
| A90V; S91P; D94G | 1 | RIF | 1 | |
| A90V; S91P; D94N | 1 | INH, RIF, STR, KAN | 1 | |
| D94A; A90V+D94A | 1 | INH, RIF, KAN, AMI, PAS | 1 | |
| D94A; A90V+D94A | M | INH, RIF, KAN, AMI, PAS | 1 | |
| D94A; A90V+D94A | 1 | INH, RIF, KAN, AMI | 1 | |
| D94N; D94G; D94Y | 1 | INH, RIF, STR, KAN, AMI, CAP | 1 | |
| D94N; D94G; D94Y | 1 | INH, RIF, STR, KAN, CAP, PAS | 1 | |
| D94N; D94G; S91P+D94N | 1 | INH, STR | 1 | |
| Total no. of isolates | 54 |
The QRDR type is shown. The wild type (W) and the mutation in codon 90, 91, or 94 of the gyrA gene is shown.
The mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) type is shown: 1 type or multiple (M) types.
The combined resistance profiles determined by drug susceptibility testing (DST) are shown. MDR-TB strains are shown in boldface type. Drug abbreviations: INH, isoniazid; RIF, rifampin; STR, streptomycin; PAS, p-aminosalicyclic acid; KAN, kanamycin; AMI, amikacin; CAP, capreomycin; EMB, ethambutol.
Here, heteroresistant isolates were obtained from patients who had received anti-TB treatment with regimens including FQ (levofloxacin or gatifloxacin). These cases were treatment failure or relapse after initial treatment with the standardized regimen of the DOTS strategy (DOTS stands for directly observed treatment, short course). FQ was used as a major component of multidrug regimens in the subsequent treatment of these cases, in combination with a first-line drug (e.g., INH, RIF, EMB, and pyrazinamide [PZA]) and an aminoglycoside or polypeptide (e.g., kanamycin [KAN], amikacin [AMI], and capreomycin [CAP]). Among the cases (74.4% [40/54]) treated with FQ over a short, 3- to 6-month period, the proportions of group I and group II isolates were 70.2% and 29.8%, respectively. Meanwhile, for the cases treated with FQ for several phases and for a total duration of up to 2 years or continuously treated with an FQ-containing regimen for more than 1 year, the proportions of group I and group II isolates changed and were 34.4% and 65.6%, respectively. Due to selection pressures from FQ, resistant derivatives became dominant after coexisting with the susceptible strain (wild type) for a period of time, which was designated the heteroresistance stage.
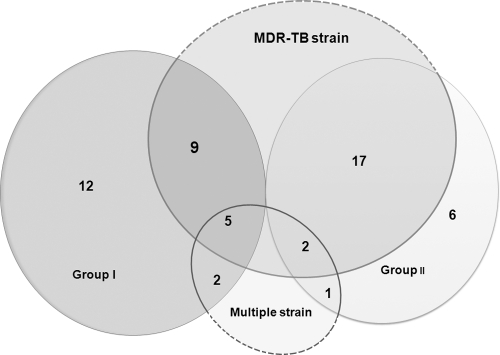
The occurrence of heteroresistance in M. tuberculosis is related to two different mechanisms: superinfection with two different strains and the segregation of a single strain. These mechanisms are related to transmission of drug-resistant strains in the region with a high incidence of TB as well as inadequate TB therapy (7). The mycobacterial interspersed repetitive-unit–variable-number tandem-repeat typing method helps distinguish between these two situations among clinical isolates. Thus, molecular genotyping was performed with 24 mycobacterial interspersed repetitive-unit loci as described previously (16). Apart from the 10 isolates that were found to contain multiple strains, the majority of heteroresistant isolates (81.5% [44/54]) were infected with one strain containing a single variable number of tandem-repeat genotype (Table 1). This finding means that the mixture of different QRDR genotype strains is due to the segregation of a single strain. Over one-half of the isolates were categorized as group II with 88.5% of isolates with the majority (73.9% [17/23]) from MDR-TB patients (Fig. 1). Among the group I isolates, 42.9% (9/21) containing a single strain were from MDR-TB patients. In one respect, group I displayed early drug resistance, and group II isolates were determined to be in an intermediate stage to full drug resistance. FQ resistance is reported in patients with a high rate of MDR-TB, and detection of FQ heteroresistance in clinical isolates would be helpful to analyze the cause of drug resistance development and decrease the emergence of XDR-TB.
Fig 1.
Distribution of MDR-TB strains and multiple strains among heteroresistant isolates. The number of strains in the various groups or categories is shown.
The rate of heteroresistance is related to the level of TB prevalence in certain regions. Commonly, heteroresistance in M. tuberculosis is detected in 9 to 20% of clinical isolates (2, 14). It has been detected in 25.8% of clinical specimens with resistance to isoniazid and rifampin in Samara, Russian Federation, where a high incidence of TB can be found (13). Of clinical isolates found to be heteroresistant to FQ in Vietnam, 31.2% contained Beijing family strains, which display high levels of FQ resistance in TB patients (4). Our data showed that heteroresistance emerged in 23% of isolates, with the majority due to infection with a single strain. In addition, the high frequency of heteroresistance that occurred in those MDR-TB isolates revealed the improper use of FQ leading to drug resistance and would also increase the possibility of XDR-TB occurrence. The rate that was found in this study is detecting only mutations in gyrA, and the actual rate would be slightly higher if it also detected mutations in gyrB. As drug resistance-related mutation in gyrB is seldom observed in clinical isolates, heteroresistance to it is not detected in this paper.
FQ is used for many bacterial infections, and empirical treatments and inadequate therapies for TB are popular in China. The accurate detection of drug-resistant strains and evaluation of treatment quality are urgently required to decrease the emergence of drug-resistant strains. This paper presents the first time the diversity of subpopulations of FQ-resistant strains simultaneously presented in the same patient was analyzed. Heteroresistant isolates with a mixture of various genotypes reflected different stages of resistance development in the course of treatment. Identification of this phenomenon in patients would provide a reference for estimating the treatment quality with a particular regimen. Thus, further detection of heteroresistance in clinical isolates, especially among MDR-TB patients, would provide valuable information about the molecular characterization of M. tuberculosis for guiding TB treatment and controlling the development of drug resistance.
ACKNOWLEDGMENT
This study was supported by the National Major Science and Technology Project for the Prevention and Treatment of AIDS and Viral Hepatitis and Other Major Infectious Diseases (2008ZX10003-005).
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Adjers-Koskela K, Katila ML. 2003. Susceptibility testing with the manual mycobacteria growth indicator tube (MGIT) and the MGIT 960 system provides rapid and reliable verification of multidrug-resistant tuberculosis. J. Clin. Microbiol. 41:1235–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakravorty S, et al. 2011. Rapid detection of fluoroquinolone-resistant and heteroresistant Mycobacterium tuberculosis by use of sloppy molecular beacons and dual melting-temperature codes in a real-time PCR assay. J. Clin. Microbiol. 49:932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cullen MM, Sam NE, Kanduma EG, McHugh TD, Gillespie SH. 2006. Direct detection of heteroresistance in Mycobacterium tuberculosis using molecular techniques. J. Med. Microbiol. 55:1157–1158 [DOI] [PubMed] [Google Scholar]
- 4. Duong DA, et al. 2009. Beijing genotype of Mycobacterium tuberculosis is significantly associated with high-level fluoroquinolone resistance in Vietnam. Antimicrob. Agents Chemother. 53:4835–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guillemin I, Jarlier V, Cambau E. 1998. Correlation between quinolone susceptibility patterns and sequences in the A and B subunits of DNA gyrase in mycobacteria. Antimicrob. Agents Chemother. 42:2084–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He GX, et al. 2010. Availability of second-line drugs and anti-tuberculosis drug susceptibility testing in China: a situational analysis. Int. J. Tuberc. Lung Dis. 14:884–889 [PubMed] [Google Scholar]
- 7. Hofmann-Thiel S, et al. 2009. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur. Respir. J. 33:368–374 [DOI] [PubMed] [Google Scholar]
- 8. Kam KM, et al. 2006. Stepwise decrease in moxifloxacin susceptibility amongst clinical isolates of multidrug-resistant Mycobacterium tuberculosis: correlation with ofloxacin susceptibility. Microb. Drug Resist. 12:7–11 [DOI] [PubMed] [Google Scholar]
- 9. Karahan ZC, Akar N. 2005. Restriction endonuclease analysis as a solution for determining rifampin resistance mutations by automated DNA sequencing in heteroresistant Mycobacterium tuberculosis strains. Microb. Drug Resist. 11:137–140 [DOI] [PubMed] [Google Scholar]
- 10. Kocagoz T, et al. 1996. Gyrase mutations in laboratory-selected, fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 40:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laszlo A, Rahman M, Raviglione M, Bustreo F. 1997. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Laboratory Network: first round of proficiency testing. Int. J. Tuberc. Lung Dis. 1:231–238 [PubMed] [Google Scholar]
- 12. Morand B, Muhlemann K. 2007. Heteroresistance to penicillin in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 104:14098–14103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nikolayevskyy V, et al. 2009. Performance of the Genotype MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin. Pathol. 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rinder H, Mieskes KT, Loscher T. 2001. Heteroresistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 5:339–345 [PubMed] [Google Scholar]
- 15. Shi R, Zhang J, Li C, Kazumi Y, Sugawara I. 2006. Emergence of ofloxacin resistance in Mycobacterium tuberculosis clinical isolates from China as determined by gyrA mutation analysis using denaturing high-pressure liquid chromatography and DNA sequencing. J. Clin. Microbiol. 44:4566–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Supply P, et al. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:4498–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takiff HE, et al. 1996. Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc. Natl. Acad. Sci. U. S. A. 93:362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takiff HE, et al. 1994. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization 2011. Global tuberculosis control: WHO report 2011. World Health Organization, Geneva, Switzerland [Google Scholar]
- 20. World Health Organization 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO/HTM/TB/2008.392. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 21. Xu P, et al. 2009. Prevalence of fluoroquinolone resistance among tuberculosis patients in Shanghai, China. Antimicrob. Agents Chemother. 53:3170–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]