Abstract
GenoType MTBDRplus is a molecular assay for detection of Mycobacterium tuberculosis and drug resistance. Assay performance as applied directly to consecutive unselected sputum samples has not been established. The objective of this study was to determine the accuracy of the MTBDRplus test for direct detection of M. tuberculosis (in sputum) and for drug resistance in consecutively submitted sputum samples. In this cross-sectional study in South Africa, one sputum specimen from each person suspected of having pulmonary tuberculosis was tested by smear microscopy, direct MTBDRplus, and Mycobacterial Growth Indicator Tube (MGIT) culture with MGIT drug susceptibility testing. MGIT results were the reference standard. We tested 2,510 sputum samples, and 529 (21.1%) were positive for M. tuberculosis by MGIT. Direct MTBDRplus identified M. tuberculosis in 256 of 529 specimens (sensitivity, 48.4%; 95% confidence interval [CI], 44.1, 52.7). The sensitivity of MTBDRplus for M. tuberculosis detection by sputum smear status was as follows: smear negative, 13.7% (95% CI, 9.8, 18.4); smear scanty, 46.2% (95% CI, 19.2, 74.9); smear 1+, 69.1% (95% CI, 55.2, 80.9); smear 2+, 86.3% (95% CI, 73.7, 94.3); smear 3+, 89.8% (95% CI, 83.7, 94.2). Direct MTBDRplus testing was negative for 1,594/1,612 sputum samples that were culture negative for M. tuberculosis (specificity, 98.9%; 95% CI, 98.2, 99.3). For specimens positive for M. tuberculosis by MTBDRplus, this assay's sensitivity and specificity for rifampin resistance were 85.7% (95% CI, 57.2, 98.2) and 96.6% (95% CI, 93.2, 98.6) and for isoniazid resistance they were 62.1% (95% CI, 42.3, 79.3) and 97.9% (95% CI, 94.8, 99.4). For sputum testing, the sensitivity of MTBDRplus is directly related to the specimen's bacillary burden. Our results support recommendations that the MTBDRplus test not be used for direct testing of smear-negative or paucibacillary sputum samples.
INTRODUCTION
Laboratory strengthening and accelerated access to rapid testing for Mycobacterium tuberculosis drug resistance are important components of the Stop TB Partnership Global Plan to Stop TB 2011-2015 (32). In May 2009, the World Health Assembly urged member states to “achieve universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis.” (33). South Africa is experiencing dual epidemics of HIV infection and drug-resistant tuberculosis (TB), making rapid detection of anti-TB drug resistance a critical component of individual patient care. Facilities for M. tuberculosis culture and culture-based phenotypic drug susceptibility testing (DST) are being expanded and strengthened in South Africa. However, the turnaround time of culture-based tests diminishes their clinical impact, the contamination rates of liquid culture systems are nontrivial, and the infrastructure and human resource requirements for safe performance of culture tests are substantial (8, 11, 22).
The GenoType MTBDRplus (Hain Lifescience GmbH, Nehren, Germany) is a molecular line probe assay containing probes specific for M. tuberculosis complex, as well as probes for common rifampin (RIF) resistance-conferring mutations and a subset of the mutations conferring resistance to isoniazid (INH). For the MTBDRplus assay, a meta-analysis showed a pooled sensitivity and specificity of 98.4% and 98.9%, respectively, for detection of RIF resistance and 88.7% and 99.2%, respectively, for detection of INH resistance, although almost all of the studies included used either cultured isolates or smear-positive respiratory specimens (19). An initial validation study showed not only that MTBDRplus was accurate for detection of resistance from smear-positive respiratory specimens but also suggested that the assay had good accuracy when applied to smear-negative respiratory specimens that contained M. tuberculosis in culture; 16/20 (80%) gave interpretable results for RIF, and 14/19 (74%) gave interpretable results for INH (3).
Thibela TB is a cluster-randomized study of community-wide preventive INH therapy in the gold-mining workforce in South Africa (9, 12). The TB notification rates in the gold-mining workforce exceed 4,000/100,000 person years—severalfold higher than for South Africa as a whole—partly due to a high HIV prevalence, estimated to be 29%, and silicosis (10, 18). Extensive transmission of highly drug-resistant strains has been documented and is facilitated by congregate living and working conditions (6). Since incident TB disease was a primary outcome of the Thibela TB study, we undertook a series of initiatives to strengthen laboratory services and evaluate new TB diagnostic tests, including GenoType MTBDRplus, in the context of Thibela TB. The major scientific objectives of the present study were to determine the accuracy of the GenoType MTBDRplus test for direct (i.e., in sputum) detection of M. tuberculosis and RIF and INH resistance in sputum regardless of sputum smear microscopy status.
MATERIALS AND METHODS
Study population.
This was a cross-sectional study of a diagnostic test performed as a substudy of Thibela TB (9, 12). Participants were adults who were suspected of having pulmonary TB based on clinical symptoms/signs and/or chest X-ray findings; individuals already on anti-TB treatment were excluded. Participants were recruited (i) at the mining health services, where symptomatic individuals can self-present or be referred for TB evaluation following routine annual chest X-ray screening, and (ii) at screening for active TB prior to preventive INH therapy initiation as part of Thibela TB. Informed consent for this TB diagnostic study was obtained from each participant, and a structured interview was conducted. Information about HIV status was collected through interview. One spontaneously expectorated sputum sample was obtained from each participant for study and transported within 24 h to the study laboratory.
Laboratory algorithms.
All laboratory tests were performed at the National Health Laboratory Services laboratory in Braamfontein, Johannesburg, South Africa. Annually, this clinical service laboratory processes approximately 150,000 sputum samples and performs approximately 135,000 MGIT cultures and 40,000 MTBDRplus tests for routine clinical purposes. All study mycobacteriology procedures were performed by study-dedicated staff. Within 24 h of receipt in the laboratory, sputum samples were decontaminated using N-acetyl-l-cysteine–NaOH with a final [NaOH] of 1% (17). After centrifugation, the pellet was suspended in approximately 1.5 ml of phosphate buffer.
Conventional microbiology tests.
An auramine-stained smear of the decontaminated sediment was examined by fluorescence microscopy and graded according to WHO criteria (23). A 0.5-ml portion of the sediment was inoculated into the Bactec MGIT 960 system (BD Diagnostic Systems, Sparks, MD) using Mycobacterial Growth Indicator Tubes (MGITs; BD Diagnostic Systems). Positive cultures were confirmed as mycobacterial using Ziehl-Neelsen staining or as M. tuberculosis using an anti MPB64 monoclonal antibody assay (Capilia TB-Neo; TAUNS Laboratories, Numazu, Japan). For cultures growing M. tuberculosis, indirect DST was performed using the MGIT SIRE system (BD Diagnostic Systems); the critical concentration was 1.0 μg/ml for RIF and 0.1 μg/ml for INH.
GenoType MTBDRplus.
Testing was conducted in accordance with the manufacturer's recommendations (13) and the manufacturer-trained laboratory staff members. Training consisted of an initial 3 days of intensive laboratory training. Briefly, crude DNA was extracted from a 500-μl aliquot of the resuspended sediment that resulted from the sputum decontamination procedure. The 500-μl aliquot was centrifuged for 15 min at 10 000 × g, and then the resulting pellet was resuspended in water and incubated at 95°C for 20 min. Following further incubation in an ultrasonic bath for 15 min, each sample was centrifuged for 5 min and 5 μl was used for amplification. The multiplex PCR (50 μl/tube) was performed using Hotstar Taq DNA polymerase (Qiagen GmbH, Hilden, Germany) and 42 amplification cycles. Hybridization was performed with the GT Blot 48 or with the TwinCubator (both from Hain Lifescience GmbH). DNA extraction, PCR, and reverse hybridization steps were carried out in separate rooms to minimize contamination. Tests were performed and interpreted without knowledge of culture results. For each test strip, the banding pattern was interpreted in a two-stage process. (i) The presence or absence of M. tuberculosis was first determined, and (ii) for strips showing M. tuberculosis to be present, the susceptibilities to RIF and INH were then assessed. For detection of M. tuberculosis, results that could not be interpreted definitively (i.e., locus control test bands present but at a lower intensity than the amplification control band) after the first test run were categorized as “indeterminate-initial” and the test was repeated once using extracted DNA. If on repeat testing a definitive test result could be obtained, then it was recorded; otherwise the result was recorded as “indeterminate-final.”
DNA sequencing.
For specimens having discordant DST results by MGIT and MTBDRplus, direct DNA sequencing of the drug resistance-determining regions of the genes rpoB, katG, and inhA (including the promoter) was performed as described elsewhere (27).
Statistical methods.
Data were entered singly, source data were verified in a structured query language database, and discrepancies were checked against the source data. For detection of M. tuberculosis by MTBDRplus, sensitivity calculations included specimens for which MGIT culture grew M. tuberculosis; specificity calculations included specimens that showed either no growth or growth of nontuberculous mycobacteria (NTM), and specimens were excluded as uninformative if the MGIT culture was contaminated. All P values were generated by Fisher's exact test.
This study was reviewed and approved by ethics committees of the University of KwaZulu Natal, the London School of Hygiene and Tropical Medicine, and the Johns Hopkins University School of Medicine.
RESULTS
Sputum samples were obtained from 2,516 participants recruited from November 2008 to January 2010. Of these 2,516 participants, 2,430 (96.6%) were male, the median age was 46 years (interquartile range, 39 to 51 years), 2,223 (88.4%) were from either South Africa or Lesotho, 699 (27.8%) self-reported a history of TB disease, and 406 (16.1%) self-reported that they were HIV infected.
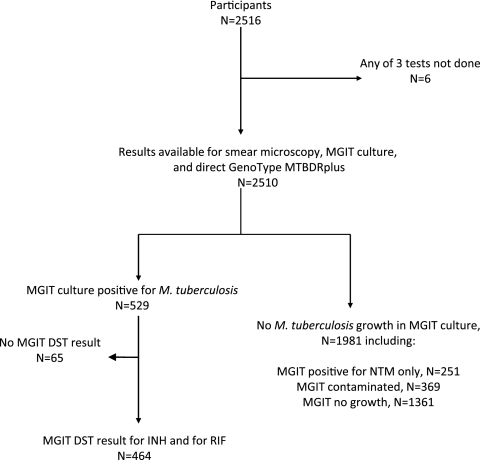
Figure 1 shows the specimen flow. There were 2,510 sputum samples that yielded smear microscopy, MGIT culture, and MTBDRplus results. MGIT culture was positive for M. tuberculosis in 529/2,510 (21.1%); 266/529 (50.3%) were smear positive, and 263/529 (49.7%) were smear negative. MGIT culture was positive for NTM (in the absence of detected MTB) in 251/2,510 (10.0%) sputum samples; 45/251 (17.9% of 251) were smear positive. Of 2,510 MGIT cultures, 369 (14.7%) were contaminated and 1,361 (54.2%) were negative for any growth.
Fig 1.
Specimen flow schematic.
The initial MTBDRplus test result was positive for M. tuberculosis in 232 (9.2%) of the 2,510 sputum samples tested, negative for M. tuberculosis in 1,975/2,510 (78.7%), and indeterminate-initial for 303/2,510 (12.1%). For the 303 indeterminate-initial specimens, on repeat testing, the result was M. tuberculosis positive for 50/303 (16.5%), M. tuberculosis negative for 235/303 (77.6%), and indeterminate-final for 18/303 (5.9%; 0.7% of 2,510). Smear-positive specimens were more likely than smear-negative specimens to be initially indeterminate (17.8% and 11.2%, respectively; P = 0.001). Similarly, specimens that were culture positive for M. tuberculosis were more likely to be initially indeterminate by MTBDRplus than were specimens that were culture positive for NTM, culture negative, or contaminated (21.6%, 6.8%, 10.1%, and 9.2%, respectively; P < 0.001). There was no correlation between indeterminate results and time from study start (data not shown).
Table 1 shows the final MTBDRplus and MGIT results for detection of M. tuberculosis in sputum. MTBDRplus correctly identified M. tuberculosis in 256 of 529 MGIT culture-positive specimens (sensitivity, 48.4%; 95% confidence interval [CI], 44.1, 52.7). The sensitivity of MTBDRplus for detection of M. tuberculosis by sputum smear grade was as follows (Fig. 2): smear negative, 36/263 (13.7%; 95% CI, 9.8, 18.4); smear scanty, 6/13 (46.2%; 95% CI, 19.2, 74.9); smear 1+, 38/55 (69.1%; 95% CI, 55.2, 80.9); smear 2+, 44/51 (86.3%; 95% CI, 73.7, 94.3); smear 3+, 132/147 (89.8%; 95% CI, 83.7, 94.2). The overall sensitivity of MTBDRplus for M. tuberculosis detection in smear-positive sputum samples was 220/266 (82.7%; 95% CI, 77.6, 87.1). Of 1,612 specimens culture negative for M. tuberculosis by MGIT (and not contaminated), the MTBDRplus test was negative in 1,594 (specificity, 98.9%; 95% CI, 98.2, 99.3). MTBDRplus specificity was lower among specimens that were smear positive and culture negative for M. tuberculosis than among specimens smear negative and culture negative for M. tuberculosis (95.4% versus 99.0%, respectively; P = 0.033). MTBDRplus was positive in 13 noncontaminated MGIT specimens that were culture negative for M. tuberculosis (4 were culture positive for NTM without growth of M. tuberculosis, although additional studies to exclude the presence of M. tuberculosis were not performed). The apparent decrement in specificity for smear microscopy grade 3+ sputum samples (Fig. 2) reflected a group of 13 smear-positive sputum samples that were culture negative for M. tuberculosis, among which MTBDRplus was positive in 2 sputum samples that grew NTM in culture. When contaminated MGIT specimens were excluded, the MTBDRplus positive predictive value for M. tuberculosis was 256/269 (95.2%; 95% CI, 91.9, 97.4) and the negative predictive value was 1,594/1,857 (85.8%; 95% CI, 84.2, 87.4). The MTBDRplus test was positive for M. tuberculosis in 11/369 (3.0%) sputum samples for which the MGIT culture was contaminated.
Table 1.
Final results for detection of M. tuberculosis by GenoType MTBDRplus and MGIT culture in 2,510 tested sputum specimens
| MTBDRplus result | No. (%)a of MGIT cultures |
Total | |||
|---|---|---|---|---|---|
| M. tuberculosis present | No M. tuberculosis present |
||||
| NTM only | Contaminated | Negative | |||
| M. tuberculosis present | 256 (48.4) | 4 (1.6) | 11 (3.0) | 9 (0.7) | 280 |
| No M. tuberculosis present | 263 (49.7) | 247 (98.4) | 355 (96.2) | 1,347 (99.0) | 2,212 |
| Indeterminate-final | 10 (1.9) | 0 (0) | 3 (0.8) | 5 (0.4) | 18 |
| Total | 529 (100) | 251 (100) | 369 (100) | 1,361 (100) | 2,510 |
Percentages shown are those of the column total.
Fig 2.
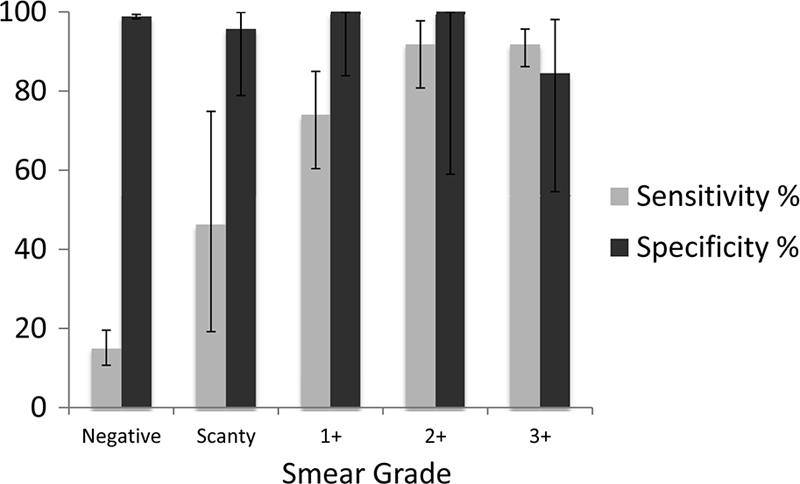
Sensitivity and specificity of the direct GenoType MTBDRplus test, stratified by sputum smear microscopy grade and using MGIT culture as the reference comparator. Vertical bars represent 95% CIs.
Of 529 sputum samples that ultimately grew M. tuberculosis in MGIT culture, conventional MGIT DST was performed on 464 M. tuberculosis isolates. Of these isolates, 24/464 (5.2%) were resistant to RIF and 58/464 (12.5%) were resistant to INH. With respect to patterns of resistance, 23/464 (5.0%) were multidrug resistant (MDR), 1/464 (0.2%) was RIF monoresistant, 35/464 (7.5%) were INH monoresistant, and 405/464 (87.3%) were susceptible to both drugs. The 65 cultures without MGIT DST consisted of 47 mixed infections (M. tuberculosis plus NTM), 13 that were contaminated on DST culture, and 5 that failed to grow on DST culture.
MTBDRplus DST results were interpreted for 255 sputum samples that were positive for M. tuberculosis by the direct MTBDRplus test. As shown in Table 2, MTBDRplus, performed on sputum, identified as RIF resistant 12 of 24 specimens that grew M. tuberculosis determined to be RIF resistant by MGIT phenotypic testing (MTBDRplus sensitivity, 50.0%; 95% CI, 29.1, 70.9). When the analysis was restricted to specimens that were positive for M. tuberculosis by MTBDRplus, that test's sensitivity for RIF resistance was 12/14 (85.7%; 95% CI, 57.2, 98.2) and its specificity for RIF resistance was 200/207 (96.6%; 95% CI, 93.2, 98.6). For the two specimens that were RIF resistant by MGIT but RIF susceptible by MTBDRplus, sequencing showed wild-type rpoB genes and was therefore concordant with the MTBDRplus test. For the two specimens that were RIF susceptible by MGIT but RIF resistant by MTBDRplus, sequencing confirmed the rpoB mutation (S531L) detected by MTBDRplus in both. For seven sputum samples determined to contain M. tuberculosis by MTBDRplus, the RIF susceptibility status could not be determined definitively with that test, in most cases due to uncertainty about the presence of bands for one or more wild-type rpoB probes; each of the M. tuberculosis isolates was susceptible to RIF by phenotypic DST.
Table 2.
DST results for direct GenoType MTBDRplus testing and indirect MGIT culture for RIF and INH
| MTBDRplus result | No. (%)a of MGIT cultures |
Total | ||
|---|---|---|---|---|
| Susceptible | Resistant | Not done | ||
| RIF | ||||
| M. tuberculosis positive, RIF susceptible | 200 (45.5) | 2 (8.3) | 26 (40.0) | 228 |
| M. tuberculosis positive, RIF resistant | 2 (0.5) | 12 (50.0) | 5 (7.7) | 19 |
| M. tuberculosis positive, RIF indeterminate-final | 7 (1.6) | 0 (0) | 1 (1.5) | 8 |
| M. tuberculosis negative | 231 (52.5) | 10 (41.7) | 33 (50.8) | 274 |
| Total | 440 (100) | 24 (100) | 65 (100) | 529 |
| INH | ||||
| M. tuberculosis positive, INH susceptible | 190 (46.8) | 11 (19.0) | 25 (38.5) | 226 |
| M. tuberculosis positive, INH resistant | 2 (0.5) | 18 (31.0) | 6 (9.2) | 26 |
| M. tuberculosis positive, INH indeterminate-final | 2 (0.5) | 0 (0) | 1 (1.5) | 3 |
| M. tuberculosis negative | 212 (52.2) | 29 (0.5) | 33 (50.8) | 274 |
| Total | 406 (100) | 58 (100) | 65 (100) | 529 |
Percentages shown are those of the column total.
Overall, MTBDRplus identified as INH resistant 18 of 58 specimens resistant by MGIT (Table 2; sensitivity, 31.0%; 95% CI, 19.5, 44.5). When the analysis was restricted to specimens that were positive for M. tuberculosis by MTBDRplus, that test's sensitivity was 18/29 (62.1%; 95% CI, 42.3, 79.3) and its specificity was 190/194 (97.9%; 95% CI, 94.8, 99.4). Of the 11 specimens that were INH resistant by MGIT yet susceptible by MTBDRplus, sequencing was performed for 9; 8 showed wild-type katG and wild-type inhA, and 1 had an inhA mutation (C15T). Sequencing was performed for one of the two specimens that were INH susceptible by MGIT yet resistant by MTBDRplus; DNA sequencing confirmed the katG S315T and inhA C15T mutations detected by MTBDRplus.
Of the 23 specimens that were identified as containing MDR M. tuberculosis by MGIT testing, MTBDRplus correctly identified 11 as MDR (sensitivity, 47.8%; 95% CI, 26.8, 69.4). When the analysis was restricted to specimens that were positive for M. tuberculosis by MTBDRplus, that test's sensitivity was 11/13 (84.6%; 95% CI, 54.6, 98.1) and its specificity was 202/203 (99.5%; 95% CI, 97.3, 100.0). Of these 11 specimens, 7 (63.6%) had the katG S315T mutation, 2 (18.2%) had the inhA C15T mutation, and 2 (18.2%) had both the katG S315T and inhA C15T mutations; 8 (72.7%) had the rpoB S531L mutation, and 3 (27.3%) had the rpoB S516L mutation.
DISCUSSION
Our study, undertaken in a setting of high HIV and TB prevalence, assessed the accuracy of MTBDRplus for detection of M. tuberculosis and drug resistance when performed directly on all sputum samples regardless of smear status. The sensitivity of MTBDRplus for detection of M. tuberculosis increased as the smear grade increased, up to a smear grade of 2+, reflecting an association between assay sensitivity and sputum bacillary burden.
Importantly from a clinical perspective, over one-quarter of M. tuberculosis culture-positive specimens of smear microscopy grade 1+ and over one-half of culture-positive specimens of smear grade “scanty” were negative by MTBDRplus. This test's relatively low sensitivity to M. tuberculosis in smear grade scanty and 1+ specimens means that caution must be used in interpreting as “TB negative” those sputum samples that are smear grade scanty or 1+ but MTBDRplus negative, and additional testing of those sputum samples is required. The WHO has endorsed the use of line probe assays for the rapid screening of patients at risk for MDR TB (31); the recommended use is limited to culture isolates and smear-positive respiratory specimens. The relatively low sensitivity of MTBDRplus for smear-positive sputum samples of smear grade <2+ may have implications for cost-effectiveness if the assay is used on all smear-positive pulmonary specimens.
From an implementation perspective, we found that the MTBDRplus test was challenging to perform and interpret when run directly on all submitted sputum samples, as approximately 12% of tests could not be interpreted on initial testing and had to be repeated. This rate of uninterpretable tests is similar to the 5 to 12% uninterpretable rates reported by others for MTBDR tests performed directly on sputum samples (2, 14, 20, 21, 28). In our study, the majority of indeterminate-initial tests were interpretable on repeat testing and the proportion of MTB-positive test results among indeterminate-initial test results (16.5%) was higher than that for initial test results overall (9.2%). The lack of an association between indeterminate results and time from study start implies that staff competency and experience performing the MTBDRplus test were not the sole drivers of indeterminate results. Rather, several factors may have been involved, including low bacillary burdens in some specimens (2, 20, 21). On the other hand, DNA cross-contamination resulting in falsely positive MTBDRplus results—a concern raised about the use of the test on smear-negative specimens—occurred infrequently at most, as evidenced by an overall specificity for M. tuberculosis detection of 98.9% in our study. In addition, when applied to sputum samples, the MTBDRplus test did not “overcall” resistance, since in our study the specificity estimates for RIF and INH susceptibility testing were similar to those determined in a meta-analysis (19). In our experience, however, extreme care was required in interpreting RIF results in the absence of an overt rpoB mutation probe band, since band intensities were sometimes very faint for rpoB wild-type probes 6, 7, and 8 (data not shown).
With respect to DST, performing MTBDRplus on all submitted sputum samples was inefficient since it detected half or fewer than half of the sputum samples containing RIF- and/or INH-resistant M. tuberculosis as determined by phenotypic DST. When considering only those specimens for which the MTBDRplus test was positive for M. tuberculosis, the assay still failed to detect INH resistance in over one-third of sputum samples containing INH-resistant isolates, and MTBDRplus failed to detect RIF resistance (and MDR M. tuberculosis) in about 15% of the sputum samples containing RIF-resistant or MDR M. tuberculosis isolates. Although the number of drug-resistant specimens in our study was small, the demonstrated imperfect sensitivity of MTBDRplus highlights the limitations of performing MTBDRplus directly on sputum, as well as the limitations of molecular assays containing a finite number of probes. For INH, DNA sequencing of available isolates showed that some discrepant (MGIT resistant, MTBDRplus susceptible) isolates had wild-type alleles for katG and inhA, the two genes represented in the MTBDRplus test; DNA sequencing to test for the presence of INH resistance-conferring mutations in other genes was not undertaken.
Our study also highlights an Achilles' heel of MGIT culture, namely, contamination. Fifteen percent of MGIT cultures were contaminated, thereby diminishing the diagnostic yield of this methodology, which has excellent analytical sensitivity (7, 16, 25, 26, 30). Rates of MGIT culture contamination similar to those in our study have been reported for other studies conducted in developing-country settings (8, 22, 25, 29). The MTBDRplus is sensitive for detection of M. tuberculosis and RIF resistance when performed on cultured isolates (1, 3, 5, 15, 24). However, when MTBDRplus is used as an indirect test, the diagnostic yield would be expected to be reduced by culture contamination since some contaminated cultures may not be suitable for MTBDRplus testing. Other molecular methodologies, such as Xpert MTB/RIF, which incorporates heminested PCR amplification and molecular beacon detection of RIF resistance-conferring mutations in rpoB, may be able to overcome the problems of poor analytical sensitivity and contamination (4).
Overall, our study supports the current recommendation (31) not to apply MTBDRplus testing to smear-negative respiratory specimens, and it provides new detail with respect to the accuracy of MTBDRplus when it is applied to smear-positive sputum samples that contain relatively few bacilli. Cost-effectiveness studies may help clarify the subset of smear-positive clinical respiratory specimens best suited for MTBDRplus testing. Finally, there is a need for investigation of the impact of an MTBDRplus testing program on patient and TB program outcomes, given the potential for this test to result in a more rapid testing turnaround time and potentially faster initiation of appropriate treatment than conventional culture-based phenotypic DST, and the relative merits of line probe versus emerging molecular beacon assays.
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. National Institutes of Health (RO1 AI51528 to S.E.D.) and the Bill and Melinda Gates Foundation (to G.J.C.).
We gratefully acknowledge the study participants for their time, interest, and willingness to share information with study staff. We also thank the many individuals who made this study possible, including study staff who recruited and interviewed participants, and Cynthia Kekana, Gilla Kaplan, and Barry Kreiswirth for helpful discussions.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Anek-Vorapong R, et al. 2010. Validation of the GenoType MTBDRplus assay for detection of MDR-TB in a public health laboratory in Thailand. BMC Infect. Dis. 10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bang D, Bengård Andersen A, Thomsen VØ. 2006. Rapid genotypic detection of rifampin- and isoniazid-resistant Mycobacterium tuberculosis directly in clinical specimens. J. Clin. Microbiol. 44:2605–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. 2008. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am. J. Respir. Crit. Care Med. 177:787–792 [DOI] [PubMed] [Google Scholar]
- 4. Boehme CC, et al. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bwanga F, Joloba ML, Haile M, Hoffner S. 2010. Evaluation of seven tests for the rapid detection of multidrug-resistant tuberculosis in Uganda. Int. J. Tuberc. Lung Dis. 14:890–895 [PubMed] [Google Scholar]
- 6. Calver AD, et al. 2010. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg. Infect. Dis. 16:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chien HP, Yu MC, Wu MH, Lin TP, Luh KT. 2000. Comparison of the BACTEC MGIT 960 with Löwenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Int. J. Tuberc. Lung Dis. 4:866–870 [PubMed] [Google Scholar]
- 8. Chihota VN, et al. 2010. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int. J. Tuberc. Lung Dis. 14:1024–1031 [PubMed] [Google Scholar]
- 9. Churchyard GJ, et al. 2010. Symptom and chest radiographic screening for infectious tuberculosis prior to starting isoniazid preventive therapy: yield and proportion missed at screening. AIDS 24(Suppl. 5):S19–S27 [DOI] [PubMed] [Google Scholar]
- 10. Corbett EL, et al. 2002. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin. Infect. Dis. 34:1251–1258 [DOI] [PubMed] [Google Scholar]
- 11. Dowdy DW, et al. 2008. Impact and cost-effectiveness of culture for diagnosis of tuberculosis in HIV-infected Brazilian adults. PLoS One 3:e4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fielding KL, et al. 2011. Thibela TB: Design and methods or a cluster randomised trial of the effect of community-wide isoniazid preventive therapy on tuberculosis among gold miners in South Africa. Contemp Clin. Trials 32:382–392 [DOI] [PubMed] [Google Scholar]
- 13. Hain Lifescience GmbH GenoType MTBDRplus 1.0 product insert. Hain Lifescience GmbH, Nehren, Germany: http://www.hain-lifescience.com [Google Scholar]
- 14. Hillemann D, Rüsch-Gerdes S, Richter E. 2006. Application of the GenoType MTBDR assay directly on sputum specimens. Int. J. Tuberc. Lung Dis. 10:1057–1059 [PubMed] [Google Scholar]
- 15. Hillemann D, Rüsch-Gerdes S, Richter E. 2007. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 45:2635–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Idigoras P, Beristain X, Iturzaeta A, Vicente D, Perez-Trallero E. 2000. Comparison of the automated nonradiometric Bactec MGIT 960 system with Lowenstein-Jensen Coletsos and Middlebrook 7H11 solid media for recovery of mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 19:350–354 [DOI] [PubMed] [Google Scholar]
- 17. Kent PT, Kubica GP. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control, Atlanta, GA [Google Scholar]
- 18. Lewis JJ, et al. 2009. HIV infection does not affect active case finding of tuberculosis in South African gold miners. Am. J. Respir. Crit. Care Med. 180:1271–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ling D, Zwerling A, Pai M. 2008. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur. Respir. J. 32:1165–1174 [DOI] [PubMed] [Google Scholar]
- 20. Miotto P, Piana F, Cirillo DM, Migliori GB. 2008. Genotype MTBDRplus: a further step toward rapid identification of drug-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 46:393–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mironova S, et al. 25 October 2011. Performance of the GenoType MTBDRplus assay in routine settings: a multicenter study. Eur. J. Clin. Microbiol. Infect. Dis. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 22. Muyoyeta M, et al. 2009. Comparison of four culture systems for Mycobacterium tuberculosis in the Zambian National Reference Laboratory. Int. J. Tuberc. Lung Dis. 13:460–465 [PubMed] [Google Scholar]
- 23. Narvaiz de Kantor I, et al. 1998. Laboratory services in TB control: microscopy, part II. WHO/TB/98.258. World Health Organization, Geneva, Switzerland: http://wwwn.cdc.gov/dls/ILA/documents/lstc2.pdf [Google Scholar]
- 24. Nikolayevskyy V, et al. 2009. Performance of the Genotype MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin. Pathol. 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otu J, et al. 2008. Comparative evaluation of BACTEC MGIT 960 with BACTEC 9000 MB and LJ for isolation of mycobacteria in The Gambia. J. Infect. Dev. Ctries. 2:200–205 [DOI] [PubMed] [Google Scholar]
- 26. Parrish N, Dionne K, Sweeney A, Hedgepeth A, Carroll K. 2009. Differences in time to detection and recovery of Mycobacterium spp. between the MGIT 960 and the BacT/ALERT MB automated culture systems. Diagn. Microbiol. Infect. Dis. 63:342–345 [DOI] [PubMed] [Google Scholar]
- 27. Ramaswamy S, Musser JM. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 79:3–29 [DOI] [PubMed] [Google Scholar]
- 28. Rigouts L, et al. 2011. Evaluation of the Genotype MTBDRplus assay as a tool for drug resistance surveys. Int. J. Tuberc. Lung Dis. 15:959–965 [DOI] [PubMed] [Google Scholar]
- 29. Verweij KE, et al. 2010. Application of modern microbiological diagnostic methods for tuberculosis in Macha, Zambia. Int. J. Tuberc. Lung Dis. 14:1127–1131 [PubMed] [Google Scholar]
- 30. Williams-Bouyer N, Yorke R, Lee HI, Woods GL. 2000. Comparison of the BACTEC MGIT 960 and ESP culture system II for growth and detection of mycobacteria. J. Clin. Microbiol. 38:4167–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization 2008. Policy statement. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB). World Health Organization, Geneva, Switzerland: http://www.who.int/tb/features_archive/policy_statement.pdf Accessed 8 January 2011 [Google Scholar]
- 32. World Health Organization 2010. The global plan to stop TB 2011-2015: transforming the fight towards elimination of tuberculosis. World Health Organization, Geneva, Switzerland: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf Accessed 8 January 2011 [Google Scholar]
- 33. World Health Organization 2009. 62nd World Health Assembly. WHA62/2009/REC/1. World Health Organization, Geneva, Switzerland: http://apps.who.int/gb/ebwha/pdf_files/WHA62-REC1-en-P1.pdf [Google Scholar]