Abstract
The anaerobic isolation of anginosus group streptococci (AGS) from respiratory specimens containing diverse microbiota using a semiselective blood agar medium incorporating nalidixic acid and sulfamethazine (NAS) is described. AGS were detected in 60% of tested sputa from patients with cystic fibrosis, chronic obstructive pulmonary disease, and bronchiectasis. This demonstrates NAS to be a diagnostic tool for detecting AGS within the complex microbial communities associated with chronic lung disorders.
TEXT
Respiratory infections in patients with chronic lung disorders (CLDs) are a frequent and important cause of morbidity. In patients with cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and bronchiectasis, recurrent intermittent inflammatory events or pulmonary “exacerbations” (PE) contribute to the progressive decline in lung function that is characteristic of these diseases. Among the triggers of PE, changes in the dynamics of the commensal and colonizing microbial flora are increasingly recognized as important (1, 2, 4, 6, 9). In CF PE, a potential role of the oropharyngeal commensal anginosus group streptococci (AGS) (Streptococcus anginosus, Streptococcus intermedius, Streptococcus constellatus subsp. constellatus, and Streptococcus constellatus subsp. pharyngis) has been highlighted. AGS, which are often overlooked as oropharyngeal contaminants in respiratory samples, have been shown to proliferate within the lung prior to the onset of and during PE. Furthermore, a better response to antimicrobial therapy has been observed when agents targeting AGS rather than traditionally recognized pathogens, such as Pseudomonas aeruginosa, have been used to treat PE (5, 6). The increased recognition of AGS in the pathophysiology of PE and chronic lung disease highlights the need for effective methods for routine isolation, quantification, and identification. We have previously employed a simple, semiselective agar containing nalidixic acid and sulfamethazine (NAS agar) to recover and enumerate all three AGS species from other diverse microbial consortia, including oral specimens such as saliva and dental plaque (11). Here we report on the use of NAS agar under anaerobic conditions to selectively isolate AGS from the sputa of patients suffering from CLDs.
In initial studies, growths of reference and laboratory strains of AGS and other oropharyngeal streptococci were compared using NAS agar and the recently described semiselective McKay medium (7). NAS plates consisted of 40 g liter−1 sensitivity test agar (STA) (STA Lab012; Lab M Ltd., Lancs, United Kingdom), 30 μg ml−1 nalidixic acid (Sigma-Aldrich), 1,000 μg ml−1 sulfamethazine (Sigma-Aldrich), and 6% defibrinated horse blood (TCS biosciences Ltd., Bucks, United Kingdom). Both media were tested for growth of the following strains: Streptococcus intermedius (NCDO 2227T and strain UNS38), S. anginosus (NCTC10713T and strain 3a), S. constellatus subsp. constellatus (NCDO 2226T and strain 13a), S. constellatus subsp. pharyngis (MM9889aT), Streptococcus sanguinis (NCTC 7863T), Streptococcus salivarius (NCTC 8168T), Streptococcus oralis (NCTC 11427T), Streptococcus mitis (NS51T), Streptococcus mutans (NCTC 10449T), Streptococcus pneumoniae (strain 9V), and Streptococcus vestibularis (MM1T). Plates were incubated in an anaerobic atmosphere (80% N2, 10% H2, 10% CO2) or in 5% CO2 at 37°C for 72 h. All type strains of AGS species and subspecies grew on NAS and McKay agar in both anaerobic and 5% CO2 atmospheres. Streptococcus mutans strain NCTC 10449T also grew on both agars in these atmospheres, although this species has a distinct colonial morphology (white, matte, and opaque), thereby precluding confusion with AGS on NAS agar (Fig. 1). Streptococcus salivarius strain NCTC 8618T grew only on McKay agar incubated in 5% CO2. No other type or reference strain was able to grow on either NAS or McKay agar when incubated anaerobically or in 5% CO2 in these studies. A quantitative comparison of AGS recovery on NAS agar, McKay agar, and several nonselective blood agar media incubated anaerobically was performed. The strains listed above were diluted in a 10-fold series and plated onto the semiselective agars and the following nonselective agars containing 6% (vol/vol) defibrinated horse blood (TCS biosciences Ltd., Bucks, United Kingdom): brain heart infusion agar (Oxoid Ltd., Hampshire, United Kingdom), blood agar base no. 2 (Oxoid Ltd.), and Columbia agar (Oxoid Ltd.). NAS agar showed AGS recovery comparable to that of McKay agar and the nonselective agar media tested, with a ≤0.2 log10 difference between the numbers of CFU per ml obtained from all agars.
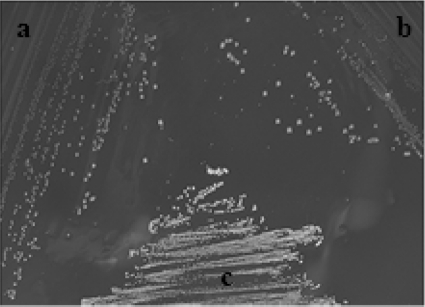
Fig 1.
S. anginosus strain NCTC 10713 (a), S. constellatus strain 13a (b), and S. mutans strain NCTC 10449 (c) on NAS agar, showing the distinct colonial morphology of S. mutans on this medium.
NAS medium was then used with a total of 25 sputa obtained from patients with CLDs attending Barts and The London NHS Trust. These consisted of 10 CF (5 outpatients and 5 exacerbations), 11 bronchiectasis (8 outpatients and 3 exacerbations), and 4 COPD (2 outpatients and 2 exacerbations) patients. Patients with exacerbations were undergoing treatment in hospital with intravenous antibiotics, whereas outpatients had stable respiratory function and were attending the clinic only for routine follow-up appointments. Sputa were liquefied in an equal volume of Sputasol (Oxoid Ltd.), and serial dilutions (20 μl) in saline were plated onto NAS and McKay agars as well as Columbia blood, chocolate, MacConkey, Pseudomonas isolation, and Pseudomonas cepacia agars according to the laboratory's standard operating procedure. Plates were incubated in 5% CO2 at 37°C for 48 h (blood, chocolate, and MacConkey) or 72 h. NAS and McKay agar plates were incubated anaerobically for 72 h at 37°C.
Colonies recovered from the CLD samples on NAS plates were subcultured and identified further by phenotypic and molecular techniques. Biochemical identification was undertaken using 4-methyl-umbelliferyl (4-MU)-linked fluorogenic substrates (Sigma) for the detection of β-N-acetylgalactosaminidase, β-N-acetylglucosaminidase, α-arabinosidase, α-l-fucosidase, β-d-fucosidase, α-d-galactosidase, β-d-galactosidase, α-d-glucosidase, β-d-glucosidase, β-d-glucuronidase, and sialidase (neuraminidase) activity (10, 12). AGS-specific PCR was performed using primers specific for the penicillin-binding protein 2B gene (pbp2b), and isolates were identified to the species and subspecies levels by amplification of the hyaluronate lyase, 16S RNA, and intermedilysin (ily) genes as described previously (8). Hemolysis after 24 h and 48 h of anaerobic incubation was determined on layered blood agar plates (blood agar no. 2; Oxoid, Ltd., Hants, United Kingdom) containing 6% defibrinated horse blood (TCS biosciences Ltd., Bucks, United Kingdom). Lancefield serological grouping was carried out using a commercial kit (streptococcal grouping kit DR0585A; Oxoid Ltd., Hants, United Kingdom).
AGS were isolated from 15/25 sputa, including 6/10 CF cases, 3/4 COPD cases, and 6/11 bronchiectasis cases (overall prevalence rate = 60%), on NAS agar. Both NAS and McKay agars allowed AGS detection from sputa containing other microbial taxa (P. aeruginosa/nonfermentative Gram-negative rods [range, 0 to 107 CFU ml−1], Enterobacteriaceae [range, 0 to 103 CFU ml−1], yeasts [range, 0 to 105 CFU ml−1], Staphylococcus aureus [range, 0 to 102 CFU ml−1], and Aspergillus fumigatus [range, 0 to 102 CFU ml−1]) which were unable to grow on either of these media under anaerobic conditions. Using the protocol described above for processing sputa, NAS agar permitted routine isolation of AGS with a potential lower detection limit of 100 CFU ml−1. Anaerobic incubation of sputa on NAS agar typically yielded 3 to 4 distinct colony morphotypes per plate which were counted, and representative colonies were subcultured for identification. Over the course of the study, AGS constituted 46/107 (42.9%) of NAS-selected isolates that were screened by the phenotypic and molecular techniques described. Of the 61 non-AGS isolates, virtually all (60/61) were unidentified Gram-positive cocci except a single isolate of S. mutans. As observed by Maeda et al. (3), Streptococcus anginosus was the AGS species most frequently isolated from CF sputa, while Streptococcus intermedius was not isolated from any of the CLD specimens. In the study of Sibley et al. (7), S. anginosus was also isolated most frequently, with S. intermedius comprising 34% of the AGS isolated. In the present study, Streptococcus anginosus was detected in 13/25 (52%) sputa, S. constellatus subsp. constellatus was detected in 8/25 (32%) sputa, and S. anginosus and S. constellatus subsp. constellatus were detected together in 6/25 (24%) sputa; the species combination was also the most frequently occurring combination found by Sibley et al. (7). Streptococcus anginosus strains were more heterogeneous than S. constellatus strains with respect to Lancefield group reaction and hemolysis on blood agar (Table 1). All 8 S. constellatus strains isolated were nongroupable and alpha-hemolytic.
Table 1.
Characteristics of AGS from patients with CLDs
| CLD | No. of strains of each species in each Lancefield groupa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
S. anginosus (n = 15) |
S. constellatus (n = 8)b |
|||||||||
| NG | A | C | F | G | NG | A | C | F | G | |
| CF | 2 | 0 | 0 | 2c | 1 | 3 | 0 | 0 | 0 | 0 |
| COPD | 3d | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Bronchiectasis | 3 | 0 | 2 | 1 | 1 | 3 | 0 | 0 | 0 | 0 |
NG, nongroupable.
All strains of S. constellatus were alpha-hemolytic.
One strain was beta-hemolytic.
One strain was nonhemolytic.
In summary, NAS agar incubated anaerobically appeared to be a relatively simple strategy for isolating and quantifying AGS from these challenging clinical specimens. We believe that this represents an important additional diagnostic tool which can be used by clinical microbiologists to monitor the involvement of AGS in respiratory disease and used in research to study the microbial dynamics of CLD infections.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 50:1477–1491 [DOI] [PubMed] [Google Scholar]
- 2. Grimwood K. 2011. Airway microbiology and host defences in paediatric non-CF bronchiectasis. Paediatr. Respir. Rev. 12:111–118 [DOI] [PubMed] [Google Scholar]
- 3. Maeda Y, et al. 2011. Population structure and characterization of viridans group streptococci (VGS) including Streptococcus pneumoniae isolated from adult patients with cystic fibrosis (CF). J. Cyst. Fibros. 10:133–139 [DOI] [PubMed] [Google Scholar]
- 4. Mojon P. 2002. Oral health and respiratory infection. J. Can. Dent. Assoc. 68:340–345 [PubMed] [Google Scholar]
- 5. Parkins MD, Sibley CD, Surrette MG, Rabin HR. 2008. The Streptococcus milleri group—an unrecognized cause of disease in cystic fibrosis. Pediatr. Pulmonol. 43:490–497 [DOI] [PubMed] [Google Scholar]
- 6. Sibley CD, et al. 2008. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 105:15070–15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibley CD, et al. 2010. McKay agar enables routine quantification of the ‘Streptococcus milleri’ group in cystic fibrosis patients. J. Med. Microbiol. 59:534–540 [DOI] [PubMed] [Google Scholar]
- 8. Takao A, Nagamune H, Maeda N. 2004. Identification of the anginosus group within the genus Streptococcus using polymerase chain reaction. FEMS Microbiol. Lett. 233:83–89 [DOI] [PubMed] [Google Scholar]
- 9. Tunney MM, Field H, et al. 2008. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 177:995–1001 [DOI] [PubMed] [Google Scholar]
- 10. Whiley RA, Fraser H, Hardie JM, Beighton D. 1990. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the “Streptococcus milleri group.” J. Clin. Microbiol. 28:1497–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whiley RA, Freemantle L, Beighton D, Radford JR, Hardie JM. 1993. Isolation, identification and prevalence of Streptococcus anginosus, S. intermedius and S. constellatus from the human mouth. Microb. Ecol. Health Dis. 6:285–291 [Google Scholar]
- 12. Whiley RA, Hall LMC, Hardie JM, Beighton D. 1999. A study of small-colony, β-haemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int. J. Syst. Bacteriol. 49:1443–1449 [DOI] [PubMed] [Google Scholar]