Governing the state of mitosis.
The drastic changes that are needed for nuclear and cellular division are imposed by cyclin B-Cdk1, monarch of mitosis, as it becomes active and phosphorylates its numerous substrates. For the orders of cyclin B-Cdk1 to be effectively executed, however, different members of a cabinet of mitotic kinases, including Aurora A and Polo-like kinase 1 (Plk1), must participate. Recently, a particular role has emerged for the kinase Greatwall (Gwl), first identified as a regulator of mitosis in Drosophila melanogaster (1). When active, Gwl phosphorylates two related small proteins, α-endosulfine (Ensa) and Arpp19, that then block activity of the B55-subunit-associated form of protein phosphatase 2A (PP2A) (5, 6). Because PP2A undermines the effects of cyclin B-Cdk1, keeping PP2A in check is needed for decisive mitotic entry and support of the mitotic state. Gwl could help to prevent insubordination by PP2A, but it has been unclear how Gwl activity is regulated. In this issue, Blake-Hodek and colleagues investigate what contributes to Gwl activation in mitosis. They provide evidence that cyclin B-Cdk1 (MPF in Xenopus laevis), with a good helping hand from its small Cdk-binding partner Cks (p9 in Xenopus), phosphorylates Gwl directly. This priming event then facilitates Gwl phosphorylation of its own C terminus, which subsequently induces a strong increase of its kinase activity toward Ensa (2). Such a fine example of loyalty (self-activation upon a direct order to block rebellious PP2A) would make Gwl the best servant of cyclin B-Cdk1 (Fig. 1A). In this model, increasingly active Gwl would also facilitate the well-known positive feedback at the G2-to-mitosis transition, maintaining phosphorylation of Cdc25 and Wee1 and thus boosting cyclin B-Cdk1 activation further (Fig. 1B). It is still unclear, however, whether some Gwl molecules could first gain activity earlier in G2, acting truly upstream of the feedback leading to cyclin B-Cdk1 activation. Perhaps there is a role for other regulatory kinases in activating Gwl. For instance, Vigneron et al., who also studied Gwl phosphorylation in detail earlier, found that Plk1 could govern Gwl activation (10). The study by Blake-Hodek et al. questions whether there is a role for Plk1 but does allow for a model in which Gwl takes orders from a relative closer to the royal leader, cyclin A-Cdk-Cks (4). Perhaps complexes between cyclin A, Cdk, and Cks that accumulate during G2 (12) could influence the correct timing of Gwl activation (Fig. 1B). A role for mitotic cyclin-Cdk complexes in Gwl activation also includes a timely mechanism for Gwl downregulation by the end of metaphase, when cyclin B is destroyed. Now, reappearing PP2A activity would help to promptly erase the emperor's traces and perhaps those of its servants.
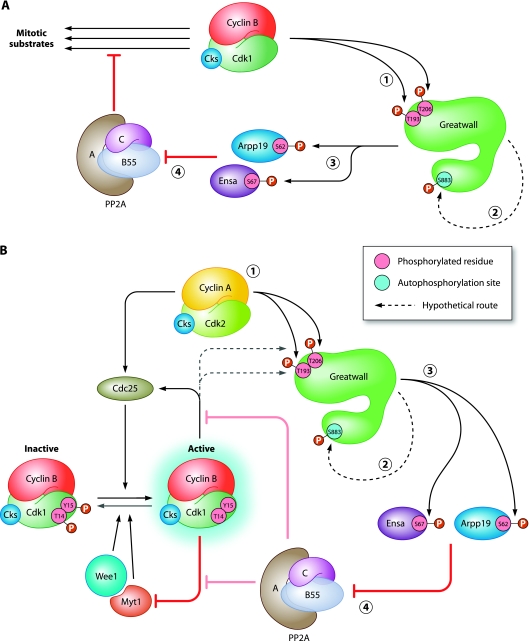
Fig 1.
Model for the regulation of Greatwall activation. (A) Greatwall reinforces the effects of cyclin B-Cdk1. Once activated, cyclin B-Cdk1 phosphorylates Gwl on multiple residues, including the critical residues Thr193 and Thr206 (step 1). Consequently, Gwl autophosphorylates its Ser883 residue (step 2), rendering Gwl fully active. Next, Gwl phosphorylates the small inhibitory proteins Ensa and Arpp19 (step 3), thereby blocking PP2A-B55 activity (step 4). This helps cyclin B-Cdk1 to execute its phosphorylation program and stably govern the mitotic state. The sites Thr193, Thr206, and Ser883 refer to the Xenopus Gwl numbering. (B) Greatwall is part of the cyclin B-Cdk1 activation loop. Cyclin A-Cdk2 supports the activation of cyclin B-Cdk1 by phosphorylations of both Cdc25 and Gwl. Once phosphorylated on residues Thr193 and Thr206 (step 1), Gwl undergoes autophosphorylation on Ser883, which further activates its kinase activity (step 2). Subsequently, Gwl phosphorylates its targets Ensa and Arpp19 (step 3), thereby downregulating PP2A-B55 activity toward cyclin B-Cdk1 substrates (step 4). Accumulating active complexes of cyclin B-Cdk1 catalyze the self-activating chain reaction by activating Cdc25, inhibiting Wee1, and strengthening PP2A inhibition by Gwl.
Gwl as an atypical AGC kinase.
Both reports about the mechanisms of Gwl activation show that, despite bearing resemblance to the AGC family of kinases (including protein kinase A [PKA], PKC, and PKB/c-AKT), Gwl is also different (2, 10). For one thing, Gwl has a supersized T loop (∼500 amino acids) separating its N- and C-terminal catalytic subdomains. This element in Gwl is poorly conserved between species (hence coined NCMR, for nonconserved middle region, by Blake-Hodek et al. [2]) and appears largely dispensable for Gwl functionality, at least in vitro (2, 10). Nevertheless, many mitotic phosphorylation sites were found scattered throughout the Gwl NCMRs of different species. This may hint at a broader role for phosphorylation of this region, such as a gross charge change, which could, speculatively, modify interactions with regulatory factors in cells. In vitro, cyclin B-Cdk1-Cks activates Gwl quite well, but specific activity falls just short of that of Gwl retrieved from insect cells which were artificially forced into mitosis. It remains to be seen whether the action of an additional factor, such as a kinase apart from Cdk and Gwl itself, must be invoked to explain full Gwl activation in normal mitosis. Some indication that another Gwl-regulatory factor is still missing comes from the observation by both groups that Gwl contains an N-terminally located, functional hydrophobic pocket, characteristically found to influence activation in other AGC kinases. Remarkably, however, Gwl, like another atypical AGC kinase, PDK1, lacks a hydrophobic C-terminal motif which would normally fold into the hydrophobic pocket for intramolecular stabilization of the active state. Vigneron et al. postulated, therefore, that perhaps a more canonical AGC kinase, such as RSK2, might lend its phosphorylated hydrophobic motif to assist in Gwl activation (10), an idea that had also been put forward to explain PDK1 activation by RSK2 (3).
Gwl phosphorylation events.
Mass spectrometry analysis revealed that Xenopus laevis Gwl is hyperphosphorylated during mitosis, whereas only very few phosphorylation sites are detected in interphase (e.g., Ser465 and Thr725 in Xenopus numbering) (2, 10). Almost half of the mitotic Gwl phosphorylation sites identified are Ser/Thr-Pro motifs, fitting the cyclin-Cdk target consensus. Blake-Hodek and colleagues identified phosphorylation of Thr193 and Thr206, as well as Ser883, to be essential for Gwl activation in mitosis, whereas Vigneron et al. focused on Ser883 (Ser875 in human Gwl) as the key site. Thr193 and Thr206 are proline-directed sites targeted by MPF in vitro, but Ser883 does not fit known kinase consensus sites. Now, Blake-Hodek et al. provide good evidence that Ser883 could be the target of an intramolecular Gwl autophosphorylation event, providing that the activation loop sites Thr193 and Thr206 become phosphorylated by MPF as a priming step (2). They find that Plx1 (Xenopus Plk1) can also phosphorylate Gwl but only on nonconserved residues within the NCMR. In contrast, Vigneron et al. showed that the site equivalent to Ser883 in human Gwl (Ser875) can be phosphorylated by Plx1 (10). Nevertheless, they also found that a partially active mutant of human Gwl (encoded by a cDNA resembling the dominant Scant allele of the Drosophila Gwl gene) acquires phosphorylation on Ser875 in vitro, in line with the possibility of Gwl autophosphorylation (10). Although Plk1 may support Gwl activation in vitro, there is also genetic evidence for antagonistic interactions between these kinases (1, 7). In summary, while the roles of Plk1 or other kinases in Gwl activation remain unsettled, MPF-mediated priming followed by Gwl autoactivation seems an attractive model that fits well into established positive and negative mitotic pathways.
Gwl: foolproof to phosphomimetics.
Aspartate (Asp) and glutamine (Glu), even though negatively charged, are obviously not identical to phosphorylated Ser/Thr residues. Still, it is somewhat surprising that none of the critical Gwl phosphorylation sites could be mimicked by Asp/Glu to create a constitutively active mutant (2, 10). Combining the mutations Ser101Asp and Thr193Glu did render Gwl slightly active, approaching ∼15% of the maximal activity observed in mitosis, but this was unexpected given that Ser101 was not identified as a stable phosphorylation site by mass spectrometry (2). Here, the authors speculate that Ser101Glu might stabilize an active conformation of Gwl that, in combination with the critical Thr193 site, enhances Gwl autoactivation. Some kind of stabilization of the active Gwl conformation seems a relevant matter, so perhaps, in vivo, an independent factor participates after all to keep Gwl in shape once it is active, which would help to explain further why the tested amino acid substitutions alone are insufficient.
Conclusions.
The emerging picture is that Gwl is activated by mitotic kinases and thereby reinforces their effects. The proposed autoactivation step would create a further feed-forward effect appropriate to a bistable regulation of mitotic entry. Further studies of the substrates of Gwl in different organisms may shed more light on the pathways that are involved. Saccharomyces cerevisiae Gwl Rim15 bears resemblance to Gwl and phosphorylates Igo1 and Igo2 (orthologs of Ensa/Arpp19), yet plays a role after mitosis in stabilizing G0-regulatory mRNAs (9). In Schizosaccharomyces pombe, overexpression of the Gwl look-alike Cek1 rescues cut8-563 mutants from cytokinesis failure upon incomplete sister chromatid separation (8). Interestingly, human Gwl plays a role in successful anaphase and completion of cytokinesis, too (11). Altogether, knowing who activates Gwl, and who obeys its orders in return will help us understand better how the distinct consecutive events in mitotic entry and mitotic exit are coordinated.
ACKNOWLEDGMENTS
Our work on the G2/M transition is supported by a research grant from the Netherlands Cancer Society (KWF 2008-4135).
We thank our lab members for support and inspiring discussions.
Footnotes
Published ahead of print 5 March 2012
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1. Archambault V, Zhao X, White-Cooper H, Carpenter AT, Glover DM. 2007. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 3: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blake-Hodek KA, et al. 2012. Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol. Cell. Biol. 32: 1337–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frödin M, et al. 2002. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 21: 5396–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fung TK, Ma HT, Poon RY. 2007. Specialized roles of the two mitotic cyclins in somatic cells: cyclin A as an activator of M phase-promoting factor. Mol. Biol. Cell 18: 1861–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gharbi-Ayachi A, et al. 2010. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330: 1673–1677 [DOI] [PubMed] [Google Scholar]
- 6. Mochida S, Maslen SL, Skehel M, Hunt T. 2010. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330: 1670–1673 [DOI] [PubMed] [Google Scholar]
- 7. Rangone H, et al. 2011. Suppression of scant identifies Endos as a substrate of greatwall kinase and a negative regulator of protein phosphatase 2A in mitosis. PLoS Genet. 7: e1002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samejima I, Yanagida M. 1994. Identification of cut8+ and cek1+, a novel protein kinase gene, which complement a fission yeast mutation that blocks anaphase. Mol. Cell. Biol. 14: 6361–6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talarek N, et al. 2010. Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5′-3′ mRNA decay pathway. Mol. Cell 38: 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vigneron S, et al. 2011. Characterization of the mechanisms controlling Greatwall activity. Mol. Cell. Biol. 31: 2262–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voets E, Wolthuis RM. 2010. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 9: 3591–3601 [DOI] [PubMed] [Google Scholar]
- 12. Wolthuis R, et al. 2008. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell 30: 290–302 [DOI] [PubMed] [Google Scholar]