Abstract
GRTH, a testis-specific member of the DEAD-box family of RNA helicases essential for spermatogenesis, is present in Leydig cells (LC) and germ cells. In LC, it exerts an autocrine negative regulation on androgen production induced by gonadotropin. GRTH is transcriptionally upregulated by gonadotropin via cyclic AMP/androgen through androgen receptors (AR). For studies of GRTH regulation by androgen in LC, we utilized in vitro/in vivo models. Androgen-induced GRTH expression was prevented by an AR antagonist. Two putative atypical ARE half-sites are present at bp −200 and −827 (ARE1 and ARE2). Point mutation of ARE2 prevented androgen-induced AR binding/function and upregulation of GRTH transcription. Chromatin immunoprecipitation (ChIP) assays showed recruitment of AR, SRC-1, Med-1, transcription factor IIB (TFIIB), and polymerase II (PolII) to GRTH ARE2 (bp −980/−702) and to the promoter region (bp −80/+63). ChIP3C assays revealed short-range chromosomal looping between AR/ARE2 and the core transcriptional machinery at the promoter. Knockdown of Med-1 and/or SRC-1 demonstrated the presence of a nonproductive complex which included AR, TFIIB, and PolII and the essential role of these coactivators in the transcriptional activation of GRTH. Our findings provide new insights into the molecular mechanism of androgen-regulated transcription in LC.
INTRODUCTION
Androgens (A) are steroid hormones that determine the expression of the male phenotype and are essential for the development of secondary sex characteristics and the initiation and maintenance of spermatogenesis (4, 12). Testosterone and dihydrotestosterone (DHT) are active A produced mainly in the Leydig cells (LC) of the testis. Their production is induced by the action of luteinizing hormone through its cognate receptor on the cell membrane of LC. The testis is not only the main source of A, but it is also a key target for A actions (31) that are mediated by the A receptor (AR) expressed in LC and Sertoli cells (4). The AR, a member of the nuclear receptor family, regulates gene transcription in several target organs. A induces AR activation and its translocation to the nucleus of target cells, where it binds the A response element(s) (ARE) of A-regulated genes and recruits/cross talks with transcription factors, coactivators, histone acetyltransferase enzymes, and components of the general transcription machinery to activate transcription (2).
GRTH/DDX25 is a testis-specific member of the DEAD-box family of RNA helicases present in LC and germ cells (26). It is a multifunctional protein that is essential for the completion of spermatogenesis (7, 25, 29). Males lacking GRTH are sterile due to the absence of sperm resulting from failure of round spermatids to elongate (30). In the LC, GRTH/DDX25 has a negative effect on the mRNA stability of the steroidogenic regulatory protein (StAR) with consequent reduction of mitochondrial cholesterol and A production induced by gonadotropin. Testosterone production by LC of GRTH-null mice is highly magnified over that of the wild type (WT) upon gonadotropin stimulation in vivo and in vitro due to removal of GRTH's negative effect on the StAR message half-life (9). These recent studies have revealed that GRTH has a regulatory role in gonadotropin-induced A production by LC.
GRTH is the only member of the RNA helicase family of proteins to be hormonally regulated, and it is developmentally regulated in pubertal and adult testes (26). Physiological studies demonstrated that GRTH in LC is transcriptionally upregulated by human chorionic gonadotropin (hCG). Induction of GRTH expression by hCG is mediated via a second messenger (cyclic AMP [cAMP]) and A at the transcriptional level, presumably by direct or indirect actions of A through AR present in LC (24). Such hCG-induced increases were prevented when LC were preincubated with a mixture of enzyme inhibitors of the steroidogenic pathway (26) that effectively abolished A production. Similar to hCG and cAMP, DHT significantly increased the expression of GRTH. This action of A was confirmed by in vivo studies of animals treated with the AR inhibitor flutamide, which prevented the GRTH increases induced by hCG (24). Studies with transgenic mice carrying sequential deletions of 5′-flanking sequences of the GRTH gene showed that the 1-kb (bp −1085/+63) transgene-directed expression was required for hCG-induced gene activation. This stimulation was blocked by flutamide treatment (28).
There are two potential nonconsensus ARE half-sites (TGTCCC), at bp −827 and −200, in the 1-kb 5′-flanking region of the GRTH gene (27) that resemble functional sites of the human secretory component (sc) gene (11). To elucidate the mechanism of A action of GRTH, we employed the GRTH-expressing hypothalamic neuron cell line GT1-7 stably expressing the AR and adult LC of mice treated in vivo with gonadotropin to induce endogenous production of A. These studies have provided new insights into A-induced AR control of GRTH transcription/expression.
MATERIALS AND METHODS
Animal treatment and LC preparations and culture.
Adult C57BL/6-SV129J strain mice (Charles River Laboratories Inc., Wilmington, MA) were housed under pathogen-free, temperature- and light-controlled conditions (20°C; alternating light-dark cycle with 14 h of light and 10 h of darkness). All animal studies were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. For in vivo studies to explore gonadotropin-mediated AR, coactivator and components of the preinitiation complex (PIC), and polymerase II (PolII) recruitment to the GRTH promoter in LC, adult male mice were pretreated with 0.5 mg of the AR antagonist flutamide 2-methyl-N[4-nitro-3-(trifluoromethyl)-phenyl]propanamide (Sigma-Aldrich, St. Louis, MO) twice a day for 3 days; this was followed by 2 injections of flutamide 12 h apart and a single subcutaneous 0.5-μg dose of hCG (Organon, Roseland, NJ) 24 h before sacrifice. Animals were killed by asphyxiation with CO2 and decapitated 24 h after hCG or the vehicle (control) treatment. LC were prepared by collagenase dispersion and purified by centrifugal elutriation as previously described (25).
We also utilized primary cultures of mouse LC to explore the role of Med-1 and SRC-1 in A-induced GRTH transcription. LC were cultured as previously described (26). Briefly, cells were cultured in medium 199 with 0.1% bovine serum albumin in the presence of Med-1, SRC-1, or scrambled small interfering RNAs (siRNAs). At 24 h after transfection, cells were incubated with inhibitors of steroid biosynthesis (aminoglutethimide at 100 μg/ml, cyanoketone at 1 μM, and spironolactone at 10 μM; Sigma) for 1 h and kept throughout the culture. One hour later, cells were cultured in the presence or absence of DHT at 10−8 M for 20 h. Subsequently, cells were evaluated for mRNA content and subjected to chromatin immunoprecipitation (ChIP) assays as described below.
Reagents, antibodies, and plasmids.
DHT was obtained from Steraloids Inc., Newport, RI. Flutamide and nilutamide were obtained from Sigma-Aldrich. The mouse GRTH promoter/luciferase reporter gene p−1084/+63, p−501/+63, and p−65/+63 constructs were previously described (27). To mutate the ARE half-site located at bp −827 and −200, the site-directed mutagenesis kit from Stratagene (La Jolla, CA) was used. AR expression vector was from GeneCopoeia Inc., Rockville, MD. The probasin promoter (270 bp)-luciferase pGL3 construct was kindly provided by Zoran Culig, Innsbruck Medical University, Innsbruck, Austria (19). The rabbit polyclonal antibody against GRTH peptide (amino acids 466 to 467) purified by affinity chromatography and used in this study was previously described (24). The antibody against AR (AG-21) was from Millipore (Billerica, MA). Antibodies against histone deacetylase 1 (HDAC1), PolII, ARA54, ARA55, ARA70, GATA2, FoxA1, ETS-1, and transcription factor IIB (TFIIB) were from Santa Cruz Biotechnology, Santa Cruz, CA. Antibody against SRC-1 was from BD Bioscience, San Jose, CA. Antibodies against Med-1 and Oct-1 were from Abcam, Cambridge, MA. Antibodies against β-actin and Flag were from Sigma-Aldrich.
Generation of a GT1-7 cell line expressing the AR (GT1-7AR).
Immortalized hypothalamic neuronal GT1-7 cells (20) that express GRTH (27) were kindly provided by Pamela Mellon, University of California, San Diego. These cells, which express very low levels of AR, were stably transfected with an AR expression vector (GeneCopoeia Inc., Rockville, MD). Full-length AR cDNA was cloned into the pReceiver vector, which contains the neomycin resistance gene (GeneCopoeia) in frame with a C-terminal tag encoding the Flag epitope. The construct was transfected into GT1-7 cells cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM) in the presence of 10% fetal bovine serum using Lipofectamine TM2000 reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's protocol. Cells were selected by treatment with Geneticin (Invitrogen) at 600 μg/ml for 2 weeks to obtain stable transfectants. Clones were expanded and tested for AR expression by Western blot analyses using antibodies against AR and Flag.
Cell culture, transfection, and reporter gene assay.
GT1-7 controls and GT1-7AR cells were maintained in DMEM-F12 (Invitrogen) supplemented with 10% fetal bovine serum. For transfection experiments, 40,000 cells were culture in 12-well plates under very low serum and steroid-free conditions in phenol red-free medium without supplemental growth factors with 5 and 1% dextran charcoal-treated fetal bovine serum for 3 days at each serum concentration. Cells were then transiently cotransfected with 0.25 μg of the 5′-flanking region of GRTH with the reporter pGL3 gene or the empty vector (basal control) and 50 ng of the pCMVSport/β-galactosidase construct (for normalization) using Lipofectamine 2000 reagent (Invitrogen) in accordance with the procedures recommended by the manufacturer. Sixteen hours later, DHT at 10−8 M or the vehicle was added, and luciferase activity was determined 24 h later.
Site-directed mutagenesis.
Mutagenesis of the ARE half-site was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The construct p−1085/+63GL was used as the template. We used primers AAAGCTCGCGGGTTTCCCGCACAGCTGCGCCCCGCAC (forward) and CGCAGCTGTGCGGGAAACCCGCGAGCTTTCCGTTTGAC (reverse) to create p−1085/+ARE1X, CTAAGAGATTGAGATCCTTTCCCAACATCGTTTAATTAACAG (forward) and CTGTTAATTAAACGATGTTGGGAAAGGATCTCAATCTCTTAG (reverse) to create p−1085/+ARE2X, TGAAAACTAAGAGAGCTCGCTCCTGTCCCAACATCGTTTAATTAACAGAAATTTAACC (forward) and AACGATGTTGGGACAGGAGCGAGCTCTCTTAGTTTTCATATAGGGTAGCCAGC (reverse) to create 5′m3-ARE, and ATTGAGATCCTGTCCCAACCAGCTGTAATTAACAGAAATTTAACCTTGAGAGTCG (forward) and GTTAAATTTCTGTTAATTACAGCTGGTTGGGACAGGATCTCAATCTCTTAGTTTTG (reverse) to create 3′m3-ARE. All mutations were confirmed by sequencing.
Real-time reverse transcription (RT)-PCR.
Total RNA was isolated from GT1-7C and GT1-7AR cells cultured in the presence or absence of DHT (0 to 10 nM) for 24 h or from LC from mice treated with the buffer control, hCG, flutamide, or hCG-flutamide using TRIzol (Invitrogen). One microgram of the total RNA was used for the RT reaction in a final volume of 20 μl using the Superscript II First Strand RT synthesis kit (Invitrogen). One-step real-time RT-quantitative PCR (RT-qPCR) was carried out using SYBR green QPCR Master Mix and an ABI 7500 sequence detection system (Applied Biosynthesis, Foster City, CA). The specific primers for amplification of GRTH mRNA were TCCAAAATCCAAGAGATGGC (forward) and GAACTTGCCCATCCTTTCAA (reverse). The primers for β-actin were GGTACCACCATGTACC CAGG (forward) and GAAAGGGTGTAAAACGCAGC (reverse). The primers for Med-1 were CCCTGTGAATG ACTCCCTTGTG (forward) and CCTCATCGTCACAGGAATGGAC (reverse). The primers for SRC-1 were GAGCGGCATAAAATTCTGCACCG (forward) and CACTGACACA GCAGTAGAGGCT (reverse). The cycling program was denaturation at 95°C for 10 min followed by 30 cycles of 95°C for 15 s and 60°C for 1 min. The specificity of the PCR products was verified by melting curve and agarose gel analyses. Values for each gene were normalized to the expression levels of β-actin. At least five independent experiments were performed to demonstrate reproducibility.
Whole-cell lysate and nuclear protein preparation and Western blot analysis.
Whole-cell lysates were prepared using radioimmunoprecipitation assay buffer (Thermo Scientific, Rockford, IL). The cytosolic and nuclear fractions from the treated and nontreated cells were isolated using the Pierce NE-PER nuclear protein extraction kit (Thermo Scientific). All of the preparations were performed in the presence of 1× Complete protease inhibitor mixture (Roche Applied Science, Mannheim, Germany) and 1× Halt phosphatase inhibitor mixture (Thermo Scientific).
DNA affinity precipitation assays (DAPA).
DAPA were performed as previously described (6). For these studies, we used the following 5′-end biotin-labeled sense and antisense oligonucleotides containing GRTH 5′-flanking sequence with WT ARE2: −836/−812, TTGAGATCCTGTCCCAACATCGTTT and its mutant form (change underlined) TTGAGATCCTTTCCCAACATCGTTT. For ARE1, we used the following: −209/−183, GCTCGCGGGTGTCCCGCACAGCTGCG; −852/−818, TATGAAAACTAAGAGATTGAGATCCTGTCCCAACA; −831/−797, ATCCTGTCCCAACATCGTTTAATTAACAGAAATTT; −78/−39, CATTGGCCACTGAGCCGAGCCGGCGCTGCCCCGCGCGGGG; −49/−10, CGCGCGGGGGTGGCAGCACGTGCCAGGGGCGAGAGCGGA; −20/+21, AGAGCGGAGACCGCAGCTATGGCGTCGTTACTTTGGGGA; +12/+52, CTTTGGGGAGGCGACGCCGGGGCGGCGGAGAGCGAGCGGC; +40/+63-Intron17bp, AGAGCGAGCGGCTGAACAGCCACGTAACTGCCGCTGAGCA. The ARE half-site of the prostate-specific antigen (PSA) promoter (AAATATGATAGCATCTTGTTCTTAGTCTTT) (14) was used as a positive control. The oligomers were annealed and purified by DNA extraction. Nuclear extracts (50 μg) were preincubated with 20 μl of streptavidin-agarose (Promega, Madison, WI) for 1 h at 4°C with rotation. The supernatant collected by centrifugation was incubated with 0.5 μg of wild or mutant biotin-labeled probed in binding buffer (20 mM HEPES pH 7.9, 50 mM NaCl, 1 mM DTT, 0.01% NP-40 and 5% Glycerol) overnight at 4°C with gentle rotation. DNA-protein complexes were washed five times with binding buffer; pellet was resuspended in 40 μl of 2× protein sample buffer (Bio-Rad, Hercules, CA) and then boiled for 10 min to dissociate complexes. Proteins were resolved by polyacrylamide gel electrophoresis, followed by Western blot detection with specific antibodies.
ChIP and ChIP3C assays.
ChIP assays were performed using MAGnify ChIP System from Invitrogen in accordance with the manufacturer's instruction. Briefly, 2 × 105 cells (LC or GT1-7 cells) were cross-linked with 1% (vol/vol) formaldehyde for 10 min at 37°C and then incubated with 125 mM glycine for 5 min. Cross-linked cells were washed with phosphate-buffered saline and resuspended in lysis buffer, and chromatin was sheared to a size of 500 bp (average) by sonication with 13 pulses at 30% power using a Misonix sonicator 4000 (Misonix, Farmingdale, NY). The sheared chromatin diluted 10-fold with dilution buffer was immunoprecipitated overnight with 2 μg of the antibodies of interest or normal IgG precoupled to Dynabeads. After extensive washes with immunoprecipitation (IP) buffer 1 and IP buffer 2, the antibody-Dynabead complexes were reverse cross-linked and DNA was purified with DNA purification magnetic beads. The relative binding of the proteins of interest to the GRTH 5′-flanking gene sequence was quantitatively analyzed by real-time PCR assay of the precipitated DNA and input DNA using SYBR green Master Mix in an ABI 7500 sequence detection system. The primers utilized for amplification of the GRTH gene sequence span the −980/−702, −588/−353, −290/−150, and −80/+63-Intron17bp regions. ChIP3C/ChIP-loop assays were performed as described previously (5, 33), with minor modifications. Briefly, antibody-specific immunoprecipitated chromatin was obtained as described for ChIP assays. Chromatin bound to Dynabeads was digested with the restriction enzyme BtgI, ligated with T4 DNA ligase (Promega), eluted, and reverse cross-linked. After purification, the ChIP3C material was detected for short-range interaction with a set of primers (−980/−960 and intron17bp) spanning the region from the AR binding region (−827/−822) to the GRTH transcriptional start site.
siRNA analyses.
Validated siRNAs designed to knock down the endogenous expression of Med-1, SRC-1, GATA2, Oct-1, FoxA1, and ETS-1 and scrambled control siRNAs were purchased from Ambion (Applied Biosystems). Transfections of siRNA to GT1-7AR cells were carried out with siPORTNeoFX reagent (Ambion, Applied Biosystems) as previously described (17, 37). Briefly, diluted siPORTNeoFX were incubated with the indicated siRNA (30 nM) in Opti-MEM I reduced-serum medium (Invitrogen) at room temperature for 10 min. The transfection complexes formed were then dispensed into 12-well plates and overlaid with cells at a density of 5 × 105/ml. Twenty-four hours after transfection, cells were replaced with normal growth medium and cultured for an additional 40 h before harvesting. For reporter gene assays, the GRTH gene promoter/reporter gene (p−1085/+63) was transfected using Lipofectamine 2000 at 24 h after siRNA transfection. Sixteen hours later, DHT at 10−8 M or the vehicle was added, and luciferase activity was determined 24 h later.
Statistical analysis.
The significance of the differences between groups in all studies was determined by the nonparametric Kruskal-Wallis test followed by Dunn's multiple-comparison test using Prism statistical software version 4.
RESULTS
GRTH expression is upregulated by A in GT1-7 cells stably expressing the AR.
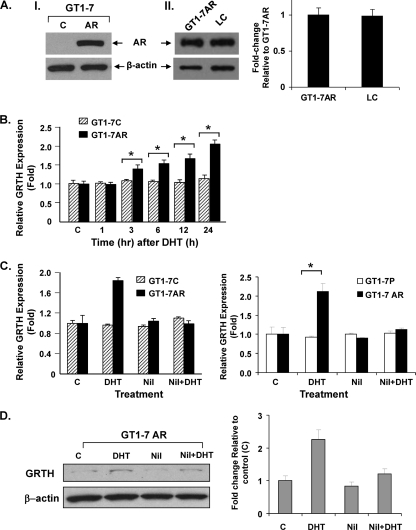
To investigate the transcriptional mechanism by which GRTH is regulated by A in LC, we stably transfected GT1-7 cells with an AR expression vector. GT1-7 cells are immortalized hypothalamic neuronal cells that express low levels of GRTH mRNA in basal conditions and lack AR expression. GT1-7 cell lines stably expressing the AR (GT1-7AR) showed high levels of AR compared to cells with the empty vector (control) (Fig. 1A, part I). GT1-7AR levels of AR were similar to those observed in mouse LC (Fig. 1A, part II). Treatment of GT1-7AR with A (DHT at 10−8 M) for 1 to 24 h produced significant upregulation of GRTH mRNA expression at 3 h (P < 0.05), and further increases were observed thereafter, reaching 2 times that of the controls at 24 h (Fig. 1B). However, A did not increase GRTH mRNA expression in GT1-7 cells expressing the empty vector (control) (Fig. 1C left panel) or GT1-7 parental cells ((Fig. 1C, right panel). The A-induced increases were prevented by treatment of cells with the AR antagonist nilutamide for 1 h prior to the addition of A (Fig. 1C). Increases in GRTH expression induced by A were also observed at the protein level (Fig. 1D). Taken together, these results demonstrate that GRTH is upregulated by A through AR in GT1-7 cells.
Fig 1.
GRTH expression is transcriptionally upregulated by DHT in GT1-7 cells stably expressing AR. (A, part I) Western blot analysis of extracts from GT1-7 cells expressing AR (GT1-7AR) or control cells (lane C) with the empty vector. β-Actin expression was used as a loading control. (A, part II, left half) Western blot analysis comparing AR levels in GT1-7AR cells and mouse LC. (A, part II, right half) Signals from three independent experiments were quantified and normalized to β-actin. The AR value in LC is presented as n-fold change relative to that of GT1-7AR. The values are means ± standard errors. (B) Real-time qPCR analysis of GRTH expressed in GT1-7AR (black bars) and GT1-7 cells (GT1-7C, striped bars) following treatment with the vehicle or DHT at 10−8 M for 1 to 24 h. (C, left half) Effect of the AR antagonist nilutamide (Nil) on DHT-induced GRTH mRNA expression in GT1-7 cells stably expressing AR (GT1-7AR). GT1-7 cells transfected with the empty vector were used as a control group (GT1-7C). (C, right half) GRTH expression in parental GT1-7 (GT1-7P) and GT1-7 cells stably expressing AR (GT1-7AR). Cells were treated with nilutamide at 1 μM for 1 h prior to incubation with DHT at 10−8 M for 24 h, after which GRTH expression was assessed by RT-qPCR. Results normalized to β-actin are expressed as n-fold over the control (cells treated with the vehicle only). Each value represents the mean ± the standard error of four separate experiments, each performed in triplicate. (D) Effect of A on GRTH protein expression. (Left half) Western blot analysis of extracts from GT1-7AR cells treated with DHT in the presence or absence of nilutamide. β-Actin was used as a loading control. (Right half) Diagram of n-fold changes due to various treatments (DHT, Nil, and Nil plus DHT) relative to the values of control cells treated with the vehicle only (bar C). Signals were quantitated and normalized to β-actin. Values are means ± standard errors. *, P < 0.05.
Identification of functional ARE essential for DHT-induced GRTH promoter activity in GT1-7 AR cells.
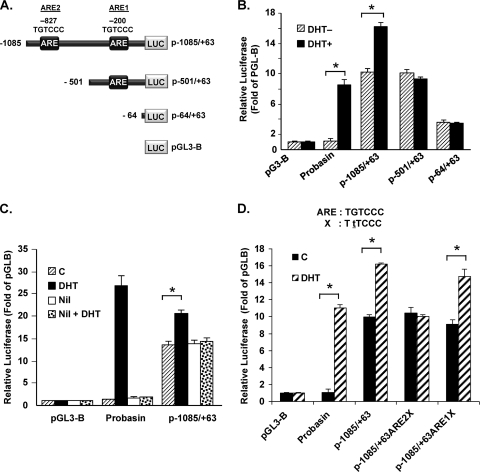
Green fluorescent protein reporter gene expression directed by the GRTH 5′-flanking sequences extending 1 kb was markedly increased by in vivo hCG treatment in LC (28). Computational analysis of the GRTH promoter sequence revealed the presence of two ARE half-sites located at bp −827/−822 and −200/−195 relative to the ATG codon. To further investigate GRTH expression induced by A, deletion constructs of the GRTH 5′-flanking region/promoter with the luciferase reporter gene were transiently transfected into GT1-7AR cells. This included p−1085/+63, which contains both AREs at −827 and −200; p−501/+63, with only one ARE at −200; and p−64/+63, with no AREs (Fig. 2A). pGL3Basic (empty vector) was used as a negative control. The promoter region of the gene for probasin, a gene known to be regulated by A, was used as a positive control. The promoter activity of p−1085/+63 was significantly increased by DHT treatment in GT1-7 cells expressing AR (Fig. 2B). However, no A induction was observed with either p−501/+63 or p−64/+63, which indicates that the region from −1085 to −501 is responsible for this regulation. To investigate whether the observed activation is mediated by AR, we treated GT1-7 AR cells transiently transfected with the p1085/+63 construct with DHT following preincubation with the AR antagonist nilutamide or the buffer control. Nilutamide prevented the activation of GRTH promoter activity by DHT, indicating that the induction by DHT is mediated by AR (Fig. 2C). Moreover, point mutation of the ARE half-site sequence TGTCCC to TTTCCC at −827 prevented DHT-induced promoter activity (Fig. 2D), indicating the essential role of this site in the observed activation. Furthermore, point mutation of the ARE1 half-site located at −200/−195 did not affect DHT-induced promoter activity, which confirms that this element is not functional. Taken together, these results indicate that GRTH is regulated by A in GT1-7 AR cells and that this process is mediated by AR, presumably by its association with the distal ARE half-site present within the 5′-flanking sequence of the GRTH gene.
Fig 2.
Identification of functional ARE essential for DHT-induced GRTH promoter activity in GT1-7AR cells. (A) Schematic representation of GRTH-luciferase expression constructs p−1085/+63GL and 5′deletions. p−1085/+63GL comprises the GRTH minimal promoter region (bp −205/+63) relative to the ATG translational codon (+1) and 5′-flanking sequences with ARE half-sites located at bp −827 and −201 (boxes). (B) Serial deletion constructs indicated in panel A were transfected into GT1-7AR cells in the presence (black bars) or absence (striped bars) of DHT at 10−8 M. Luciferase activity is expressed relative to that of pGL3basic (empty vector). (C) DHT-induced promoter activity was also assessed in the presence or absence of nilutamide (Nil) treatment as described in the legend to Fig. 1. (D) Effect of a point mutation within the ARE half-site located at −827 or at −200 on DHT-induced promoter activity of p−1085/+63. WT ARE and the mutant form (X, with the mutated base underlined) are shown at the top. Luciferase activity is expressed relative to that of pGL3-B (empty vector) in all cases. The probasin construct (see Materials and Methods) was the positive control. Results are the means ± the standard errors of three individual experiments done in triplicate. *, P < 0.01.
AR binds to the ARE site at bp −827/−822 of the GRTH 5′-flanking region in response to DHT by DAPA.
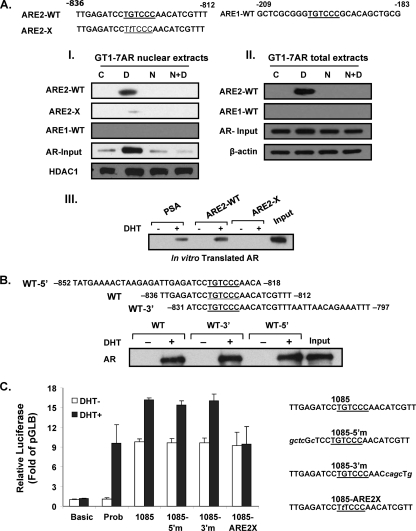
To determine whether AR binds to the GRTH 5′-flanking region, we performed DAPA using a GRTH probe that contains the ARE half-site at −827/−822 or at −200/−195. This was incubated with nuclear extracts (Fig. 3A, I) or total extracts (Fig. 3A, II) from GT1-7 AR cells treated with the vehicle control, DHT, nilutamide, or a combination of the latter two. DHT induced AR binding to the GRTH probe that contains −827/−822 ARE2 but not to −200/−195 ARE1, and this binding was prevented by preincubation of the cells with nilutamide in both total and nuclear extracts (Fig. 3A, I and II). Most important, point mutation of the ARE2 half-site almost abolished AR binding to the probe. Moreover, similar results were obtained when these GRTH probes were incubated with in vitro-translated AR (Fig. 3A, III). This indicated that the AR protein binds directly to the ARE half-site located at −827/−822 and that no other protein/transcription factor is mediating its binding.
Fig 3.
AR recruitment to the ARE half-site at −827/−822. DAPA were performed using probes for the WT ARE sites (underlined) with sequences 5′- and 3′-flanking nucleotides (10 bp). The ARE1-WT probe (−209/−183), the ARE2-WT probe (−836/−812), or a mutant form with a point mutation (G to T) in the ARE site at −826 (ARE2-X) was incubated with nuclear extracts (panel I) or total extracts (panel II) of GT1-7AR cells treated with the vehicle, DHT [D] (10−8 M), nilutamide [N] (1 μM), DHT plus nilutamide (N+D), or in in vitro-translated AR (panel III) in the presence or absence of DHT at 10−8 M. DNA-protein complexes were precipitated with streptavidin resin and subjected to Western blot analysis. Levels of AR are shown as the input using HDAC1 as a loading control for nuclear extracts or β-actin as a loading control for total extracts. Input, 10% in vitro-translated AR. Probasin (PSA), ARE2 WT (ARE2-WT), or mutant ARE2 (ARE2-X) was used as the probe. (B) Exploration of flanking sequence requirement for association of AR upon treatment with DHT to ARE2 located at bp −827/−822 of GRTH. DAPA analysis was performed using probes GRTH-WT (−836/−812), GRTH-WT-5′ (−852/−818), and GRTH-WT-3′ (−831/−797) with in vitro-translated AR in the presence or absence of DHT at 10−8 M. Ten percent in vitro-translated AR was included as the input. (C) Effects of mutations in 5′- or 3′-flanking sequence adjacent to ARE2 at −827 and sequences flanking nonfunctional ARE1 (italic) on DHT-induced promoter activity of p−1085/+63. WT p−1085/+63GL (1085) with the WT ARE (underlined) and mutations of the adjacent 5′ (5′m) and 3′ sequences (3′m) are shown on the right. Luciferase activity is expressed relative to that of pGL3 basic in all cases. The probasin construct (Prob) was used as a positive control. Results are the means ± the standard errors of three individual experiments done in triplicate.
Lack of effect on binding and function of sequences adjacent to the functional consensus ARE half-site.
AR dimerization is produced as a consequence of its activation by DHT (4). Generally the A/AR binds as a homodimer to an hexameric consensus DNA half-site repeat sequence spaced by 3 bp. In the case of GRTH, where only a half-site is present, the contribution of adjacent sequences to the binding of AR homodimer partners required further investigation. To study the impact of immediately adjacent sequences 5′ or 3′ to the ARE half-site located at −827/−822 on AR binding, we used probes containing the ARE 5′ or 3′ extensions and only 4 nucleotides (nt) in the 3′ or 5′ direction, respectively, for DAPA analysis. The DHT-induced AR association with these probes was essentially similar to that of the WT (−836/−812) (Fig. 3B). To further investigate the participation of the adjacent sequences 5′ and/or 3′ to the ARE half-site located at −827/−822, we performed functional studies. For these studies, we mutated the 5′ and 3′ adjacent sequences of the functional ARE2 site located at −827/−822 to the flanking sequences of the nonfunctional ARE1 half-site located at −200/−195 while leaving the immediately adjacent 3 nt on the 3′ and 5′ sides of ARE2 as in the WT and determined the transcriptional activity induced by DHT. As shown in Fig. 3C, none of the mutations introduced affected DHT-induced transcriptional activity except for the single-point mutation within functional ARE2. This suggests that the ARE2 half-site located at −827/−822 is mainly involved in DHT-induced promoter activity and weak interactions, if present, at 3′ and 5′ adjacent sequences do not appear to be required for productive transcription.
Recruitment of AR, SRC1, TFIIB, and PolII to GRTH gene promoter/5′-flanking region in GT1-7AR cells upon in vitro treatment with DHT.
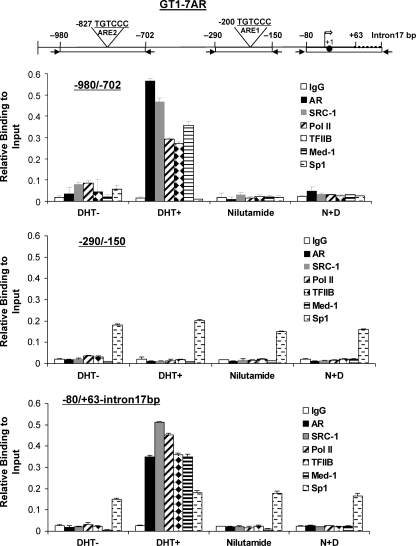
To further investigate the involvement of the ARE half-site located at −827/822 of the GRTH gene in DHT-induced GRTH expression, we performed ChIP assays with GT1-7 AR cells treated with the vehicle control, DHT, nilutamide, or a combination of the latter two. Three pairs of primers were used for this study, including (i) the −980/−702 primer, which encompasses the distal ARE half-site located at −827/−822; (ii) the −290/−150 primer, which covers the proximal nonfunctional ARE half-site; and (iii) the −80/+63-intron17bp primer, which encompasses the GRTH transcriptional start site region (Fig. 4). Recruitment of AR was detected in the −980/−702 region in the presence of DHT. Moreover, this was prevented by pretreatment of cells with nilutamide. No AR recruitment was observed at the −290/−150 region, which confirmed that the AR half-site located at −201/−195 is nonfunctional. Sp1 recruitment to the −290/−150 region was included as a positive control. An Sp1 functional site at −163/−155 which binds Sp1/Sp3 and is responsible for GRTH basal promoter activity was previously demonstrated in our laboratory (27). In addition, recruitment of Sp1 to the −80/+63-intron17bp region, where two putative Sp1 binding sites are present that were not explored in our previous study, is shown in Fig. 4 (bottom). In both cases, Sp1 recruitment was not affected by DHT treatment. Surprisingly, DHT induced AR recruitment to the −80/+63-intron17bp region, where no ARE was detected by sequence analysis or functional studies. ChIP assays showed, in addition to AR recruitment, recruitment of the SRC-1 coactivator Med-1 and the transcriptional machinery components TFII-B and PolII to the two regions of the 5′-flanking sequence of the GRTH gene, and the recruitment of all of these proteins was prevented by nilutamide. This implied that AR is part of a complex formed by AR and the SRC-1 coactivator Med-1 and the transcriptional machinery components. In addition, the crucial role of AR in the formation of the complex was demonstrated.
Fig 4.
Recruitment of AR, SRC-1, Med-1, Sp1, TFIIB, and PolII to the GRTH gene promoter/5′-flanking region in GT1-7AR cells upon in vitro treatment with DHT. Schematic representation of the locations of the GRTH gene regions (bp −980/−702, −290/−150, and −80/+63-intron17bp) analyzed by PCR for ChIP analysis (Top). (Bottom) ChIP analysis of GT1-7AR treated with the vehicle (DHT−), DHT at 10−8 M (DHT+), (N) at 1 μM, or DHT plus nilutamide (N+D). Recruitment of AR, SRC-1, Med-1, TFIIB, Sp1, and PolII to the −980/−702, −290/−150, and −80/+63-intron17bp regions was assessed by real-time PCR and expressed as a percentage of the total input DNA. The broken line in the diagram at the top is the 17-bp intron, the solid circle is the ATG codon at position +1, and the open arrow is the transcriptional start site. Results are the means ± the standard errors of three individual experiments done in triplicate.
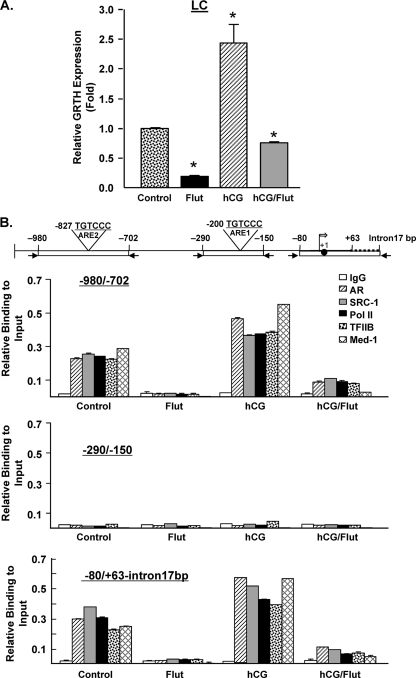
Recruitment of AR, SRC-1, Med-1, TFII B, and PolII to GRTH promoter/5′-flanking sequences in LC upon in vivo treatment with DHT and AR antagonist.
To investigate if the recruitment demonstrated in GT1-7AR cells is also observed in LC, we treated mice with hCG, flutamide (AR antagonist), or a combination of the two (see Materials and Methods). Untreated mice were used as a control. Analysis of LC from these groups by RT-qPCR confirmed that hCG treatment upregulated GRTH expression and flutamide decreased basal GRTH expression by 80% and also blocked hCG-induced GRTH expression (Fig. 5A). ChIP assays (Fig. 5B) showed recruitment of AR, SRC-1, Med-1, TFIIB, and PolII at both regions −980/−702 and −80/+63-intron17bp in control untreated animals, and this was prevented by flutamide treatment. The recruitment in the controls is explained by the effect of endogenous A present in these cells, and the blockade observed by the antagonist treatment indicates that this process is mediated by AR. Recruitment of AR, SRC-1, Med-1, TFIIB, and PolII was further increased by hCG and markedly reduced by flutamide treatment (Fig. 5B). These results confirmed our previous results with GT1-7AR cells in an in vivo system of physiological relevance. No recruitment of any of the proteins studied was observed at the −290/−150 region in any of the treatments, which confirms that the ARE half-site located at −200/−195 is not functional. These findings suggest the formation of a chromosomal loop between the upstream and promoter regions.
Fig 5.
Recruitment of AR, SRC-1, TFIIB, and PolII to GRTH promoter/5′-flanking sequences in LC upon in vivo treatment with DHT. Mice were treated with flutamide (Flut) (0.5 mg twice a day) for 2 days prior to treatment with hCG (0.5 μg), flutamide alone, or a combination of the two. Mice treated with the vehicle were used as a control. LC were prepared as described in Materials and Methods. (A) GRTH expression was assessed by RT-qPCR and normalized to β-actin expression. Results are expressed relative to those of untreated control cells. *, P < 0.01 (B) ChIP analysis using antibodies against IgG, AR, SRC-1, Med-1, PolII, and TFIIB. Real-time PCR to assess the recruitment of proteins to these regions was performed using primers for the −980/−702, −290/−150, and −80/+63-intron17bp regions (top). The broken line in the diagram at the top is the 17-bp intron, the solid circle is the ATG codon at position +1, and the open arrow is the transcriptional start site. Results are expressed as percentages of the total input DNA. Means ± standard errors of three individual experiments done in triplicate are shown.
Chromosomal loop formation as a result of interaction betweenthe −980/−702 region and the GRTH transcriptional start site.
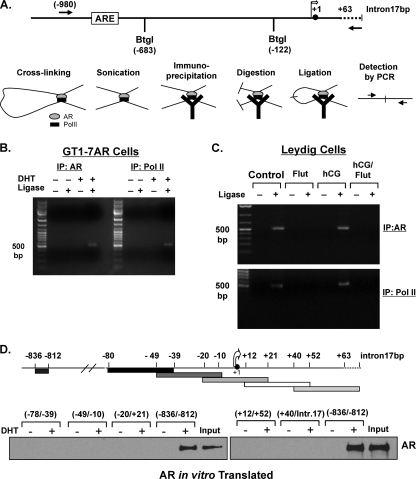
To further investigate if there is an interaction between the −980/−702 region and the GRTH transcriptional start site within the 5′-flanking sequence of the GRTH gene, we used a ChIP-coupled 3C/ChIP-loop assay. In this assay, which is a variation of the 3C assay, an immunoprecipitation step is included to enrich for a factor(s) bound to chromatin (21). Ligation products were detected by PCR using the primer pairs indicated in Fig. 6A. We used antibodies against AR and PolII, and the ligation product was detected in both cases only when cells were treated with DHT and ligase was included in the reaction mixture. In this case, the DHT/AR-induced formation of a chromosomal loop between the −980/−702 region and the GRTH transcriptional start site (−80/+63-intron17bp) in GT1-7AR cells is clearly shown (Fig. 6B).
Fig 6.
AR, coactivators, and PolII form a chromosomal loop between AR-bound ARE located at −827 and the GRTH transcriptional start site in GT1-7AR and mouse LC. (A) Schematic diagram showing the primers and restriction enzyme used in the 3C assay at the GRTH 5′-flanking sequence (−980/intron17bp). The diagram depicts the process of the ChIP3C/ChIP-loop assay. Y, antibody. Restriction enzyme BtgI was used for digestion. (B) 3C assays were performed with GT1-7AR cells treated with or without DHT for 24 h using AR (left) and PolII (right) antibodies. Ligation products were detected by PCR using the primers indicated in panel A. (C) 3C assay performed with LC from mice treated with control (untreated mice), flutamide (Flut), hCG, or hCG/Flut for 24 h using AR (top) or PolII antibodies (bottom). Ligation products were detected by PCR using the primers indicated in panel A. (D) DAPA analysis of the GRTH transcriptional start site region. GRTH probes (−78/−39, −49/−10, −20/+21, +12/+52, and +40/+intron17bp) were incubated with in vitro-translated AR in the presence or absence of DHT at 10−8 M precipitated with streptavidin resin, and DNA-protein complexes were analyzed by Western blot analysis. A GRTH probe (−836/−812) was included as a positive control. The broken line in the diagram at the top is the 17-bp intron, the solid circle is the ATG codon at position +1, and the open arrow is the transcriptional start site. IP, immunoprecipitate.
To confirm these results in vivo, we performed a ChIP-coupled 3C/ChIP-loop assay with LC from mice treated with the control, hCG, flutamide, or a combination of the latter two (Fig. 6C). The ligation product was detected in cells from control and hCG-treated mice only in the presence of ligase, which indicates the specificity of the reaction. Also, flutamide treatment prevents loop formation, which indicates that nonactivated AR is unable to bind ARE2 or recruit the coactivator Med-1 and the transcriptional machinery components required to promote GRTH transcription.
Some AR target genes have more than one region of AR binding. PSA contains three ARE located in two different regions known as promoter and enhancer regions (23). It has been demonstrated that AR is recruited to both region. This is followed by recruitment of AR coactivators and transcriptional machinery components to initiate PSA transcription (33). To further investigate the recruitment of AR, we used DAPA experiments employing in vitro-translated AR to rule out A-induced association of AR to the GRTH transcriptional start site region. To cover the region amplified by PCR in the ChIP assays at −80/+63Intron17bp (Fig. 6D, upper panel), we used five different DNA probes. As shown in Fig. 6D (bottom), AR recruitment was not observed with any of these probes, which indicated that transcriptional machinery components bind to this site and associate with AR, SRC-1, and Med-1, forming a chromosomal loop.
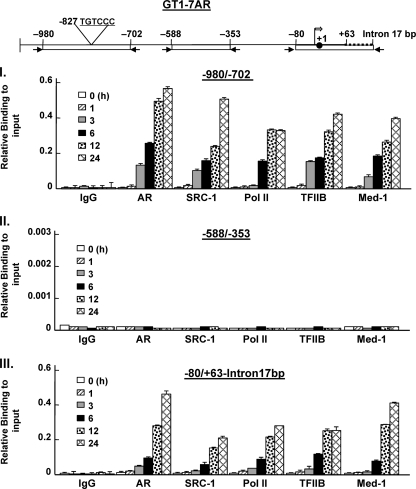
SRC-1 coactivator/Med-1 and transcriptional machinery components are recruited after 3 h of DHT-induced complex formation treatment in GT1-7 AR cells.
To further investigate the manner by which AR and associated proteins are recruited to form the protein complex on the GRTH promoter, we performed a time course study of protein recruitment to different regions of the GRTH 5′-flanking/promoter in GT1-7AR cells treated with DHT at 10−8 M for 0 to 24 h. Analysis of ChIP assays showed that AR, SRC-1, Med-1, TFIIB, and PolII are recruited after 3 h of treatment in both −980/−702 and −80 +63/intron17bp (Fig. 7, parts I and III, respectively). The levels of recruitment increased over 24 h. No recruitment of any of the relevant proteins, including PolII, was found at −588/−353 (Fig. 7, part II) which indicates the absence of PolII tracking from the ARE half-site at −827 to the transcriptional start site.
Fig 7.
Time course of recruitment of AR, coactivator, and components of the transcriptional machinery to ARE and the promoter region after DHT treatment. GT1-7AR cells were treated with DHT at 10−8 M for 1 to 24 h. ChIP assays were performed as previously described using the antibodies indicated. DNA precipitated was analyzed by real-time PCR with the primers indicated at the top. The broken line in the diagram at the top is the 17-bp intron, the solid circle is the ATG codon at position +1, and the open arrow is the transcriptional start site. The results are presented as percentages of the input. Results are means ± standard errors.
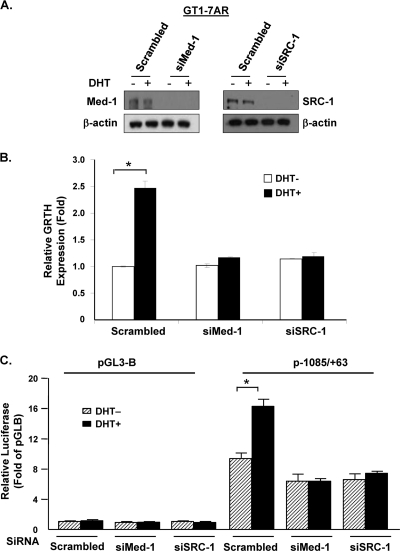
Med-1 and SRC-1 coactivators are essential for transcriptional activation and expression of the GRTH gene in LC.
In an effort to elucidate whether Med-1 and/or SRC-1 participate in DHT-induced GRTH transcription, Med-1 or SRC-1 expression was knocked down by siRNA in GT1-7AR cells (Fig. 8A) and GRTH expression was measured after 24 h of DHT treatment. Figure 8B shows that both siRNAs prevented the DHT-induced GRTH mRNA expression observed in the scrambled control (SC). Furthermore, the promoter activity of the GRTH p−1085/+63 construct, which is induced by DHT treatment, was abolished in the presence of siRNAs against Med-1 and SRC-1 (Fig. 8C), which indicates that these coactivators are essential for DHT-induced GRTH expression in GT1-7AR cells.
Fig 8.
AR coactivators SRC-1 and Med-1 are essential for DTH-induced GRTH transcription and expression. (A) GT1-7AR cells were transfected with siRNAs against Med-1 (siMed-1) and SRC-1 (siSRC-1) or control scrambled siRNA. Knockdown was assessed by Western blot analysis (A) using β-actin as a loading control. (B) Real-time qPCR analysis of GRTH expression in GT1-7AR cells transfected with scrambled, Med-1, or SRC-1 siRNA following treatment with the vehicle or DHT at 10−8 M for 24 h. Results (normalized to β-actin) are expressed as n-fold differences from the control (scrambled siRNA-transfected cells treated with the vehicle only). (C) The p−1085/+63 construct was transfected into GT1-7AR cells in the presence (black bars) or absence (striped bars) of DHT at 10−8 M. Luciferase activity is expressed relative to that of pGL3-B (empty vector) in all cases. Results are the means ± the standard errors of three individual experiments done in triplicate. *, P < 0.05.
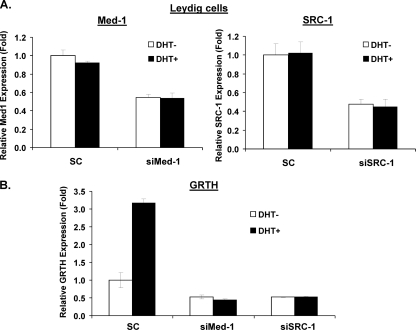
In order to confirm the findings described above, we utilized LC in primary culture. Med-1 and SRC-1 siRNAs reduced the expression of the respective mRNAs by 50 and 55%, respectively (Fig. 9A). Med-1 and SRC-1 knockdown prevented the DHT-induced GRTH expression observed in the scrambled control (Fig. 9B). This confirms that Med-1 and SRC-1 play an essential role in DHT-induced GRTH expression in LC. The Med-1 and SRC-1 siRNAs also reduced the basal levels of GRTH, which are explained by the effect of residual endogenous A.
Fig 9.
SRC-1 and Med-1 are essential for DHT-induced GRTH transcription and expression in mouse LC primary culture. LC were prepared as described in Materials and Methods. Cells were cultured in the presence of siRNA against Med-1 (siMed-1) or SRC-1 (siSRC-1) or control scrambled siRNA (SC) for 24 h. Cells were incubated with inhibitors of steroid biosynthesis (see Materials and Methods) for 1 h and then treated with the vehicle or DHT at 10−8 M for 20 h. (A) Knockdown of Med-1 or SRC-1 was assessed by real-time qPCR. (B) Real-time qPCR analysis of GRTH mRNA expression in GT1-7AR cells transfected with scrambled, Med-1, or SRC-1 siRNA. Results (normalized to β-actin) are expressed as n-fold differences from the control (scrambled cells treated with the vehicle only).
Med-1 and SRC-1 coactivators in DHT-induced complex assembly.
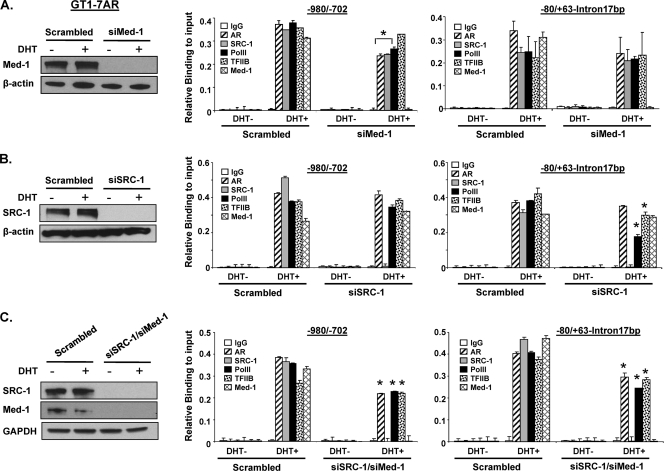
We subsequently determined the roles of Med-1 and SRC-1 in complex assembly by ChIP assay with the GT1-7 AR (Fig. 10) and LC in primary culture (Fig. 11) after knockdown of endogenous Med-1 and/or SRC-1. Forty-eight hours after siRNA transfection, cells were treated with DHT at 10−8 M for 24 h. Recruitment of AR, SRC-1, PolII, TFIIB, and Med-1 was assessed by real-time PCR. In GT1-7AR cells, ChIP analysis showed that recruitment of AR, PolII, and TFIIB was induced by DHT treatment in the scrambled, Med-1, SRC-1, and simultaneous siRNAs groups at the −980/−702 and −80/+63-intron17bp regions (Fig. 10A and C, respectively). This recruitment is attenuated in Med-1 siRNA-transfected cells compare to scrambled cells (Fig. 10A) in both regions. However, reduction of PolII was only observed in siSRC-1 at −80/+63-intron17bp. Simultaneous knockdown of Med-1 and SRC-1 reduced significantly the recruitment of AR, PolII, and TFIIB to both regions (Fig. 10C). This suggests that Med-1 and SRC-1 facilitate the recruitment of AR, PolII, and TFIIB to GRTH regulatory regions.
Fig 10.
Effect of knockdown of Med-1 and/or SRC-1 on AR, TFIIB, and PolII recruitment to the GRTH promoter/5′-flanking region in GT1-7AR cells. GT1-7AR cells were transfected with siRNA against Med-1 (siMed-1) (A, left side), SRC-1 (siSRC-1) (B, left side), or siSRC-1 and siMed-1 combined (C, left side). Scrambled siRNA was used as a control. Knockdown was assessed by Western blot analysis using β-actin (A and B, left side) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (C, left side) as a loading control. ChIP assays were performed with these cells, and recruitment of AR, SRC-1, Med-1, TFIIB, and PolII to the −980/−702 and −80/+63-intron17bp regions was assessed by real-time PCR. Results are the means ± the standard errors of three individual experiments done in triplicate, *, P < 0.05.
Fig 11.
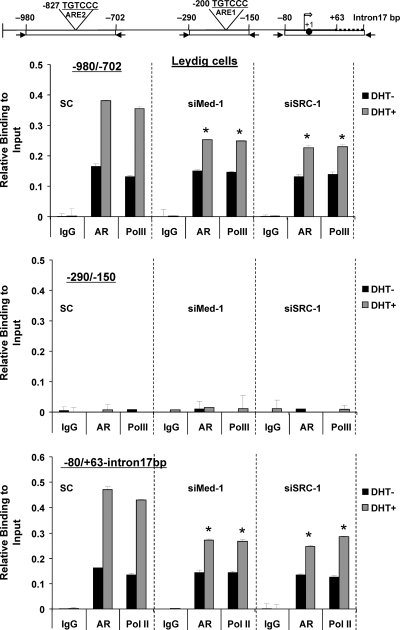
Effect of knockdown of Med-1 and/or SRC-1 on AR, TFIIB, and PolII recruitment to the GRTH promoter/5′-flanking region in primary cultures of mouse LC. Cells cultured in the presence of siRNA against Med-1 (siMed-1) or SRC-1 (siSRC-1) or control scrambled siRNA and treated with inhibitors of steroid biosynthesis (see Materials and Methods) and DHT as described in the legend to Fig. 9. ChIP assay was performed with these cells, and recruitment of AR and PolII to the −980/−702, −290/−150, and −80/+63-intron17bp regions was assessed by real-time PCR. Results are the means ± the standard errors of three individual experiments done in triplicate. *, P < 0.05.
In LC, recruitment of AR and PolII was observed in untreated cells which are explained by the effect of the residual endogenous A. Importantly, AR and PolII recruitments are significantly increased by DHT treatment in the scrambled control at both regions −980/702 (Fig. 11, top panel) and −80/+63-intron17bp (Fig. 11, bottom). Once again, no recruitment was observed at −290/−150 region (Fig. 11, middle panel). Recruitment of AR and PolII was also increased by DHT treatment in the presence of Med-1 siRNA or SRC-1 siRNA at both regions compared to that in the scrambled control. These results further indicate that Med-1 and SRC-1 significantly enhanced the recruitment of AR and PolII to regulatory regions of the GRTH gene (sc versus Med-1 or SRC-1 siRNA at −80/−702 and −80/+63-Intron17bp), which is essential for AR-mediated GRTH transcription in LC (Fig. 11; also Fig. 8 and 9).
GATA2, ETS-1, Oct-1, and FoxA1 do not participate in DHT-induced GRTH expression.
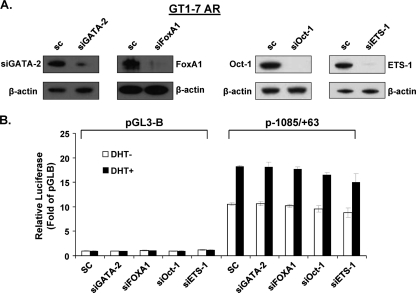
Some transcription factors have been reported to play important collaborative roles in AR function in prostate cancer (34), including GATA2 (3), Oct-1 (15), ETS-1 (35), and Forkhead BoxA1 (FoxA1) (10). We found putative binding sites for these transcription factors in the vicinity of the AR half-site (i.e., GATA2 at −514/−509 and −508/−503; Oct-1 at −882/−875, −514/−509, and −508/−503; and ETS-1 at −1083/−1074; FoxA1 at −939/−931, −824/−816, −809/−801, −787/−779, and −581/−573), either of which could play a role in the efficiency of AR binding to its site and/or monomer-dimer partner association and secondary DNA interactions. To study whether these transcription factors participate in DHT-induced GRTH transcription, the expression of these genes was knocked down by their corresponding siRNA (Fig. 12 A) in GT1-7AR cells and GRTH promoter activity was then assessed after 24 h of DHT treatment. None of these transcription factors participate in DHT-induced GRTH promoter activity. Thus, we can exclude their participation in GRTH transcription.
Fig 12.
GATA2, FoxA1, Oct-1, or ETS-1 does not participate in DHT-induced GRTH expression. GT1-7AR cells were transfected with siRNA against GATA2 (siGATA2), FoxA1 (siFoxA1), Oct-1 (siOct-1), or ETS-1 (siETS-1). Scrambled siRNA was used as a control. (A) Knockdown was assessed by Western blot analysis using β-actin as the loading control. (B) The p−1085/+63 construct was transfected into GT1-7AR cells in the presence or absence of DHT at 10−8 M. Luciferase activity is expressed relative to that of pGL3-B (empty vector) in all cases. Results are the means ± the standard errors of three individual experiments done in triplicate. *, P < 0.05.
DISCUSSION
In this report, we describe the mechanism by which A regulates the expression of GRTH in LC through cognate receptors present in these cells. We demonstrated that A (DHT) induced GRTH transcription only in the presence of an AR half-site, and this effect was inhibited by the AR antagonist flutamide. The functional nonconsensus ARE receptor half-site (5′-TGTCCC-3′) resides at −827 relative to the GRTH translational start site, while the identical ARE half-site downstream at −200 is inoperative and could not substitute for the upstream site in its absence.
The classic ARE DNA binding site consists of two inverted repeats (AGAACA) with a 3-nt spacer (34). However, some AR target genes contain noncanonical ARE sites within their promoter/5′-flanking or upstream sequences (enhancers), including single half-sites or half-sites separated by more than a 3-nt spacer (18, 34). The nucleotide sequence of the functional ARE2 site in GRTH resembles the sequences identified in the A-specific enhancer region 3.5 kb upstream of the transcription start site of the human sc gene (5′-TGTTCT-3′) (32) and is identical to that of the 3′ half-site of the two core ARE sites separated by 3 nt residing in the first exon of this gene (11). In the sc gene, the G on the ARE half-site located in the first exon is required for the transcription of sc induced by A/AR. Point mutation of G to T in the ARE half-site at −827 of the GRTH gene abolished AR binding and A-induced promoter activity (Fig. 2D). In addition, permutations of sequences adjacent to the downstream nonfunctional ARE site did not affect A/AR binding and activation of the functional ARE site at −827. This suggests that this half-site is sufficient for A-induced AR binding and GRTH transcriptional activation (Fig. 3C and D). We have excluded the participation of several transcription factors known to facilitate AR binding to its site with putative binding sites in the vicinity of the ARE2 half-site (i.e., GATA2, Oct-1, ETS-1, Forkhead box A1) in DHT-induced GRTH promoter activity (Fig. 12).
ChIP analysis showed that AR was recruited to the ARE half-site upon DHT treatment in vitro or upon hCG treatment in vivo (Fig. 4 and 5B, respectively). In addition, recruitment of SRC-1 (36) and Med-1 (1) was observed. We could not detect recruitment of other AR coactivators (ARA54, ARA55, and ARA70) (8, 13, 22) or SRC-2 (16). It is worth noting that AR, SRC-1, and Med-1 were also recruited to the −80/intron17bp region and recruitment of TFIIB and PolII was also observed at both sites, which suggested interaction between AR bound to the ARE half-site located at −827 and transcriptional machinery in the region comprising start sites and adjacent sequences of the GRTH TATA-less gene. ChIP assays and ChIP-coupled 3C/ChIP-loop studies demonstrated short-range physical interaction between the ARE half-site located at −827 and the GRTH transcriptional site in vitro and in vivo (Fig. 6B and C). AR regulation of the PSA-encoding gene in prostate cancer involves both the promoter and enhancer regions where AR is recruited together with essential coactivators and the formation of a long-range chromosomal loop (34) with inclusion of PolII. Here we conclusively demonstrated that AR is directly bound to the ARE half-site located at −827 but not to the GRTH transcriptional start site (Fig. 6D); thus, its association with the promoter region must involve A-induced AR interaction with a member(s) of the PIC. Association of both coactivators Med-1 and SRC-1 was found to be essential for the activation of GRTH gene transcription in GT1-7AR cells and in LC in primary culture (Fig. 8 and 9). Moreover, recruitment of AR and PolII was reduced when these coactivators were knock down, which indicates that Med-1 and SRC-1 facilitate their recruitment to regulatory regions of the GRTH gene in LC to induce GRTH transcription in response to A.
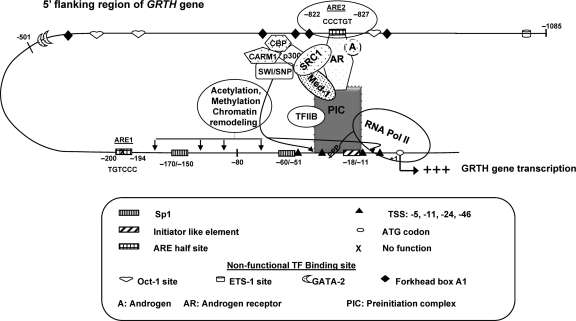
In the case of GRTH enhancer-promoter communication, temporal ChIP analysis of AR, coactivators, and PolII over 24 h of exposure of cells in culture or in vivo treatment of mice with A (DHT) demonstrated that exclusively chromosomal looping is involved since PolII tracking from ARE2 to the transcriptional start site was not observed in temporal studies (Fig. 7). This contrasted with the A regulation of the PSA-encoding gene, where looping and PolII tracking from the enhancer to the promoter (33) were observed in prostate cancer cells. Thus, upon treatment, hCG-stimulated endogenous A production in vivo induces AR activation and its binding to the ARE half-site at −827. This association and changes in the recruitment of SRC-1 and Med-1 provide the essential functional components which, in concert with the molecular bridge formed by AR (bound to the ARE half-site), the components of the PIC, TFIIB, and PolII, elicit GRTH transcription. Of interest is the finding that SRC-1 and Med-1 are not required for looping and recruitment of PolII and TFIIB to the linking complex. However, these coactivators are essential for maximal recruitment of the AR, TFIIB, and PolII to the complex and transcriptional activation of the GRTH gene (Fig. 8 to 11). Moreover, these coactivators may provide the changes in chromatin structure in the promoter region necessary to elicit transcriptional activation (Fig. 13). Such changes presumably occur through coactivator association with the p300/CBP-associated factor and/or coactivator-associated arginine methyltransferase with the participation of chromatin-remodeling complexes (Fig. 13).
Fig 13.
Model of A action on GRTH gene transcription in mouse LC. In vivo and in vitro, A activates the AR (A/AR). The A/AR binds to the functional ARE2 site at −827/−822 and interacts with a member(s) of the PIC and PolII through short-range chromosomal looping. SRC-1 and Med-1, which associates with the AR, are required for functional activation of transcription of the loop complex of the GRTH gene induced by A. Oct-1, ETS, GATA2, and Forkhead box A1 are nonfunctional putative TF binding sites.
In summary, our findings describe the mechanism by which AR regulates the expression of the GRTH gene in LC. These studies reinforce the concept that A/AR signaling in LC, through its activation of GRTH transcription, participates in an autocrine regulation mechanism with a major impact on LC steroidogenic function (9). The combined expression of these two genes in the LC is essential for the regulation of early steps of steroidogenesis in these cells and for A production induced by gonadotropin in the male.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Belakavadi M, Fondell JD. 2006. Role of the mediator complex in nuclear hormone receptor signaling. Rev. Physiol. Biochem. Pharmacol. 156: 23–43 [DOI] [PubMed] [Google Scholar]
- 2. Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. 2010. Molecular cell biology of androgen receptor signalling. Int. J. Biochem. Cell Biol. 42: 813–827 [DOI] [PubMed] [Google Scholar]
- 3. Briegel K, et al. 1993. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 7: 1097–1109 [DOI] [PubMed] [Google Scholar]
- 4. Collins LL, et al. 2003. The androgen receptor in spermatogenesis. Cytogenet. Genome Res. 103: 299–301 [DOI] [PubMed] [Google Scholar]
- 5. Dekker J, Rippe K, Dekker M, Kleckner N. 2002. Capturing chromosome conformation. Science 295: 1306–1311 [DOI] [PubMed] [Google Scholar]
- 6. Dong J, Tsai-Morris CH, Dufau ML. 2006. A novel estradiol/estrogen receptor alpha-dependent transcriptional mechanism controls expression of the human prolactin receptor. J. Biol. Chem. 281: 18825–18836 [DOI] [PubMed] [Google Scholar]
- 7. Dufau ML, Tsai-Morris CH. 2007. Gonadotropin-regulated testicular helicase (GRTH/DDX25): an essential regulator of spermatogenesis. Trends Endocrinol. Metab. 18: 314–320 [DOI] [PubMed] [Google Scholar]
- 8. Fujimoto N, et al. 1999. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J. Biol. Chem. 274: 8316–8321 [DOI] [PubMed] [Google Scholar]
- 9. Fukushima M, Villar J, Tsai-Morris C-H, Dufau ML. 2011. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), a negative regulator of luteinizing/chorionic gonadotropin hormone-induced steroidogenesis in Leydig cells. Central role of steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 286: 29932–29940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao N, et al. 2003. The role of hepatocyte nuclear factor-3 alpha (Forkhead box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol. Endocrinol. 17: 1484–1507 [DOI] [PubMed] [Google Scholar]
- 11. Haelens A, et al. 1999. The first exon of the human sc gene contains an androgen responsive unit and an interferon regulatory factor element. Mol. Cell. Endocrinol. 153: 91–102 [DOI] [PubMed] [Google Scholar]
- 12. Holdcraft RW, Braun RE. 2004. Hormonal regulation of spermatogenesis. Int. J. Androl. 27: 335–342 [DOI] [PubMed] [Google Scholar]
- 13. Kang HY, Yeh S, Fujimoto N, Chang C. 1999. Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor. J. Biol. Chem. 274: 8570–8576 [DOI] [PubMed] [Google Scholar]
- 14. Kasper S, et al. 1994. Cooperative binding of androgen receptors to two DNA sequences is required for androgen induction of the probasin gene. J. Biol. Chem. 269: 31763–31769 [PubMed] [Google Scholar]
- 15. Kutoh E, Stromstedt PE, Poellinger L. 1992. Functional interference between the ubiquitous and constitutive octamer transcription factor 1 (OTF-1) and the glucocorticoid receptor by direct protein-protein interaction involving the homeo subdomain of OTF-1. Mol. Cell. Biol. 12: 4960–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li X, Wong J, Tsai SY, Tsai MJ, O'Malley BW. 2003. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol. Cell. Biol. 23: 3763–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liao M, Zhang Y, Dufau ML. 2008. Protein kinase Calpha-induced derepression of the human luteinizing hormone receptor gene transcription through ERK-mediated release of HDAC1/Sin3A repressor complex from Sp1 sites. Mol. Endocrinol. 22: 1449–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin B, et al. 2009. Integrated expression profiling and ChIP-seq analyses of the growth inhibition response program of the androgen receptor. PLoS One 4: e6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malinowska K, et al. 2009. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr. Relat. Cancer 16: 155–169 [DOI] [PubMed] [Google Scholar]
- 20. Mellon PL, et al. 1990. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5: 1–10 [DOI] [PubMed] [Google Scholar]
- 21. Pan YF, et al. 2008. Regulation of estrogen receptor-mediated long range transcription via evolutionarily conserved distal response elements. J. Biol. Chem. 283: 32977–32988 [DOI] [PubMed] [Google Scholar]
- 22. Peng Y, et al. 2008. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70. Am. J. Pathol. 172: 225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. 2004. Structural basis of androgen receptor binding to selective androgen response elements. Proc. Natl. Acad. Sci. U. S. A. 101: 4758–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheng Y, Tsai-Morris CH, Dufau ML. 2003. Cell-specific and hormone-regulated expression of gonadotropin-regulated testicular RNA helicase gene (GRTH/Ddx25) resulting from alternative utilization of translation initiation codons in the rat testis. J. Biol. Chem. 278: 27796–27803 [DOI] [PubMed] [Google Scholar]
- 25. Sheng Y, Tsai-Morris CH, Gutti R, Maeda Y, Dufau ML. 2006. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J. Biol. Chem. 281: 35048–35056 [DOI] [PubMed] [Google Scholar]
- 26. Tang PZ, Tsai-Morris CH, Dufau ML. 1999. A novel gonadotropin-regulated testicular RNA helicase. A new member of the dead-box family. J. Biol. Chem. 274: 37932–37940 [DOI] [PubMed] [Google Scholar]
- 27. Tsai-Morris CH, Lei S, Jiang Q, Sheng Y, Dufau ML. 2004. Genomic organization and transcriptional analysis of gonadotropin-regulated testicular RNA helicase-GRTH/DDX25 gene. Gene 331: 83–94 [DOI] [PubMed] [Google Scholar]
- 28. Tsai-Morris CH, et al. 2010. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) gene: cell-specific expression and transcriptional regulation by androgen in transgenic mouse testis. J. Cell. Biochem. 109: 1142–1147 [DOI] [PubMed] [Google Scholar]
- 29. Tsai-Morris CH, Sheng Y, Gutti RK, Tang PZ, Dufau ML. 2010. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25): a multifunctional protein essential for spermatogenesis. J. Androl. 31: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsai-Morris CH, Sheng Y, Lee E, Lei KJ, Dufau ML. 2004. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl. Acad. Sci. U. S. A. 101: 6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K. 2010. Androgens and spermatogenesis: lessons from transgenic mouse models. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 1537–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verrijdt G, et al. 1999. Androgen specificity of a response unit upstream of the human secretory component gene is mediated by differential receptor binding to an essential androgen response element. Mol. Endocrinol. 13: 1558–1570 [DOI] [PubMed] [Google Scholar]
- 33. Wang Q, Carroll JS, Brown M. 2005. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell 19: 631–642 [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, et al. 2007. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 27: 380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei GH, et al. 2010. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 29: 2147–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. York B, O'Malley BW. 2010. Steroid receptor coactivator (SRC) family: masters of systems biology. J. Biol. Chem. 285: 38743–38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Liao M, Dufau ML. 2006. Phosphatidylinositol 3-kinase/protein kinase Cζ-induced phosphorylation of Sp1 and p107 repressor release have a critical role in histone deacetylase inhibitor-mediated depression of transcription of the luteinizing hormone receptor gene. Mol. Cell. Biol. 26: 6748–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]