Abstract
The Mediator subunit MED1 is essential for mammary gland development and lactation, whose contribution through direct interaction with estrogen receptors (ERs) is restricted to involvement in pubertal mammary gland development and luminal cell differentiation. Here, we provide evidence that the MED24-containing submodule of Mediator functionally communicates specifically with MED1 in pubertal mammary gland development. Mammary glands from MED1/MED24 double heterozygous knockout mice showed profound retardation in ductal branching during puberty, while single haploinsufficient glands developed normally. DNA synthesis of both luminal and basal cells were impaired in double mutant mice, and the expression of ER-targeted genes encoding E2F1 and cyclin D1, which promote progression through the G1/S phase of the cell cycle, was attenuated. Luciferase reporter assays employing double mutant mouse embryonic fibroblasts showed selective impairment in ER functions. Various breast carcinoma cell lines expressed abundant amounts of MED1, MED24, and MED30, and attenuated expression of MED1 and MED24 in breast carcinoma cells led to attenuated DNA synthesis and growth. These results indicate functional communications between the MED1 subunit and the MED24-containing submodule that mediate estrogen receptor functions and growth of both normal mammary epithelial cells and breast carcinoma cells.
INTRODUCTION
Nuclear receptors, which include steroid and nonsteroid hormone receptors, comprise a superfamily of DNA-bound transcriptional regulators that are activated in response to specific small lipophilic ligands and that play major physiological roles in cell growth, differentiation, and homeostasis (reviewed in references 10 and 25). Estrogen receptor α (ERα) is the key activator that leads to growth of the mammary glands during adolescence, as well as during pregnancy, in response to elevated plasma estrogen levels. Among the hormone-responsive genes transcribed under the control of ERα is another steroid hormone receptor, progesterone receptor (PR), which in concert with ERα, plays an important role in mammary gland development (5).
The metazoan Mediator/TRAP coactivator complex is a master transcriptional coregulator composed of about 30 subunits and is structurally subdivided into head, body, and tail modules. It constitutes a subcomplex of the RNA polymerase II holoenzyme and integrates a wide variety of intracellular signals through specific interactions of activators with specific Mediator subunits that reside predominantly at its tail module (reviewed in references 4, 6, 15, 20, and 24). We have proposed a multistep model for nuclear receptor-induced transcriptional activation (15). In this model, histone-modifying coactivators that possess either histone acetyltransferase or histone methyltransferase activities first interact with ligand-bound nuclear receptors, and chromatin structure is subsequently relaxed. Then an exchange of coactivators takes place and the Mediator is bound to nuclear receptors through two canonical LxxLL nuclear receptor recognition motifs (NR boxes) of the MED1/TRAP220 subunit. Finally, a preinitiation complex (PIC) is formed through the Mediator-bound RNA polymerase II. The rapid and cyclic exchange of coactivators is characteristic of ERα, a steroid receptor (18, 33), and contrasts with the slow and stepwise exchange of coactivators that is characteristic of thyroid hormone receptor α (TRα), a nonsteroid receptor (34).
Studies with mouse models using conditional knockout genes have shown that the MED1 subunit of the transcriptional Mediator is essential for both mammary gland development and lactation (17), while more recent genetic studies using LxxLL-mutant Med1 cDNA knock-in [Med1(LX) KI] mice showed that the contribution of direct interaction of MED1 with estrogen receptors is restricted to involvement in pubertal mammary gland development and luminal cell differentiation (19). The phenotypic differences in these studies suggest that, apart from its role as a nuclear receptor-specific coactivator, MED1 has yet-unidentified roles (probably through coactivation of other activators) in mammary gland physiology. Furthermore, the role of Mediator subunits other than MED1 in mammary gland physiology has not yet been explored.
The MED1-deficient Mediator is stable, where the constitution of other Mediator subunits is unaffected, and in nuclear receptor-mediated transcriptional activation, there is a natural Mediator complex that is devoid of MED1 and constitutes an inactive form of the Mediator. MED1 predominantly exists in an active form of the Mediator associated with RNA polymerase II (42). On the other hand, MED24/TRAP100, MED23/TRAP150β, and MED16/TRAP95 constitute a submodule within the tail module of the Mediator, and this submodule becomes unstable and subject to degradation when one of its components is deficient. However, other Mediator subunits are stably incorporated in MED24-deficient Mediator (16, 37). The MED24/MED23/MED16 submodule has an important role in magnifying the effects of activators on the general transcriptional machinery, beyond its roles in regulating activator interactions and recruiting RNA polymerase II by Mediator, and dose dependency between the MED1 subunit and the MED24-containing submodule exists in embryonic development and housekeeping gene expression (16). Hence, the attenuated function of the MED24-containing submodule might diminish the effect of MED1 and result in overtly altered phenotypes in circumstances where the dose of MED1 (or the MED24-containing submodule) is critical. This might be the case in mammary gland development particularly, as MED1 appears to be crucial in this process (17, 19), and limited Med1 and/or Med24 gene doses in mice would reveal this hypothesis.
In this report, the genetic analyses of synthetic single or complex haploinsufficiency in Med1 and/or Med24 genes in mice are made use of to reveal possible functional interactions among Mediator subunits with regard to mammary gland physiology, and through analyses of MED1/MED24 double heterozygous knockout mice, we show that there is a functional communication between the MED1 subunit and the MED24-containing submodule, which specifically regulates ERα functions in relation to cell cycle progression and mammary gland development. We also show that both MED1 and MED24 play a significant role in the growth of breast carcinoma cells.
MATERIALS AND METHODS
Mice.
Med1 and Med24 knockout mice (14, 16), backcrossed at least 10 times with C57BL6 mice, were used for the experiments. To examine estradiol (E2)-stimulated mammary gland growth, 21-day-old virgin females were ovariectomized and implanted with 21-day slow-release pellets (0.025 mg; Innovative Research of America). After 21 days, inguinal glands were excised for whole-mount staining with carmine (Sigma) as described previously (19). All animal experiments were performed according to the institutional guidelines of the Animal Research Center, Kobe University, Japan.
Whole-mount staining, histology, lacZ staining, and BrdU staining.
For whole-mount staining, the inguinal mammary glands were isolated, fixed in Carnoy's fixative, and stained overnight in carmine alum (19). Samples were then cleared in xylene and mounted. For histological analysis, mounting medium was removed and the tissues were embedded in paraffin for sectioning.
For lacZ staining, the inguinal glands were isolated, fixed in 4% paraformaldehyde, 0.25% glutaraldehyde, and 0.01% NP-40 in phosphate-buffered saline (PBS) at 4°C for 2 h, and then stained in buffer containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal), 30 mM K4Fe(CN)6, 30 mM K3Fe(CN)6 · 3H2O, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% NP-40 in PBS at 30°C for 48 h. Tissues were then cleared in acetone, followed by xylene, and mounted.
For bromodeoxyuridine (BrdU) staining, BrdU (0.01 mg/g of body weight) was administered intraperitoneally. Mice were sacrificed 2 h later and perfusion fixed in 4% paraformaldehyde in PBS. Mammary inguinal glands were isolated and postfixed at 4°C for 2 h, embedded in paraffin, and sectioned. BrdU-positive cells were visualized with a BrdU in situ detection kit (BD Biosciences) according to the manufacturer's protocol, and sections were then counterstained with hematoxylin.
Immunohistochemistry.
For immunohistochemistry, deparaffinized mouse tissue sections and human breast carcinoma tumor arrays (human breast cancer/metastasis/normal tissue array; SuperBioChips, South Korea) were subjected to hydrated heating for 10 min in a pressure cooker in 10 mM citrate buffer (pH 6.0). Then the sections were incubated overnight at room temperature in anti-cleaved caspase 3 (1:100 dilution) (no. 9661; Cell Signaling Technology), anti-phospho-histone H2A.X (serine 139; γH2A.X) (5 μg/ml) (no. 07-164; Millipore), anti-MED1 (0.5 μg/ml) (SAB4502265; Sigma), or anti-MED24 (0.5 μg/ml) (SAB4503717; Sigma) rabbit polyclonal antibodies. After washing in PBS, the sections were incubated with immunoenzyme polymer reagent (Nichirei, Tokyo, Japan) at room temperature for 60 min. The reaction products were visualized in 0.05% diaminobenzidine tetrahydrochloride solution containing 0.003% hydrogen peroxide. The nuclei were lightly counterstained with Mayer's hematoxylin.
Cell culture.
Mouse embryonic fibroblasts (MEFs) were obtained from embryonic day 10.0 (E10.0) embryos. The MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C. Breast carcinoma cells (luminal A type, MCF-7 and T47D; luminal B type, BT-474 and MDA-MB-361; HER2 type, HCC1954; and basal-like type, MDA-MB-231 and BT-549) (22), obtained from ATCC, were cultured in RPMI supplemented with 10% FBS at 37°C.
For primary culture of mouse mammary epithelial cells, thoracic and inguinal glands were aseptically dissected out, minced, and digested overnight at 37°C in phenol red-free DMEM–F-12 containing 0.1% collagenase A (Roche), 100 U/ml hyaluronidase (Sigma), and 100 U/ml penicillin-streptomycin. Samples were centrifuged for 10 min at 1,500 rpm, and supernatant tissue fragments containing the fat pad and mammary glands were collected. This procedure was repeated 4 times, and the collected samples were treated with 4 U/ml DNase (Promega) for 5 min at 37°C and dispersed. Cells forming tubule structures were washed 10 times in phenol red-free DMEM–F-12, followed by brief centrifugation. The cell pellets were then suspended in growth medium (phenol red-free DMEM–F-12 containing 5 μg/ml insulin, 1 μg/ml hydrocortisone, 5 ng/ml epidermal growth factor [EGF], 100 U/ml penicillin-streptomycin, and 5% charcoal-stripped FBS) and cultured in fetuin-coated 6-well plates.
Cell growth and DNA synthesis.
For cell growth, cells (1 × 104 cells/well) were plated in 24-well plates, transfected with small interfering RNAs (siRNAs), and cultured in complete medium. Cells from replicate wells were counted after trypsinization. The cell viability was assessed by trypan blue exclusion. For DNA synthesis, the incorporation of BrdU into cells in 24-well plates, after purging for 6 h, was measured by using a cell proliferation enzyme-linked immunosorbent assay (ELISA) with BrdU (chemiluminescence) (Roche) (38).
Luciferase reporter assays.
Cells were cultured in DMEM supplemented with charcoal-stripped FBS before transfection. The cDNAs for activators (20 ng of human ERα [hERα], human PRβ [hPRβ], and Gal4-fused vitamin D receptor [VDR] and VP16), cloned into the cytomegalovirus (CMV) promoter-driven mammalian expression vector pcDNA3.1 (Invitrogen), together with the firefly luciferase reporter pGL3 (Promega), containing either 3× ER-responsive element (ERE), 4× PR-responsive element (PRE), or 5× Gal4-binding sites (100 ng), were transfected with the Renilla control luciferase vector (5 ng) into MEFs using Lipofectamine (Invitrogen). After 48 h, the reporter activities were measured using the dual-luciferase reporter assay system (Promega) and normalized to the control Renilla luciferase activity (38).
RNA interference.
For RNA interference, siRNAs (5 nM in 24-well plates) (Silencer select predesigned siRNA; Applied Biosystems) were transfected using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. For simultaneous suppression of expression of both MED1 and MED24, siRNAs for these genes were mixed and similarly transfected.
For cotransfection of siRNA with mammalian expression vectors, the human siRNA-resistant cDNAs [full-length MED1, MED1(1–602), MED1(1–703), and MED24] were constructed by mutating at three sequential codons simultaneously corresponding to the amino acid residues by using the QuikChange II site-directed mutagenesis kit (Agitent Technologies) together with the mutant oligonucleotides. The mutated sequences are available upon request. Cotransfection of siRNAs (5 nM) with siRNA-resistant cDNAs cloned into pcDNA3.1 (100 ng) was performed by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Quantitation of mRNA.
For the quantitative PCR (qPCR), total RNAs (1 μg), prepared with Isogen (Toyobo), were used to synthesize cDNAs with the ReverTra Ace qPCR reverse transcription (RT) kit (Toyobo). The expression of various mouse genes was identified by quantitative PCR (7300 real-time PCR System; Applied Biosystems). The sequences of the primers used and the PCR conditions for amplification are available upon request.
Western blot analysis.
For the Western blot analysis, total cell lysates were separated by SDS-PAGE, blotted onto a nitrocellulose membrane, and probed with polyclonal antibodies (16).
ELISA.
For quantitation of serum estradiol (E2) levels, ELISA was performed by using a 17β-estradiol ELISA kit (Tokiwa Chemical Industry).
Statistical analysis.
The significance of differences between independent means was assessed by Student's t test. We considered a P value of <0.05 as statistically significant.
RESULTS
Med1+/− Med24+/− mammary gland development is retarded during puberty.
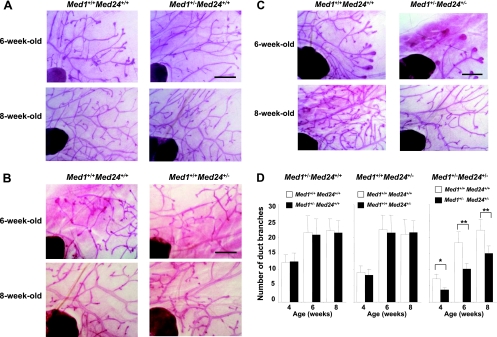
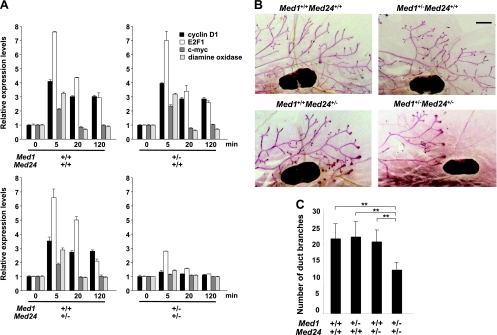
To investigate the contribution of the MED24/MED23/MED16 submodule in MED1- and ERα-mediated mammary gland development, we analyzed pubertal mammary gland development in Med1+/− Med24+/− mice. The Med1+/− single heterozygous mutant mammary gland exhibited normal ductal elongation under whole-mount staining (Fig. 1A and D), and histological examination of these preparations showed normal ductal architecture at the ages of 3, 4, and 6 weeks (data not shown). The Med24+/− single heterozygous mutant mammary gland also showed normal ductal development at these ages (Fig. 1B and D). However, the Med1+/− Med24+/− double heterozygous mutant mammary gland showed retarded ductal elongation (Fig. 1C and D), but at the ages of 3, 4, and 6 weeks, the ductal architecture was histologically indistinguishable (data not shown). These studies show that a full dosage of either the MED1 subunit or the MED24-containing submodule is necessary for normal pubertal mammary gland development and might highlight the MED24-containing submodule as an indispensable compartment for full activity of MED1-mediated ERα function in mammary gland elongation.
Fig 1.
Med1+/− Med24+/− mammary gland development is retarded during puberty. (A, B, and C) Whole-mount staining of virgin female mammary inguinal glands at the ages of 6 and 8 weeks is shown. (A) Med1+/− Med24+/+; (B) Med1+/+ Med24+/−; (C) Med1+/− Med24+/−. Scale bar, 1 mm. (D) The number of duct branches per visual field is shown. The Med1+/− Med24+/− glands show an attenuated number of duct branches. Values are means ± standard deviations (SD). *, P < 0.05; **, P < 0.01. n = 2, 4 weeks old; n = 6, 6 weeks old; n = 10, 8 weeks old.
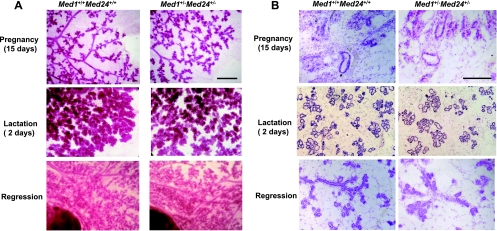
Med1+/− Med24+/− mammary glands are normal during and after pregnancy.
We asked if the Med1+/− Med24+/− mammary glands might be functionally impaired and examined the glands during pregnancy and after labor. Indeed, the whole-mount staining of the Med1+/− Med24+/− mammary glands showed normal physiological changes in morphology during pregnancy (15 days), lactation (2 days), and regression (Fig. 2A). Histological examination confirmed that the normal ductal architectures were preserved in these mammary glands (Fig. 2B). The pups nursed by Med1+/− Med24+/− females grew normally, indicating that the mammary glands are functionally normal. These findings are reminiscent of the phenotype observed in Med1(LX) KI mice (19) and contrast with those of Med1 conditional KO mice (17). Taking into account that the alteration in Med1(LX) KI mammary glands apparently represents impaired ERα function, the phenotype of the Med1+/− Med24+/− mammary glands is also highly suggestive of impaired ERα function.
Fig 2.
Med1+/− Med24+/− mammary glands are normal during and after pregnancy. (A) Whole-mount staining of female mammary inguinal glands at 15 days of pregnancy, 2 days after lactation, and during regression. Wild-type and Med1+/− Med24+/− littermate mammary glands are shown. Scale bar, 1 mm. (B) Histological examination of the same glands at 15 days of pregnancy, 2 days after lactation, and during regression. Wild-type and Med1+/− Med24+/− littermate mammary glands are shown. Scale bar, 50 μm.
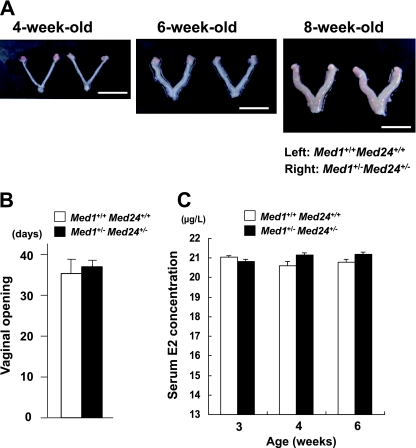
Med1+/− Med24+/− uterus develops normally during puberty.
If estrogen action was impaired, other estrogen-mediated organ development might also be impaired. Hence, uterine development in the Med1+/− Med24+/− mice was investigated, but we found that the uteri of 4-, 6-, and 8-week-old Med1+/− Med24+/− mice were indistinguishable from those of their wild-type littermates, indicating that the Med1+/− Med24+/− uterine development was normal during puberty (Fig. 3A).
Fig 3.
Uterine development, timing of puberty, and serum E2 concentration are normal in Med1+/− Med24+/− mice. (A) Wild-type and Med1+/− Med24+/− littermate uteri at ages of 4, 6, and 8 weeks are shown. Scale bar, 1 cm. (B) Days of vaginal opening of wild-type and Med1+/− Med24+/− females are comparable, indicating that Med1+/− Med24+/− females reach puberty normally. Values are means ± standard errors (SE) from 4 mice. (C) Serum E2 concentration of wild-type and Med1+/− Med24+/− females at ages of 3, 4, and 6 weeks are comparable, indicating that E2 production by Med1+/− Med24+/− ovaries is normal during puberty. Values are means ± SE from 4 mice.
Furthermore, the vaginal opening in females and the descensus testis in males were temporally comparable between wild-type and Med1+/− Med24+/− mice (Fig. 3B) (data not shown), indicating that the timing of adolescence is unaffected in Med1+/− Med24+/− mice. These results also parallel the Med1(LX) KI phenotype (19).
Normal estrogen levels in Med1+/− Med24+/− females.
To exclude the possibility that the above-mentioned phenotype in Med1+/− Med24+/− mice might reflect altered estrogen production during development, the serum E2 concentration was measured. The E2 levels in Med1+/− Med24+/− females were found to be the same as those in the wild-type littermate controls, at the ages of 3, 4, and 6 weeks (Fig. 3C), confirming that similar to the case in Med1(LX) KI mice (19), ovarian E2 production was not affected in these mice. This implies that the attenuated ERα function in Med1+/− Med24+/− female mammary glands is due to attenuated postreceptor signaling in these mice.
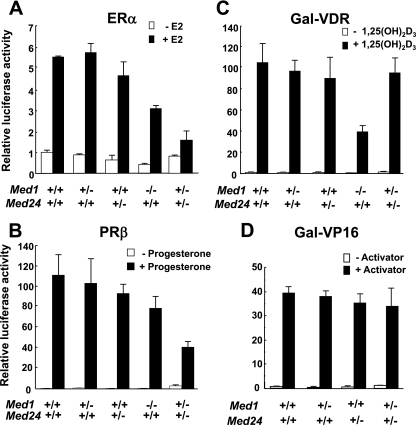
Selectively impaired functions of ectopically expressed ERα and PRβ in Med1+/− Med24+/− cells.
Since the E2 concentration was normal in Med1+/− Med24+/− females, we hypothesized that the ERα function was selectively impaired in these mice and checked various activator functions by using transient transfection and luciferase reporter assays in MEFs. The ligand-dependent functions of both ERα and PRβ that were ectopically expressed were similar in singly heterozygous Med1+/− and Med24+/− MEFs and impaired in Med1−/− MEFs, but these receptor functions were further attenuated in Med1+/− Med24+/− MEFs, being reduced to one-third of the control levels (Fig. 4A and B). In contrast, the ligand-dependent Gal-VDR function was defective in Med1−/− MEFs, being less than half of the control level, but was not impaired in Med1+/− Med24+/− MEFs (Fig. 4C). As expected, the function of Gal-VP16, which is presumably activated via the MED17/TRAP80 or MED25 subunit of Mediator (13, 26, 41), was not impaired in Med1+/− Med24+/− MEFs (Fig. 4D). These results strongly suggest that ERα and PRβ functions are selectively and profoundly impaired in Med1+/− Med24+/− cells.
Fig 4.
Defective ERα- and PRβ-driven transcriptional activation in Med1+/− Med24+/− MEFs. (A) ERα-driven transcriptional activation is mildly attenuated in Med1−/− Med24+/+ MEFs and profoundly attenuated in Med1+/− Med24+/− MEFs. Values (means ± SD from a representative experiment performed in triplicate) are plotted as fold increase against the value of Med1+/+ Med24+/+ MEFs without ligand (A to C). The ligands used are 10−8 M E2, 10−7 M progesterone, and 10−7 M 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (A to C). (B) PRβ-driven transcriptional activation is attenuated profoundly in Med1+/− Med24+/− MEFs. (C) Gal-VDR-driven transcription is attenuated in Med1−/− Med24+/+ MEFs but not affected in Med1+/− Med24+/− MEFs. (D) Gal-VP16-driven transcription is not affected in Med1+/− Med24+/− MEFs. Values (means ± SD from a representative experiment performed in triplicate) are plotted as fold increase against the value of Med1+/+ Med24+/+ MEFs without Gal4-VP16.
Defective ERα-targeted, cell-cycle-related gene expressions in Med1+/− Med24+/− primary mammary epithelial cells.
To prove that impaired mammary gland development in Med1+/− Med24+/− mice was intrinsic and to corroborate that the function of endogenous ERα on endogenous and cell growth-related gene promoters was defective in these mice, primary mammary epithelial cells were isolated from wild-type and mutant mice and cultured in vitro in the absence or presence of E2. The expression of genes directly targeted by ERα, which included genes encoding G1/S-phase transition-related proteins E2F1 and cyclin D1, as well as c-myc and diamine oxidase (DAO), was analyzed after the cells were treated with E2 for different durations (0, 5, 20, and 120 min). The expression of these genes was prominently induced in wild-type and singly Med1+/− and Med24+/− primary mammary epithelial cells, but was only weakly induced in Med1+/− Med24+/− mammary epithelial cells (Fig. 5A). Intriguingly, the response of E2F1 expression was rapid and robust, with a peak as soon as 5 min after E2 treatment, while the response of cyclin D1 was initially weaker but sustained longer (Fig. 5A). Since the gene promoter of cyclin D1 is also activated by E2F1 transcription factor (30), the sustained transcription of cyclin D1 might have been employed by E2F1 induced by E2 treatment. These results reiterate the MEF-based conclusions drawn from the luciferase reporter assays, confirming that ERα function is severely defective in Med1+/− Med24+/− cells, and suggest that impaired Med1+/− Med24+/− mammary gland development is intrinsic and apparently related to defective ligand-dependent progression of cell cycle through G1/S phase (2, 36).
Fig 5.
Med1+/− Med24+/− double haploinsufficiency blocks estrogen-dependent gene expression and estrogen-stimulated mammary duct growth. (A) Gene expression levels. Primary mammary epithelial cells were isolated from wild-type and mutant mice as indicated and treated with 10−7 M E2 for the indicated time. Total RNAs were isolated and expression of the indicated ERα target genes was measured by real-time PCR. Values (means ± SD from a representative experiment performed in triplicate) are plotted as a fold increase against the value obtained before E2 addition. (B) Mammary duct growth. Three-week-old wild-type and mutant mice as indicated were ovariectomized and implanted with slow-release E2 pellets for 21 days. Whole-mount stainings of inguinal glands from these mice are shown. Scale bar, 1 mm.
Med1+/− Med24+/− mammary ducts respond poorly to estrogen-stimulated growth in vivo.
To directly test our assertion that the defect in mammary gland development manifest in Med1+/− Med24+/− females was due to effects of estrogen-ER function, 21-day-old wild-type females, as well as both singly and double mutant females, were ovariectomized and implanted with slow-release pellets containing E2 or placebo. Twenty-one days later, uteri of both wild-type and all kinds of mutant females were enlarged by E2 treatments, as expected, and mammary ductal growth was not stimulated by placebo treatments (data not shown). Importantly, whereas a significant induction of mammary ductal growth by E2 treatment was observed in wild-type mice and singly Med1+/− and MED24+/− mice, the growth of mammary ducts was significantly inhibited in the Med1+/− MED24+/− mice despite E2 treatment (Fig. 5B). These results further confirm that the defect in Med1+/− MED24+/− mammary ductal growth is due to the defective ER function in response to estrogen.
Attenuated growth of Med1+/− Med24+/− mammary epithelial cells.
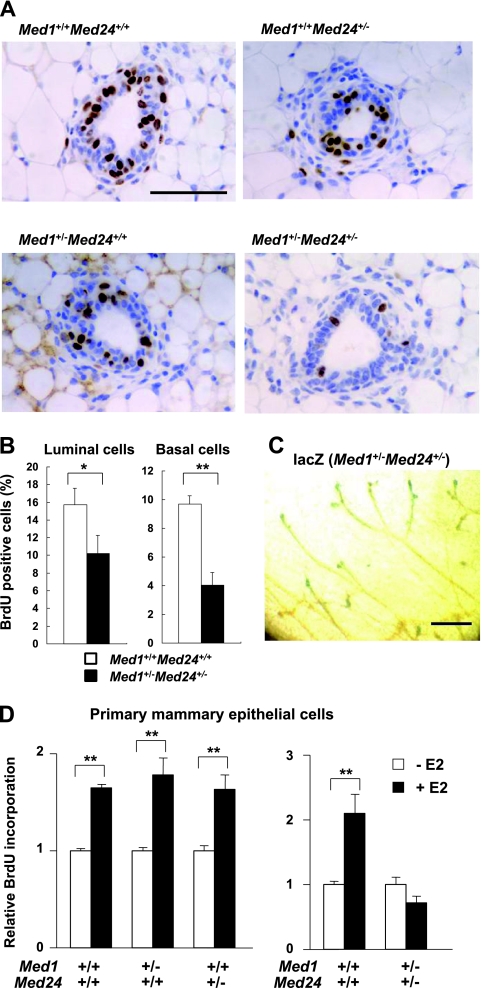
We hypothesized that Mediator is necessary for optimal mammary epithelial cell growth and analyzed DNA synthesis in vivo and in vitro. Mammary luminal cells express abundant MED1, while mammary basal cells, which are situated underneath the luminal cells, scarcely express MED1. In contrast, the expression of the core subunit MED30 is abundant in both cell types (19). When 8-week-old virgin littermate females (wild-type and mutants) were purged with BrdU for 2 h, the positivity for BrdU in singly Med1+/− and Med24+/− epithelia was similar to the one in wild-type mammary epithelia, but the positivity was significantly attenuated to approximately half of the control level in both luminal and basal cells of Med1+/− Med24+/− mammary epithelia, indicating that DNA synthesis was impaired (Fig. 6A and B).
Fig 6.
Retarded DNA synthesis of Med1+/− Med24+/− mammary epithelial cells. (A) BrdU immunohistochemical staining of mammary inguinal gland sections. After 2 h of purging 8-week-old littermate virgin females with BrdU, BrdU-positive cells are visualized. Representative sections at terminal buds are shown. Scale bar, 50 μm. (B) Percentage of BrdU-positive mammary luminal and basal cells from wild-type and Med1+/− Med24+/− mice are shown (n = 10). The values represent means ± SE (*, P < 0.05; **, P < 0.01). (C) Whole-mount lacZ staining of 8-week-old Med1+/− Med24+/− mammary inguinal gland. Terminal buds are positively stained. Scale bar, 1 mm. (D) BrdU incorporation into primary mammary epithelial cells after E2 addition. The incorporation is comparable for singly Med1+/− or Med24+/− cells (left panel) but impaired in Med1+/− Med24+/− cells (right panel). Values (means ± SD from representative experiments performed in duplicate) are plotted as a fold increase against the value obtained without E2 (**, P < 0.01).
The lacZ gene was introduced in frame into the Med1 and Med24 knockout alleles, so that it was expressed when and where MED1 and MED24 were expressed in mutant mice (14, 16). The whole-mount lacZ staining showed that expression of both MED1 and MED24 was under the detectable limit in Med1+/− or Med24+/− single heterozygous mutant mammary glands at the age of 8 weeks (data not shown). However, the Med1+/− Med24+/− mammary epithelial cells stained weakly at the elongating terminal buds at this age (Fig. 6C), suggesting that Mediator subunits, including MED1 and MED24, were most abundantly expressed in the terminal buds, where active cell division takes place. Enhanced expression of MED1 and MED24 in actively growing terminal buds further suggests the role of these subunits for optimal ductal elongation.
To determine whether the attenuated DNA synthesis was intrinsic and ligand dependent, primary mammary epithelial cells were isolated, cultured in vitro, and purged with BrdU in the absence or presence of E2. The RT-PCR analyses showed that, in these cells, expression of Med1 and Med24 mRNA paralleled the dosage of these genes, and expression of ERα mRNA was constant (data not shown). The wild-type and singly Med1+/− and Med24+/− primary mammary epithelial cells incorporated BrdU in response to E2, but Med1+/− Med24+/− cells showed no response to E2 (Fig. 6D). These results strongly suggest that MED1 and MED24 are necessary for normal ERα-mediated DNA synthesis in mammary epithelial cells.
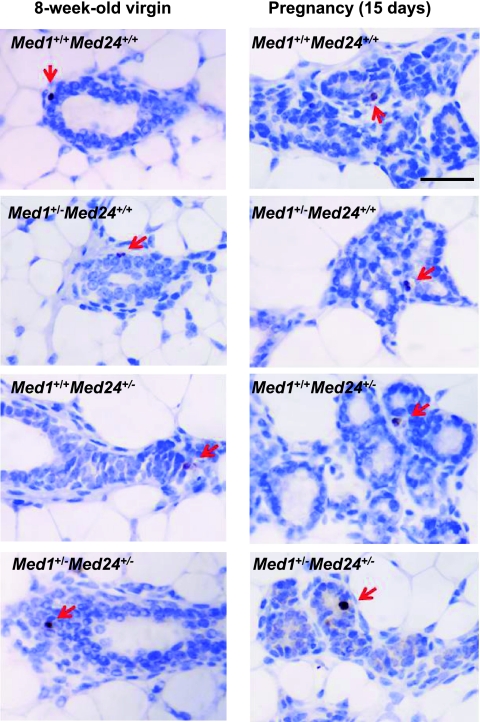
As MED1 was reported to regulate p53-dependent apoptotic events (8), we also assessed the effects of Med1 and Med24 heterozygosity on the rates of apoptosis of mammary epithelia, during both development and pregnancy. Since the caspase-dependent cascade of apoptosis is converged to the cleavage of caspase 3 (31) and γH2A.X is a specific marker of DNA double-stranded breaks (32), we demonstrated apoptotic cells by immunohistochemical analyses using anti-cleaved caspase 3 and anti-γH2A.X antibodies. The cleaved caspase 3-positive cells were rarely observed in wild-type mammary epithelial cells during both development and pregnancy, and the occurrence of positive cells in mutant glands was comparable (Fig. 7). Similar results were obtained by the analyses with an anti-γH2A.X antibody (data not shown). Therefore, the apoptotic events appear to occur normally in Med1+/− Med24+/− ductal cells.
Fig 7.
Apoptosis of wild-type and mutant mammary glands. Apoptotic cells of mammary glands from 8-week-old virgin and pregnant (15 days) females were visualized by immunohistochemistry using an anti-cleaved caspase 3 polyclonal antibody. The incidences of apoptotic cells in wild-type and (singly and double heterozygous) mutant mammary glands are comparable. Scale bar, 100 μm.
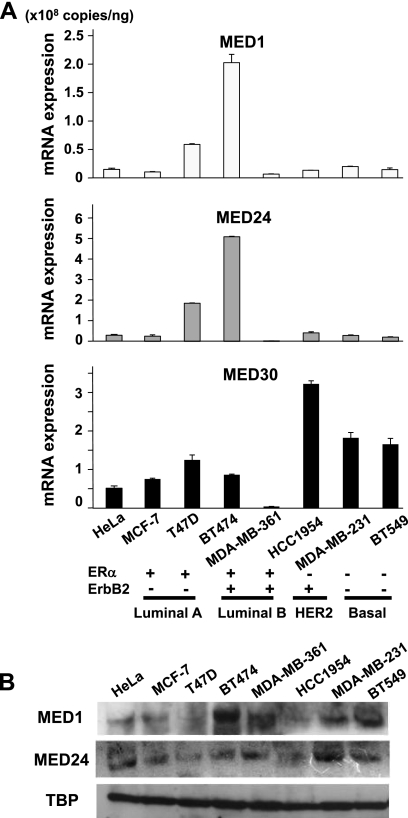
Breast carcinoma cells express abundant levels of MED1, MED24, and MED30.
Since Mediator subunits appeared necessary for normal mammary cell growth, we asked if Mediator was abundantly expressed in breast carcinoma cells and contributed to tumor cell growth as well. We also asked if MED1 expression might be enhanced in basal-like breast carcinoma cells, as mammary basal cells might be the normal counterpart of this type of carcinoma according to one hypothesis (35). Aside from the MED1 expression that is tissue-specifically controlled, we examined the expression of MED24 (in the tail module) and MED30 (a core subunit in the head module), both of which reside essentially stoichiometrically within the complex (24). Assessment of mRNA expression of Mediator subunits, including MED1, MED24, and MED30, in luminal A and B, HER2, and basal-like breast carcinoma cells revealed high and various levels of all of these subunits, and these expression levels were comparable to the ones in HeLa cells, where Mediator had been first isolated and was known to be abundant (13) (Fig. 8A). The protein levels of these subunits in these cells were also comparable (Fig. 8B). Notably, the MED1 expression in basal-like carcinoma cells (MDA-MB-231 and BT549) was as high as that one in luminal- and HER2-type carcinomas (MCF-7, MDA-MB-361, and HCC1954).
Fig 8.
Breast carcinoma cells express abundant levels of Mediator subunits. (A) Results of quantitative RT-PCR of MED1, MED24, and MED30 are shown. Values (means ± SD from a representative experiment performed in duplicate) are plotted. (B) Western blot analysis showing protein expression levels of MED1 and MED24. TATA-binding protein (TBP) was used as a control.
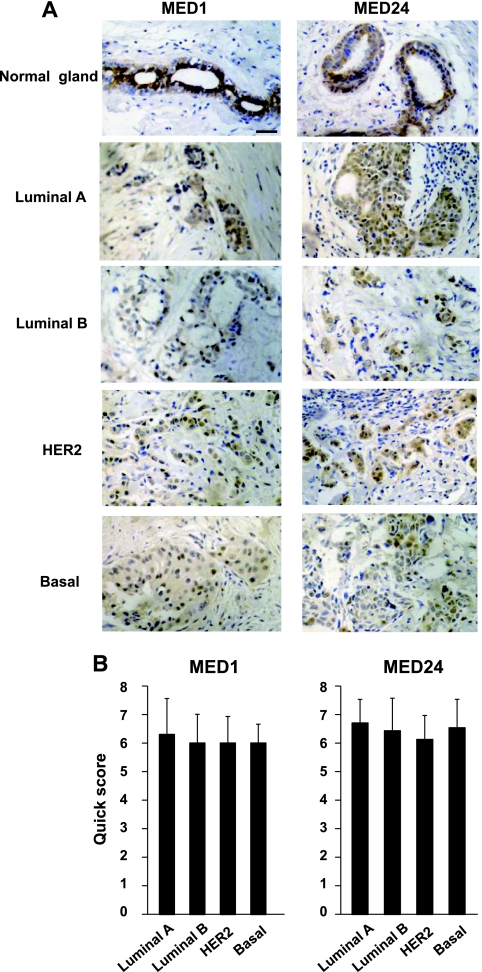
MED1 and MED24 protein expression in primary breast carcinomas was further examined by immunohistochemical analyses using stage-defined tumor arrays, and the expression levels were scored according to the quick score. This scoring system is widely used in clinic for defining the level of ERα expression in breast carcinomas (12). In normal human mammary glands, consistent with mouse glands (19), the detection of MED1 expression was restricted in luminal cells (Fig. 9A). In contrast, akin to MED30 expression in mouse glands (19), MED24 was expressed both in luminal and basal cells of normal human glands (Fig. 9A). Intriguingly, the expression of MED1 and MED24 was detected in all cases of primary breast carcinomas, although the expression levels were somewhat weaker in tumors than in normal glands. Importantly, MED1 expression in basal-like carcinomas was comparable to that in other subtypes of breast carcinomas (Fig. 9A and B). Metastatic tumors of 10 cases expressed MED1 and MED24 in the same manner as their primary origins (data not shown).
Fig 9.
MED1 and MED24 expression in primary breast carcinomas. Human breast carcinoma tumor arrays were used to show MED1 and MED24 expression in primary breast carcinomas. (A) Representative stainings of MED1 and MED24 in normal mammary glands and various types of breast carcinomas are shown. Scale bar, 100 μm. (B) Scores of MED1 and MED24 protein expression in primary carcinomas according to the quick scoring system (12). The numbers of cases are 10 (luminal A type), 7 (luminal B type), 8 (HER2 type), and 15 (basal-like type). Means ± SD are shown.
Through a search of publically available microarray databases, we also confirmed high and various levels of Med1 and Med24 mRNA expression in primary human breast carcinomas, both between luminal and basal-like types and between ER-positive and ER-negative types (GEO accession no. GSE1561 and GSE20437). These results suggested that breast carcinoma cells express abundant levels of Mediator and that MED1 appears to be stoichiometrically expressed in both the luminal and basal-like breast carcinoma cells.
ER-negative breast carcinoma cells grow MED1 and MED24 dependently.
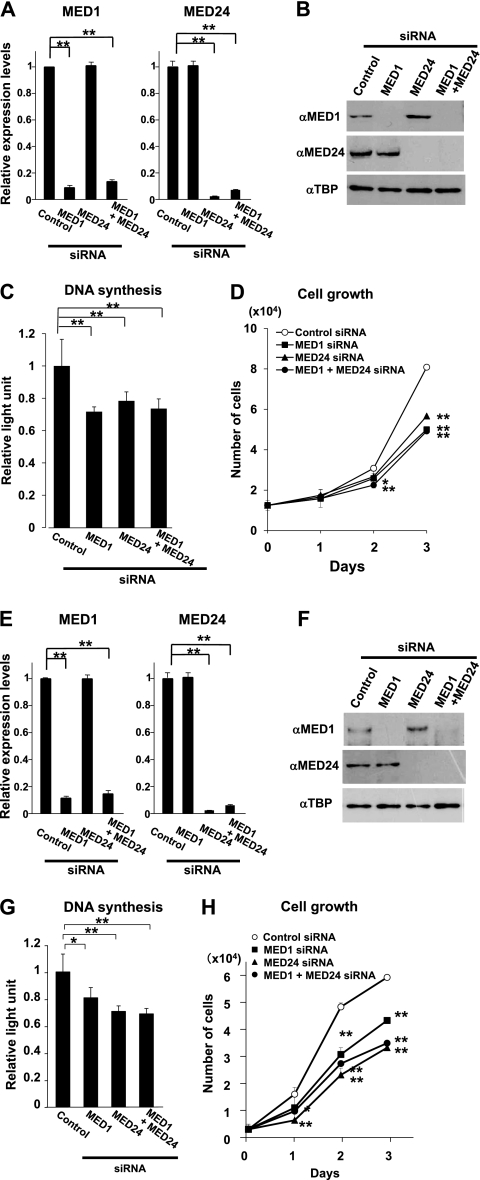
Since MED1 is involved in both intrinsic growth of embryonic cells (14) and ligand-dependent growth of MCF-7 luminal A-type breast carcinoma cells (42), we hypothesized that the enhanced expression of MED1 might also contribute to the growth of the basal-like-type breast carcinoma cells, possibly in cooperation with the MED24-containing submodule. Hence, we downregulated endogenous expression of MED1 and MED24 in BT-549 cells by means of RNA interference and analyzed the growth of these cells. When mRNA of either MED1 or MED24 was knocked down to the level of approximately one-tenth of the control level (Fig. 10A) and the protein level was likewise suppressed (Fig. 10B), DNA synthesis became approximately 70% of the control level and the cell growth was attenuated significantly (Fig. 10C and D). However, when both MED1 and MED24 were knocked down simultaneously, both the cell number and DNA synthesis were attenuated, but to the levels that were identical to those of MED1 or MED24 single knockdown cells (Fig. 10C and D). We also examined HER2-positive HCC1954 cells and obtained similar results (Fig. 10E to H). These results indicate that both the MED1 subunit and the MED24-containing submodule are involved in growth of these types of breast carcinoma cells and that MED1 and MED24 appear to constitute an identical signaling cascade.
Fig 10.
Growth of ER-negative breast carcinoma cells is MED1- and MED24-dependent. (A and E) Quantitative RT-PCR of MED1 and MED24 2 days after transfection of BT-549 (A) and HCC1954 (E) cells with siRNAs. Values (means ± SD from a representative experiment performed in duplicate) are plotted as a fold increase against the value of control scrambled siRNA (**, P < 0.01). (B and F) Western blot analyses of BT-549 (B) and HCC1954 (F) cells 2 days after transfection. αMED1, anti-MED1; αMED24, anti-MED24; αTBP, anti-TBP. (C and G) BrdU incorporation into BT-549 (C) and HCC1954 (G) cells after suppression of MED1 and MED24 expression. Values (means ± SD from a representative experiment performed in quadruplicate) are plotted as fold increase against the value of cells transfected with control siRNA (*, P < 0.05; **, P < 0.01). (D and H) Number of BT-549 (D) and HCC1954 (H) cells after transfection with siRNAs. Values are means ± SD from a representative experiment performed in triplicate (*, P < 0.05; **, P < 0.01).
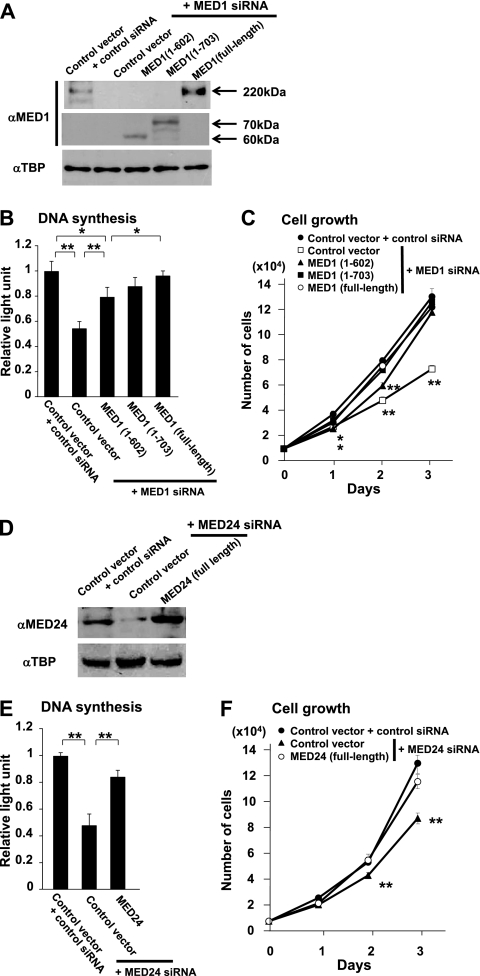
Next, to test transcriptional functions of MED1 and MED24 in detail for effects on breast cancer cell proliferation, the potential of mutant MED1 to rescue the effects of knockdown was tested first by cotransfection of siRNA and siRNA-resistant cDNA in BT-549 cells. Indeed, both full-length and N-terminal truncations of human MED1, MED1(1–602), lacking NR boxes, and MED1(1–703), having NR boxes, rescued cell growth. However, the rescue by MED1(1–602) was partial, indicating that both MED1(1–602) and MED1(603–703) domains might cooperatively play roles in mitogenicity (Fig. 11A to C). The effect of MED24 in cell growth was next confirmed by efficient rescue of cell growth by cotransfection of human siRNA-resistant Med24 cDNA in MED24-knockdown cells (Fig. 11D to F).
Fig 11.
Transcriptional functions of MED1 and MED24 for effects on proliferation of ER-negative BT-549 cells. αMED1, anti-MED1; αTBP, anti-TBP. The full-length proteins or fragments of MED1 (A to C) or MED24 (D to F) were tested to rescue the effects of knockdown of MED1 or MED24. (A and D) Western blot analyses 2 days after transfection. (B and E) BrdU incorporation 2 days after transfection. (C and F) Numbers of cells after transfection. Values are means ± SD from a representative experiment performed in triplicate (*, P < 0.05; **, P < 0.01). Values are plotted as fold increase against the value from cells transfected with control vector and siRNA (B and E).
The mRNA expression of representative ERα-targeted genes, encoding DAO, pS2 and WNT1-inducible signaling pathway protein 2 (WISP2), in BT-549 and HCC1954 cells did not change following knockdown or rescue of MED1 and/or MED24, when assessed by semiquantitative RT-PCR (data not shown). This result is consistent with the fact that these cells do not express ERα. Hence, we next aimed at testing whether enforced expression of constitutively active ERα (ERα-Y537S) (40) was able to induce expression of ERα target genes in these cells. After ERα-Y537S was exogenously expressed in BT-549 and HCC1954 cells by transfection with the ERα-Y537S mammalian expression vector prepared by site-directed mutagenesis, expression of ERα-targeted genes encoding E2F1, cyclin D1, c-myc, and DAO was assessed by quantitative RT-PCR, and cell growth was analyzed. Unexpectedly, however, expression of these genes was not increased, and consequently, neither DNA synthesis nor cell growth was enhanced after ERα-Y537S was expressed in these cells (data not shown). These results might imply that ERα-targeted endogenous gene promoters are transcriptionally inert in ERα-negative BT-549 and HCC1954 cells, possibly due to an altered epigenomic structure, such as tightly packed chromatin or altered DNA methylation status.
DISCUSSION
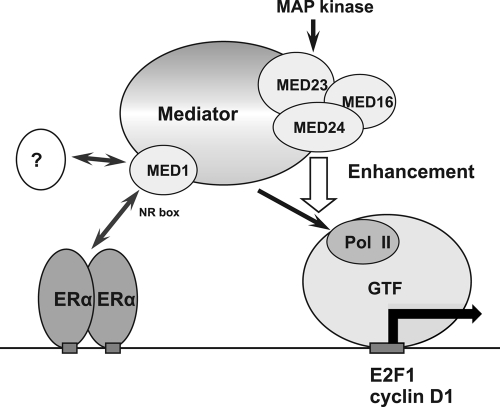
An important consideration in this study is that the role of MED1 and MED24 in cell growth is conserved between normal mammary epithelial cells and breast carcinoma cells. These functionally distinct subunits within the Mediator can be defined as a tissue-specific growth-intensifying unit. The MED24/MED23/MED16 submodule is reidentified here as a specific magnifier of the ERα-mediated coactivation function of MED1 in pubertal mammary epithelial cells (Fig. 12).
Fig 12.
Model of ERα-driven transcriptional activation in mammary epithelial cells. Interaction of MED1 with ligand-bound ERα primarily initiates postreceptor signaling, which forms PIC through recruitment of RNA polymerase II (Pol II). In this model, the MED24/MED23/MED16 submodule secondarily boosts this signal. The MAP kinase signal, which converges to MED23, may enhance the ERα-driven transcription in a trans-acting manner. Alternatively, MAP kinase-mediated phosphorylation of MED1 may enhance the incorporation of MED1 into Mediator, which may subsequently enhance PIC formation. Target promoters include genes encoding the G1/S-phase-promoting proteins E2F1 and cyclin D1. Apart from ERα, putative activators act through MED1 (probably its N terminus) to activate transcription in mammary epithelial cells during pregnancy. GTF, general transcription factor; NR box, LXXLL nuclear receptor recognition motif.
MED1 and MED24 as key molecules in normal and malignant mammary epithelial growth.
The trophic effects of the MED1 subunit and MED24-containing submodule are preceded by the phenotypes of tiny Med1−/− or Med24−/− embryos, delayed embryonic cell growth, and resultant embryonic lethality, as well as mild dwarfism of Med1+/− mice (14, 16). The difference between the ERα-related phenotypes observed in the Med1+/− Med24+/− and Med1(LX) KI mice and the distinct phenotypes in the Med1 conditional knockout mice suggests that the contributions of ERα-unrelated functions of MED1 may be more important in pregnancy-related mammary growth and lactation than in pubertal mammary growth. The specific phenotype of the Med1+/− Med24+/− basal cells, whose growth is MED1 dependent and ER independent, might be related to delayed DNA synthesis in both luminal and basal cells, the latter of which express a very small amount of MED1 that is nevertheless important for normal growth.
MED1 in the luminal A-type MCF-7 cells leads to E2-dependent cell growth (42), indicating an important role for MED1 in the ERα function of ERα-expressing breast carcinoma. Luminal B-type BT-474 cells also require MED1 for survival (21). Many breast carcinoma cases amplify genes encoding Mediator subunits, such as MED1, MED24, and MED13 (3, 28, 43), further indicating the importance of these subunits in cell growth (and perhaps tumorigenesis) of breast carcinomas. The contribution of the MED1 subunit and MED24-containing submodule in mammary epithelial cell growth is also conserved in basal-like (ERα-negative) carcinoma cells, where high expression of MED1 plays a role in ligand-independent cell growth. This contrasts with the E2-dependent growth of MCF-7 cells and indicates that the trophic effects of MED1 in BT-549 cells are mediated through hitherto unknown mechanisms that are distinct from ERα function. Since the N terminus of MED1 rescues the growth of MED1-deficient primary mammary epithelial cells immortalized by Notch4 (44), the N terminus of MED1 might be the cardinal component of MED1-mediated cell growth. Candidate activators include BRCA1 (39) and C/ERPβ (23). In this regard, it is notable that N-terminal MED1(1–602) and MED1(603–703) domains might have cooperative roles in mitogenicity (this study). The identification of the putative activator(s) that underlies this mechanism is the remaining important issue, which might contribute to the key to conquer this type of breast carcinoma.
Although MED1 is reported to be involved in p53-mediated apoptosis (8) and dapk1-associated tumor metastasis (9), the rates of apoptosis in Med1+/− Med24+/− mammary epithelia are normal, and MED1 expression in metastatic human breast carcinomas is unchanged compared to their original tumors (this study). Therefore, the roles of MED1 in apoptosis during mammary ductal growth and in breast carcinoma metastasis are unclear. One study indicates the association of an attenuated expression of MED1 with strong tumorigenic phenotype in melanoma cells (29). However, in breast carcinoma cases, the expression levels of MED1 are not apparently correlated to aggressiveness of tumors.
MED1 is an integrative and substoichiometric component of the larger (TRAP-type), and probably core (PC2-type) Mediator complexes. The Mediator complexes that do not possess the MED1 subunit can exist stably, but in nuclear receptor-mediated transcriptional activation, the Mediator that is enriched in PIC contains MED1. If this is also the case in general transcription, the limiting availability of MED1 in BT-549 (and the absence of MED1 in Med1−/− embryos) might lead to broad transcriptional attenuation and eventually retard the cell cycle.
The precise mechanism of regulating growth of mammary epithelial cells and breast carcinoma cells by the MED24/MED23/MED16 submodule is unknown. One explanation emphasizes the role of the MED24-containing submodule as a cis-acting intensifier of MED1 function (below). Another possibility is that the MED24-containing submodule mediates growth stress originating from signals distinct from the MED1 subunit. The candidate signals might include the ras-mitogen-activated protein (MAP) kinase pathway that is integrated into the MED23 subunit of the Mediator (37). The MAP kinase-MED23 axis might, additively or synergistically, function with the MED1-mediated ERα in a trans-acting manner, leading to growth in these cells. In addition, MAP kinase-dependent phosphorylation of MED1, which facilitates the recruitment of MED1 to Mediator (1), could contribute to the MED1-dependent transcriptional activation in a cis-acting manner, and attenuated MED23 function might suppress this process. Thus, it would be of interest to determine if MAP kinase modulation effectively reverses the MED1/MED24 cooperation in ERα-dependent gene transcription.
The fact that no further attenuation of growth is observed by MED1 and MED24 double knockdown of BT549 cells is probably explained by the cis-acting effect of the MED24-containing submodule, whose threshold of attenuation may be high. Thus, compared to the case in normal mammary gland development, the secondary enhancement of the MED1-mediated primary coactivation function by MED24 must be sufficient to overcome the deficiency in MED1. The high level of expression of MED1, and its significant contribution to growth could be explained as an ectopic overexpression by the tumors originating from basal cells. However, as increasing evidence now supports the hypothesis that basal-like carcinoma originates from mammary stem/precursor cells with commitment to the luminal cell lineage (27; reviewed in references 7, 11, and 22), the idea that presumably high expression of MED1 in these precursor cells is reflected in basal-like type tumors would be more favored.
The upregulated targets of liganded ERα and the MED1/MED24 unit in mammary epithelia include G1/S-phase progression-related molecules E2F1 and cyclin D1 (this study), the former of which binds to and can further activate the cyclin D1 promoter (30). Indeed, growth of ERα-positive MCF-7 cells is attenuated when expression of either E2F1 or cyclin D1 is suppressed (2, 36). These facts inevitably suggest the role of the MED1/MED24 unit in cell cycle progression in breast carcinoma cells, and most likely, in normal mammary epithelial cells as well.
The parallel and extraordinarily elevated expression of Med1 and Med24 mRNA in T47D and BT474 (Fig. 8A) might be explained by the fact that the Med1 and Med24 gene loci are very closely situated. The amplification of genes that cover these two loci, or the altered chromatin structures that facilitate these gene expressions simultaneously, might underlie the mechanism.
MED24/MED23/MED16 submodule as a specific coactivator for ERα function.
The MED24-containing submodule has been recognized to play a secondary role as a general magnifier of Mediator functions, beyond the primary activator interactions and RNA polymerase II recruitment by the Mediator. The MAP kinase-MED23 axis that mediates phosphorylation of MED1 might underlie this mechanism (above). The specific coactivation function of MED23 in E1A and the MAP kinase pathway provides a precedent for the function of the MED24-containing submodule as a specific coactivator. In this case, E1A directly interacts with MED23, and activation by E1A and the ras-MAP kinase pathway is absent in MED23-deficient ES cells (37). E1A-mediated activation is severely attenuated in MED24-deficient embryonic cells as well (16).
Here, in contrast to the activator interaction-mediated specific coactivation function of the MED24-containing submodule, this submodule apparently plays an activator- and tissue-specific coactivator function as a general coactivator submodule. The mechanism may probably be explained by specific thresholds of attenuated MED24-containing function that are specifically distinct in different activators and tissues. Hence, the relatively low threshold in pubertal mammary epithelia might induce the mammary gland-specific growth retardation in Med1+/− Med24+/− mice.
In conclusion, both the MED1 subunit and the MED24/MED23/MED16 submodule have a specific and important role in pubertal mammary gland development and the growth of breast carcinoma cells. The concentration of the MED24/MED23/MED16 submodule is the key factor for the coactivation function of the Mediator in this situation.
ACKNOWLEDGMENTS
We thank H. Matsuoka, Y. Hori, O. Horie, M. Morichika, S. Shiozawa, H. Matsuo, and members of our laboratories for helpful discussion.
This study was supported by grants from the MEXT, the Global Center for Excellence Program “Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation” from MEXT, the Japan Brain Foundation, the Takeda Science Foundation, the Sagawa Foundation for Promotion of Cancer Research, the Suzuken Memorial Foundation, and the ONO Medical Research Foundation (to M.I.) and by NIH grant DK071900 (to R.G.R.).
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Belakavadi M, Pandey PK, Vijayvargia R, Fondell JD. 2008. MED1 phosphorylation promotes its association with mediator: implications for nuclear receptor signaling. Mol. Cell. Biol. 28: 3932– 3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carroll JS, Prall OW, Musgrove EA, Sutherland RL. 2000. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130-E2F4 complexes characteristic of quiescence. J. Biol. Chem. 275: 38221– 38229 [DOI] [PubMed] [Google Scholar]
- 3. Clark J, et al. 2002. Identification of amplified and expressed genes in breast cancer by comparative hybridization onto microarrays of randomly selected cDNA clones. Genes Chromosomes Cancer 34: 104– 114 [DOI] [PubMed] [Google Scholar]
- 4. Conaway RC, Sato S, Tomomori-Sato C, Yao T, Conaway JW. 2005. The mammalian mediator complex and its role in transcriptional regulation. Trends Biochem. Sci. 30: 250– 255 [DOI] [PubMed] [Google Scholar]
- 5. Conneely OM, Jericevic BM, Lydon JP. 2003. Progesterone receptors in mammary gland development and tumorigenesis. J. Mammary Gland Biol. 8: 205– 214 [DOI] [PubMed] [Google Scholar]
- 6. Fan X, Chou DM, Struhl K. 2006. Activator-specific recruitment of Mediator in vivo. Nat. Struct. Mol. Biol. 13: 117– 120 [DOI] [PubMed] [Google Scholar]
- 7. Foulkes WD, Smith IE, Reis-Filho JS. 2010. Triple-negative breast cancer. N. Engl. J. Med. 363: 1938– 1948 [DOI] [PubMed] [Google Scholar]
- 8. Frade R, Balbo M, Barel M. 2002. RB18A regulates p-53-dependent apoptosis. Oncogene 21: 861– 866 [DOI] [PubMed] [Google Scholar]
- 9. Gade P, Singh AK, Roy SK, Reddy SP, Kalvakolanu DV. 2009. Down-regulation of the transcriptional mediator subunit Med1 contributes to the loss of expression of metastasis-associated dapk1 in human cancers and cancer cells. Int. J. Cancer 125: 1566– 1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glass CK, Rosenfeld MG. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14: 121– 141 [PubMed] [Google Scholar]
- 11. Gusterson B. 2009. Do “basal-like” breast cancers really exist? Nat. Rev. Cancer 9: 128– 134 [DOI] [PubMed] [Google Scholar]
- 12. Harvey JM, Clark GM, Osborne CK, Alfred DC. 1999. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 17: 1474– 1481 [DOI] [PubMed] [Google Scholar]
- 13. Ito M, et al. 1999. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 3: 361– 370 [DOI] [PubMed] [Google Scholar]
- 14. Ito M, Yuan C-X, Okano HJ, Darnell RB, Roeder RG. 2000. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol. Cell 5: 683– 693 [DOI] [PubMed] [Google Scholar]
- 15. Ito M, Roeder RG. 2001. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12: 127– 134 [DOI] [PubMed] [Google Scholar]
- 16. Ito M, Okano HJ, Darnell RB, Roeder RG. 2002. The TRAP100 component of the TRAP/Mediator complex is essential in broad transcriptional events and development. EMBO J. 21: 3464– 3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jia Y, et al. 2005. Peroxisome proliferator-activated receptor-binding protein null mutation results in defective mammary gland development. J. Biol. Chem. 280: 10766– 10773 [DOI] [PubMed] [Google Scholar]
- 18. Jiang C, et al. 2004. TIP30 interacts with an ERα-interacting coactivator CIA and regulates c-myc transcription. J. Biol. Chem. 279: 27781– 27789 [DOI] [PubMed] [Google Scholar]
- 19. Jiang P, et al. 2010. Key roles for MED1 LxxLL motifs in pubertal mammary gland development and luminal-cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 107: 6765– 6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kornberg RD. 2005. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 30: 235– 239 [DOI] [PubMed] [Google Scholar]
- 21. Kourtidis A, et al. 2010. An RNA interference screen identifies metabolic regulators NR1D1 and PBP as novel survival factors for breast cancer cells with the ERBB2 signature. Cancer Res. 70: 1783– 1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lerma E, Barnadas A, Prat J. 2009. Triple negative breast carcinomas: similarities and differences with basal like carcinomas. Appl. Immunohistochem. Mol. Morphol. 17: 483– 494 [DOI] [PubMed] [Google Scholar]
- 23. Li H, et al. 2008. The Med1 subunit of transcriptional mediator plays a central role in regulating CCAAT/enhancer-binding protein-beta-driven transcription in response to interferon-gamma. J. Biol. Chem. 283: 13077– 13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik S, Roeder RG. 2010. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11: 761– 772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mangelsdorf DJ, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83: 835– 839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mittler, et al. 2003. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 22: 6494– 6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molyneux G, et al. 2010. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7: 403– 417 [DOI] [PubMed] [Google Scholar]
- 28. Monni O, et al. 2001. Comprehensive copy number and gene expression profiling of the 17q23 amplicon in human breast cancer. Proc. Natl. Acad. Sci. U. S. A. 98: 5711– 5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ndong Jde L, Jean D, Rousselet N, Frade R. 2009. Down-regulation of the expression of RB18A/MED1, a cofactor of transcription, triggers strong tumorigenic phenotype of human melanoma cells. Int. J. Cancer 124: 2597– 2606 [DOI] [PubMed] [Google Scholar]
- 30. Ohtani K, DeGregori J, Nevins JR. 1995. Regulation of the cyclin E gene by transcription factor E2F1. Proc. Natl. Acad. Sci. U. S. A. 92: 12146– 12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philchenkov A. 2004. Caspases: potential targets for regulating cell death. J. Cell Mol. Med. 8: 432– 434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273: 5858– 5868 [DOI] [PubMed] [Google Scholar]
- 33. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103: 843– 852 [DOI] [PubMed] [Google Scholar]
- 34. Sharma D, Fondell JD. 2002. Ordered recruitment of histone acetyltransferases and the TRAP/Mediator complex to thyroid hormone-responsive promoters in vivo. Proc. Natl. Acad. Sci. U. S. A. 99: 7934– 7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorlie T, et al. 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. U. S. A. 100: 8418– 8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stender JD, et al. 2007. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol. Endocrinol. 21: 2112– 2123 [DOI] [PubMed] [Google Scholar]
- 37. Stevens JL, et al. 2002. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science 296: 755– 758 [DOI] [PubMed] [Google Scholar]
- 38. Sumitomo A, et al. 2010. Transcriptional Mediator subunit MED1/TRAP220 in stromal cells is involved in hematopoietic stem/progenitor cell support through osteopontin expression. Mol. Cell. Biol. 30: 4818– 4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wada O, et al. 2004. BRCA1 function mediates a TRAP/DRIP complex through direct interaction with TRAP220. Oncogene 23: 6000– 6005 [DOI] [PubMed] [Google Scholar]
- 40. Weis KE, Ekena K, Thomas JA, Lazennec G, Katzenellenbogen BS. 1996. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol. Endocrinol. 10: 1388– 1398 [DOI] [PubMed] [Google Scholar]
- 41. Yang F, DeBeaumont R, Zhou S, Näär AM. 2004. The activator-recruited cofactor/Mediator coactivator subunit ARC92 is a functionally important target of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. U. S. A. 101: 2339– 2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, et al. 2005. MED1/TRAP220 exists predominantly in a TRAP/Mediator subpopulation enriched in RNA polymerase II and is required for ER-mediated transcription. Mol. Cell 19: 89– 100 [DOI] [PubMed] [Google Scholar]
- 43. Zhu Y, et al. 1999. Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/PPARBP) gene in breast cancer. Proc. Natl. Acad. Sci. U. S. A. 96: 10848– 10853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu YT, et al. 2010. Peroxisome-proliferator-activated receptor-binding protein (PBP) is essential for the growth of active Notch4-immortalized mammary epithelial cells by activating SOX10 expression. Biochem. J. 425: 435– 444 [DOI] [PMC free article] [PubMed] [Google Scholar]