Abstract
A herpes simplex virus 2 (HSV-2) glycoprotein E deletion mutant (gE2-del virus) was evaluated as a replication-competent, attenuated live virus vaccine candidate. The gE2-del virus is defective in epithelial cell-to-axon spread and in anterograde transport from the neuron cell body to the axon terminus. In BALB/c and SCID mice, the gE2-del virus caused no death or disease after vaginal, intravascular, or intramuscular inoculation and was 5 orders of magnitude less virulent than wild-type virus when inoculated directly into the brain. No infectious gE2-del virus was recovered from dorsal root ganglia (DRG) after multiple routes of inoculation; however, gE2-del DNA was detected by PCR in lumbosacral DRG at a low copy number in some mice. Importantly, no recurrent vaginal shedding of gE2-del DNA was detected in immunized guinea pigs. Intramuscular immunization outperformed subcutaneous immunization in all parameters evaluated, although individual differences were not significant, and two intramuscular immunizations were more protective than one. Immunized animals had reduced vaginal disease, vaginal titers, DRG infection, recurrent genital lesions, and recurrent vaginal shedding of HSV-2 DNA; however, protection was incomplete. A combined modality immunization using live virus and HSV-2 glycoprotein C and D subunit antigens in guinea pigs did not totally eliminate recurrent lesions or recurrent vaginal shedding of HSV-2 DNA. The gE2-del virus used as an immunotherapeutic vaccine in previously HSV-2-infected guinea pigs greatly reduced the frequency of recurrent genital lesions. Therefore, the gE2-del virus is safe, other than when injected at high titer into the brain, and is efficacious as a prophylactic and immunotherapeutic vaccine.
INTRODUCTION
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) cause common infections worldwide (33, 47). HSV-1 typically causes vesicles and ulcers on the vermillion border of the lip, and HSV-2 causes genital ulcers. HSV-1 and HSV-2 transmission occurs by contact with infected individuals, with HSV-1 acquisition typically beginning in childhood and HSV-2 acquisition occurring at the onset of sexual activity.
HSV-1 and HSV-2 have similar infection cycles, replicating initially in epithelial cells and spreading to sensory neurons of the peripheral nervous system. After the virus enters axons, it travels retrograde to the neuron cell body, where latency is established, followed by periodic reactivation. During recurrences, HSV travels along neurons in the anterograde direction to the dermatome innervated by the infected ganglion. Replication within the epithelium results in either asymptomatic virus shedding or lesions. Initial episodes of genital ulcer disease are as likely to be caused by HSV-1 as HSV-2; however, recurrences are considerably more common after HSV-2 infection (15, 23, 43). Serious sequelae of genital ulcer disease include infection of neonates during labor and delivery and a 3-fold increased risk of acquiring HIV-1 infection (10–12, 44).
A vaccine to prevent HSV-2 is a high public health priority. Preclinical studies include vaccines comprised of DNA, glycoproteins, attenuated live viruses, or combinations of these compounds (1, 4, 6–8, 18–22, 27–29, 31, 42). Placebo-controlled human trials have been performed using HSV-2 subunit glycoprotein vaccines, including HSV-2 glycoprotein D (gD2) given with alum and 2-O-deacylated-monophosphoryl lipid A (MPL) adjuvant and HSV-2 glycoprotein B (gB2) combined with gD2 administered with MF59 adjuvant (5, 14, 36). The gD2 and gB2 vaccine trial showed no protection against HSV-2 infection beyond the first 5 months after immunization (14). The first gD2-MPL/alum vaccine was effective at preventing genital lesions in HSV-1- and HSV-2-seronegative women but not in HSV-1-seropositive women or men (36). A follow-up trial to confirm the findings in seronegative women failed to show significant protection against HSV-2; however, the vaccine was effective at preventing HSV-1 genital disease (5). New vaccine approaches are warranted to improve upon these results.
Attenuated live virus vaccines have been highly effective for prevention of measles, mumps, rubella, polio, influenza, and chickenpox. The success of the varicella-zoster vaccine has renewed interest in live virus vaccines for prevention of HSV-2, a related alphaherpesvirus. Attenuated live virus vaccine candidates in preclinical testing for HSV-2 include HSV-2 dl5-29, the HSV-2 ICP0 deletion mutant 0ΔNLS, and the ICP0 deletion mutant CJ2-gD2, which was modified to express gD2 and a dominant-negative form of the gene carrying the origin of viral DNA replication (1, 18–22). In addition, a replication-competent live virus vaccine strain that is defective in immune evasion has entered phase 1 human trials (Immunovex; Biovex Inc.).
We previously reported for mouse models that an HSV-1 gE deletion virus, NS-gEnull, is safe and effective as a replication-competent, attenuated live virus vaccine that is defective in neuronal spread (9). An HSV-2 gE deletion strain, gE2-del virus, is also replication competent and defective in neuronal spread (46). Studies reported here indicate that the gE2-del virus is safe when administered intramuscularly (i.m.), intravaginally (IVAG), by flank scarification, or intravenously (i.v.). The vaccine candidate greatly reduces HSV-2 genital disease in mice and guinea pigs as a prophylactic vaccine and, importantly, reduces the frequency of recurrent genital lesions in guinea pigs when administered as a therapeutic vaccine.
MATERIALS AND METHODS
Cells and virus.
Vero cells (African green monkey kidney epithelial cells) were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 mM HEPES (pH 7.3), 2 mM l-glutamine, 20 μg/ml gentamicin, and 5% heat-inactivated fetal bovine serum (FBS). HSV-2 strain 2.12 was isolated from a woman with genital ulcer disease and used at passage 8 to derive a gE2-del virus that has a green fluorescent protein (GFP2) cassette under the control of a cytomegalovirus (CMV) promoter replacing gE2 amino acids 124 to 495 (46). Virus stocks for immunization and challenge studies were prepared in Vero cells, using both cells and supernatant fluids that were subjected to freezing at −80°C, thawing, and sonication. Virus titers were determined by plaque assay on Vero cell monolayers.
Safety of gE2-del vaccine in mice.
Laboratory animals were managed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania. Five- to 6-week-old female BALB/c (NCI) and SCID (strain CB17/lcr-Prkdcscid/lcrCrl; Charles River Inc.) mice were allowed to acclimate to the animal facility for 1 week prior to handling. Mice were assessed following i.m. inoculation of the gastrocnemius (calf) muscle of a hind leg, i.v. injection of the tail vein, or IVAG inoculation. The 50% lethal dose (LD50) was calculated by the method of Reed and Muench (32). Five days prior to IVAG infection, mice received a subcutaneous (s.c.) injection of 2 mg of medroxyprogesterone (Sicor Pharmaceuticals, Inc.) in 0.9% NaCl and 10 mM HEPES (9). The LD50 after intracerebral (i.c.) inoculation was evaluated in 20-day-old female BALB/c mice injected in the frontal cerebral cortex by use of a 27-guage needle and tuberculin syringe containing 5 μl of virus (17). Disease signs included impaired mobility, hunched posture, and ruffled fur. Flank inoculations were performed by skin scarification (30). Dorsal root ganglia (DRG) were harvested for determination of virus titers and HSV-2 DNA copy numbers 4 days after infection, a time chosen based on prior studies that indicated that peak titers develop at 3 to 5 days postinfection (2, 3, 9). DRG samples were minced with small scissors and pulverized with a pestle (3, 9). After i.m. inoculation, spinal cords were harvested in addition to DRG. The limit of detection of the virus culture assay was 2 PFU per sample.
Real-time qPCR.
DRG and spinal cord samples were homogenized and assessed by real-time quantitative PCR (qPCR) using primers and probes for HSV-2 Us9 DNA and the mouse adipsin gene as previously described (4). For some assays, gE2 DNA or GFP DNA was amplified to detect sequences unique to wild-type or gE2-del virus, respectively. Primers for gE2 DNA were as follows: forward, 5′-CGTCTGGATGCGGTTTGAC-3′; and reverse, 5′-CTGGAAGCTGCGGGTGATAC-3′. The reporter dye plus probe for gE2 was 6-carboxyfluorescein (FAM)-5′-ATGCGGATCTACGAAGC-3′-MGBNFQ. Primers for GFP were as follows: forward, 5′-AGCAAAGACCCCAACGAGAA-3′; and reverse, 5′-GGCGGCGGTCACGAA-3′. The reporter dye plus probe for GFP was FAM-5′-ATCACATGGTCCTGCTGG-3′-MGBNFQ. Standard curves to determine Us9 and gE2 DNA copy numbers were run in triplicate, using 50,000, 5,000, 500, 50, and 5 copies of wild-type HSV-2 DNA (Advanced Biotechnologies). The same HSV-2 DNA preparation was used to generate the standard curves to calculate the copy numbers of Us9 and gE2 DNAs; therefore, similar DNA copy numbers are expected using these primers. The gE2-del virus DNA was used to generate a standard curve to quantify GFP copy number. DRG and vaginal swab samples that did not yield a positive signal in duplicate wells by 40 cycles were considered negative. DRG samples were considered negative if they contained <1 copy of HSV-2 DNA per 104 adipsin genes. Vaginal swab samples were considered negative if they contained <1.5 copies of HSV-2 DNA per assay (10 μl was amplified per assay; therefore, the cutoff for a negative sample was ≤150 copies/ml) (4).
Mouse immunizations and wild-type virus IVAG challenge.
Immunization studies were performed in 6- to 8-week-old female BALB/c mice at the time of first immunization (9). Mice were immunized i.m. twice at 3-week intervals with gE2-del virus or were mock immunized with Vero cell lysate. Prior to IVAG virus challenge, mice received an s.c. injection with 2 mg of medroxyprogesterone (Sicor Pharmaceuticals, Inc.) approximately 23 days after the last immunization and 5 days before infection (9). On the day of infection, the vaginal vaults were swabbed with phosphate-buffered saline (PBS)-moistened cotton swabs and 5 μl of HSV-2 MS containing 5 × 104 PFU (∼104 LD50) was introduced into the vaginal vault by use of a gel-loading pipette tip (9). A dry polyester swab was used to collect vaginal secretions on the indicated days postinfection, and titers were determined by plaque assay on Vero cells. The severity of vaginal disease was scored on a scale of 0 to 4 by assigning one point each for erythema, exudate, hair loss, and necrosis (4). Blood was collected using Goldenrod animal lancets (MEDIpoint, Inc.) by the submandibular route.
Explant cocultures of murine DRG for recovery of latent HSV-2.
At least 28 days after infection, the DRG were removed, minced with scissors, placed on Vero cells for 20 days, and observed for cytopathic effect as a marker of virus reactivation (4, 9).
Guinea pig immunizations and wild-type virus IVAG challenge.
Immunizations for prevention vaccine studies were performed in female Hartley strain guinea pigs weighing ∼225 g at the time of first immunization. Animals were immunized in the hind leg gastrocnemius muscle twice at 3-week intervals with gE2-del virus or were mock immunized with Vero cell lysate. Some guinea pigs were immunized with both gE2-del virus and gC2 and gD2 subunit antigens (4). Guinea pigs were inoculated with the gE2-del virus on days 0 and 21 and inoculated i.m. in the other hind leg gastrocnemius muscle on days 7 and 28, using 10 μg of the gC2 antigen bac-gC-2(426t) and 5 μg of the gD2 antigen bac-gD-2(306t), each mixed with 100 μg of CpG oligonucleotide (Tri-Link Inc.) and 20 μg alum (Alhydragel; Accurate Chemical and Scientific Corp.) per μg protein in 50 μl saline (4, 13, 39, 40). Mock immunizations were performed using CpG and alum alone.
Guinea pigs were challenged intravaginally with HSV-2 MS at 5 × 105 PFU (>500 LD50) in 50 μl DMEM by use of a soft catheter (4, 24, 35). Vaginal swabs were obtained for titers and were stored at −80°C until used for plaque assay. Animals were scored for disease severity on a scale of 0 to 4 by assigning 1 point for erythema, 2 points for discrete lesions, 3 points for coalesced lesions, and 4 points for ulcerative lesions (4, 24). Animals were monitored for recurrent lesions from days 15 to 60 postinfection, and vaginal swabs were obtained from days 28 to 48 to assess recurrent vaginal shedding of HSV-2 DNA.
gE2-del virus as an immunotherapeutic.
Thirty guinea pigs were infected IVAG with HSV-2 MS at 1 × 104 PFU. Thirteen of 30 guinea pigs died during the acute infection. The 17 surviving animals were divided into two comparable groups based on the severity of the acute vaginal disease. One group was assigned to receive the mock vaccine (PBS) and the other to receive 5 × 105 PFU gE2-del virus. Two i.m. immunizations were given at 51 and 81 days postinfection, and animals were scored for recurrent lesions for 7 weeks, starting 8 days after the first immunization.
ELISA and neutralization assays.
Enzyme-linked immunosorbent assay (ELISA) antibody responses were measured using bac-gC-2(426t) and bac-gD-2(306t) as antigens and serum at a starting dilution of 1:100 (4). Neutralizing antibody assays were performed by heating mouse or guinea pig serum for 30 min at 56°C to inactivate complement and incubating serial 2-fold dilutions of serum starting at a 1:20 dilution with 300 PFU of HSV-2 strain MS. Vero cells were overlaid with medium containing 0.6% low-melting-point agarose, and plaques were counted at 72 h (4).
Statistics.
P values were calculated using GraphPad Prism software. Survival results were evaluated using the log rank (Mantel-Cox) test. Analysis of variance (ANOVA) with Tukey's adjustment was used for statistical analysis when more than two groups were compared. The Mann-Whitney test for nonparametric data was used when comparing two groups. Fisher's exact test was used to evaluate the days of vaginal shedding of HSV-2 DNA and the effects of gE2-del virus immunization in reducing recurrent genital lesions.
RESULTS
Safety of gE2-del virus.
The i.m., i.v., and IVAG infections with HSV-2 2.12 (wild-type virus) or gE2-del virus were performed in BALB/c and SCID mice, while i.c. infections were performed in BALB/c mice only. The LD50 of gE2-del virus was determined using two or three serial 10-fold dilutions starting with 5 × 106 PFU, except that 5 × 105 PFU was the highest dose used for IVAG infection in BALB/c mice. Five mice were evaluated at each dose, except that 10 mice were inoculated i.c. with gE2-del virus at 5 × 106 PFU. The LD50 of HSV-2 2.12 was determined using three or more serial 10-fold dilutions ranging from 5 × 100 to 5 × 105 PFU. Five mice were included in each group, except that 10 SCID mice were inoculated i.m. with HSV-2 2.12 at 5 × 103 and 5 × 104 PFU.
BALB/c and SCID mice injected with HSV-2 2.12 died at one or more concentrations when inoculated by each route (Table 1). In contrast, no mouse inoculated with gE2-del virus died or showed signs of disease, even at 5 × 106 PFU, with the exception of mice infected i.c. The LD50 of gE2-del virus exceeded the LD50 of wild-type virus by at least 100 PFU by the i.m. route, by 500 PFU by the i.v. route, and by 10,000 PFU by the IVAG route. The LD50 of HSV-2 2.12 given i.c. was <5 PFU, while the gE2-del virus was fatal at 1.3 × 106 PFU, which is >300,000 PFU higher than the LD50 of HSV-2 2.12. None of 10 mice inoculated i.c. with uninfected Vero cell extracts died, indicating that death following i.c. infection cannot be attributed to the inoculation procedure per se. Thus, the gE2-del virus is highly attenuated for virulence compared with the parental strain.
Table 1.
LD50 of gE2-del and parental virus strains in mice
| Virus | LD50 (PFU) |
||||||
|---|---|---|---|---|---|---|---|
| Intramuscular |
Intravenous |
Intravaginal |
Intracerebral |
||||
| BALB/c | SCID | BALB/c | SCID | BALB/c | SCID | BALB/c | |
| gE2-del | >5 × 106 | >5 × 106 | >5 × 106 | >5 × 106 | >5 × 105a | >5 × 106 | 1.3 × 106 |
| HSV-2 2.12 | 1.6 × 104 | 5 × 104b | 1 × 104 | <5 × 103 | 3.4 × 101 | <5 × 101 | <5 × 100 |
| Difference in LD50 between viruses | >3 × 102 | >102 | >5 × 102 | >103 | >104 | >105 | >3 × 105 |
A titer of 5 × 105 PFU was the highest titer tested after intravaginal infection in BALB/c mice.
The result shown represents 35 SCID mice infected with HSV-2 2.12 over the range of 5 × 101 to 5 × 105 PFU, including 10 mice each at 5 × 103 and 5 × 104 PFU.
Virus titers and HSV-2 DNA copy numbers in DRG and spinal cords.
An important safety consideration for a live virus vaccine is to assess whether the candidate vaccine reaches the DRG, which is the site of latency. No infectious virus was recovered from lumbosacral DRG 4 days after i.m., flank, or IVAG inoculation of BALB/c mice with 5 × 105 PFU of gE2-del virus. Motor neurons that innervate the gastrocnemius muscle originate in the spinal cord; therefore, spinal cord tissue was harvested to assess whether gE2-del virus reached the spinal cord by retrograde spread from muscle at the inoculation site (48). No infectious virus was isolated. In contrast, infectious virus was recovered from lumbosacral DRG and spinal cords after inoculation with 100- to 100,000-fold lower titers of wild-type virus, including after i.m. infection with 5 × 103 PFU of wild-type virus, flank infection with 5 × 102 PFU of wild-type virus, and IVAG infection with 5 PFU of wild-type virus (Fig. 1A).
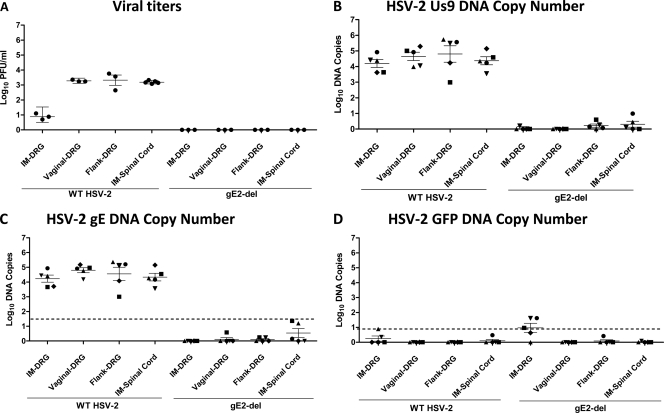
Fig 1.
DRG and spinal cord HSV-2 titers and DNA copy numbers after i.m., flank, and vaginal infections with HSV-2 2.12 or gE2-del virus. Mice were inoculated i.m. with 5 × 103 PFU, by flank scarification with 5 × 102 PFU, or by IVAG infection with 5 PFU of HSV-2 2.12. The gE2-del virus was inoculated at 5 × 105 PFU by each route. On day 4 postinfection, lumbosacral DRG were harvested. Spinal cords were also collected from mice inoculated by the i.m. route. (A) Viral titers in DRG and spinal cords (n = 3 to 5 animals per group). (B) HSV-2 Us9 DNA copy numbers. (C) HSV-2 gE DNA copy numbers. (D) HSV-2 GFP DNA copy numbers. Results shown in panels B to D are for different mice from those used for panel A. For panels B to D, the same samples were evaluated using Us9, gE2, and GFP primers and probes. Symbols used are intended to link the results for the various primers so that individual mice can be compared in panels B to D. The dotted lines in panels C and D represent the cutoff values for considering a sample positive, which are >23 copies of gE2 DNA and >8 copies of GFP DNA. Results represent geometric mean titers ± standard errors of the means (SEM).
DRG samples were analyzed for HSV-2 DNA by qPCR. Approximately 4 to 5 log10 copies of Us9 HSV-2 DNA were detected in DRG isolated from mice infected with wild-type virus (Fig. 1B). Collectively, no HSV-2 Us9 DNA was detected in 10 of 15 DRG samples obtained after i.m., IVAG, and flank infections with gE2-del virus or in 2 of 5 spinal cord samples after i.m. infection with gE2-del virus (Fig. 1B).
The low copy number of gE2-del virus DNA detected in some DRG and spinal cord samples could represent false-positive results based on contamination of specimens during the harvesting of tissues or processing of samples. To help distinguish between true- and false-positive results, DRG and spinal cord DNAs were amplified using gE2 primers and probes. The gE2 DNA is present in wild-type virus but not in gE2-del virus; therefore, detecting gE DNA in gE2-del virus samples suggests specimen contamination.
Twenty DRG and spinal cord samples harvested from mice infected with wild-type virus or gE2-del virus were assessed for gE2 DNA. The 20 samples from mice infected with wild-type virus yielded results that were almost identical using the Us9 and gE2 primers and probes (see wild-type HSV-2 in Fig. 1B and C), suggesting comparable sensitivities of the Us9 and gE2 primers and probes. The 20 samples from mice infected with gE2-del virus also yielded similar results using the Us9 and gE2 primers and probes (see gE2-del virus in Fig. 1B and C). Six samples from mice infected with gE2-del virus, which does not contain gE2 DNA, had a positive signal, with two of the samples yielding 16 and 23 copies of gE2 DNA (Fig. 1C). Therefore, these samples represent false-positive results and establish the specificity of the gE2 qPCRs as >23 copies of HSV-2 DNA. Based on this cutoff, all of the wild-type virus samples were truly positive, and specimen contamination likely accounts for the low-level positive gE2-del virus samples in Fig. 1C.
The 20 DRG and spinal cord samples from mice infected with wild-type or gE2-del virus were also evaluated for GFP DNA (Fig. 1D). Wild-type virus does not contain the GFP DNA sequence; therefore, amplification of GFP DNA from mice infected with wild-type virus represents a false-positive result. Seventeen of 20 samples from mice infected with wild-type virus were negative, while 3 samples had low-level positive readings of 2, 3, and 8 copies of GFP DNA (Fig. 1D). Therefore, the specificity of the GFP probe for a true-positive result was set at >8 copies of HSV-2 DNA. Six of 20 samples from mice infected with gE2-del virus had positive PCR results for GFP DNA (Fig. 1D). Based on >8 copies as the cutoff for a true-positive result, three DRG samples were above this cutoff after i.m. inoculation with gE2-del virus, with copy numbers of 9, 41, and 42, suggesting that these samples may be true-positive samples.
Explant cocultures of DRG are useful to assess whether HSV-2 has infected DRG and established latency (41). Lumbosacral DRG were harvested 35 days after i.m., flank, or IVAG infection with 5 × 105 PFU of gE2-del virus (3 animals per group). No virus was isolated from these nine samples. In contrast, as discussed below, 9 of 10 explant cocultures yielded virus when performed 28 days after IVAG challenge with wild-type virus of i.m. or s.c. immunized mice, indicating that the explant coculture assay is sensitive for detecting latent infection. Therefore, we failed to isolate gE2-del virus from DRG during the acute (day 4) or latent (day 35) stage of infection but did detect GFP DNA above the cutoff value in three samples after i.m. infection with gE2-del virus. Since qPCR is the most sensitive of the assays used, the conservative interpretation of the results is that small quantities of gE2-del DNA may have reached the DRG after i.m. inoculation.
Comparison of gE2-del virus immunization by i.m. and s.c. routes.
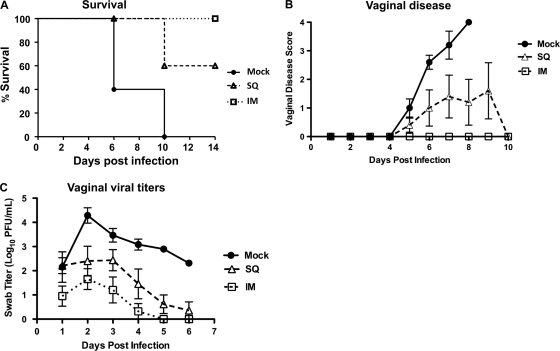
Mice were mock immunized or immunized once by the i.m. or s.c. route with 5 × 105 gE2-del virus. Twenty-eight days later, mice were challenged IVAG with 250 PFU (∼50 LD50) of HSV-2 MS. Mice immunized by the i.m. route all survived, in contrast with 60% (3 of 5 mice) survival of mice immunized s.c. and no (0 of 5 mice) survival of mock-immunized mice (P < 0.05 for comparing i.m. with mock infection; the P value was not significant for comparing i.m. and s.c. or s.c. and mock infections) (Fig. 2A). All mock-immunized mice developed severe disease, and some s.c. immunized mice developed disease; however, no mouse vaccinated i.m. developed disease at this challenge dose (P < 0.05 for comparing i.m. with mock infection; the P value was not significant for comparing i.m. and s.c. or s.c. and mock infections) (Fig. 2B). Vaginal HSV-2 swab titers were reduced following s.c. or i.m. immunization compared with mock immunization, with the lowest titers in the i.m. immunization group (the P value was not significant for comparing i.m. and s.c. infections, P < 0.001 for comparing i.m. and mock infections, and P < 0.05 for comparing s.c. and mock infections) (Fig. 2C). Explant cocultures were performed on sacral DRG at 28 days postinfection. Virus was recovered from 4 of 5 i.m. immunized mice and 5 of 5 s.c. immunized mice. The i.m. route outperformed the s.c. route for each parameter evaluated, although none of the differences between i.m. and s.c. immunization were statistically significant. However, one immunization was not sufficient to prevent the challenge virus from reaching the DRG and establishing latency as assessed by explant cocultures.
Fig 2.
Protection after i.m. or s.c. (SQ) immunization of mice. BALB/c mice were mock immunized or immunized i.m. or s.c. with 5 × 105 PFU gE2-del virus and were challenged IVAG with 250 PFU HSV-2 MS. (A) Survival kinetics. (B) Vaginal disease scores. (C) Vaginal viral titers. Each group had 5 animals. Disease scores are plotted only for surviving animals. Results in panels B and C represent means ± SEM.
Comparison of one and two i.m. immunizations with gE2-del virus.
Mice were mock immunized or immunized with 5 × 105 PFU gE2-del virus i.m. once or twice, separated by 3 weeks. Twenty-eight days after the last immunization, mice were challenged IVAG with 5 × 104 PFU of HSV-2 MS (∼104 LD50), which is 200-fold more virus than that used for the experiments in Fig. 2. All mice immunized with gE2-del virus survived, while no mock-immunized mouse survived (P < 0.01 for comparing one or two immunizations with mock immunization) (Fig. 3A). One immunization with gE2-del virus did not completely protect mice from disease, while mice that received two immunizations were totally protected (P < 0.001 for comparing one or two immunizations with mock immunization; the P value was not significant for comparing one with two immunizations) (Fig. 3B). Vaginal titers were lowest in mice that received two immunizations (P < 0.01 for comparing two immunizations with mock immunization, P < 0.05 for comparing one immunization with mock immunization, and the P value was not significant for comparing one with two immunizations) (Fig. 3C). Representative photographs from each group taken at 7 days postinfection are shown (Fig. 3D).
Fig 3.
Protection after one or two i.m. immunizations with gE2-del virus in mice. BALB/c mice were immunized once or twice at 3-week intervals and challenged IVAG with 5 × 104 PFU HSV-2 MS. (A) Survival kinetics. (B) Vaginal disease scores. (C) Vaginal viral titers. Each group had 5 animals. Results in panels B and C represent geometric means ± SEM. (D) Photographs taken on day 7 postinfection are representative of mice in each group. Scores are indicated beneath the photographs.
Antibody responses to gE2-del virus are dose dependent.
ELISA and neutralizing antibodies were measured to further assess the effects of two immunizations. Mice were immunized twice i.m. with 5 × 103, 5 × 104, or 5 × 105 PFU gE2-del virus, with immunizations given 3 weeks apart. Serum was obtained 3 weeks after the first and second immunizations. ELISA titers to gC2 and gD2 (Fig. 4A and B) and neutralizing antibody titers (Fig. 4C) were higher in mice immunized twice than in those immunized once and higher in mice immunized with 5 × 105 PFU gE2-del virus than in those immunized with lower doses. Comparisons between one and two immunizations were significant for gD2 ELISA at 5 × 105 PFU (P < 0.001), and neutralizing antibody titers were significantly different at 5 × 105 PFU (P < 0.002). Based on the protection experiments shown in Fig. 3 and the antibody responses in Fig. 4, two i.m. immunizations were used in subsequent studies.
Fig 4.
ELISA and neutralizing antibody responses to gE2-del virus. Mice were immunized i.m. once or twice with 5 × 103, 5 × 104, or 5 × 105 PFU gE2-del virus, with immunizations separated by 3 weeks. (A) ELISA antibody titers to gC2. (B) ELISA antibody titers to gD2. (C) Neutralizing antibody titers to HSV-2 MS. Horizontal lines and error bars represent mean titers ± SEM.
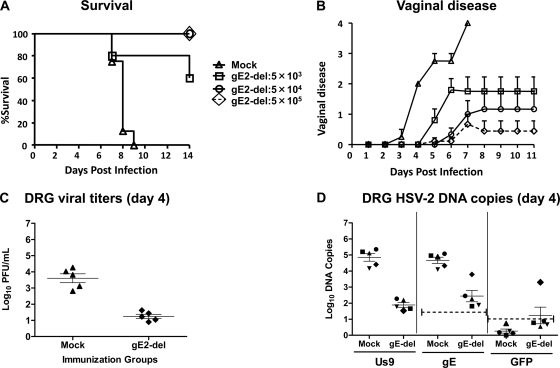
Protection against wild-type virus challenge in mice is dependent on the immunizing dose of gE2-del virus.
Mice were mock immunized or immunized twice i.m. with either 5 × 103, 5 × 104, or 5 × 105 PFU of gE2-del virus and were challenged 28 days later by IVAG inoculation of 5 × 104 PFU HSV-2 MS (∼104 LD50). No mock-immunized mice survived, while 4 of 5 (80%) mice immunized with 5 × 103 PFU gE2-del virus survived and all mice immunized with 5 × 104 or 5 × 105 PFU survived (P < 0.001 for comparing all 3 gE2-del virus doses with mock immunization; the P value was not significant for comparing 5 × 103 PFU gE2-del with 5 × 104 or 5 × 105 PFU gE2del) (Fig. 5A). Protection against vaginal disease after wild-type virus infection correlated with the immunization dose used, with the best protection at the highest dose and the poorest protection at the lowest dose (P < 0.05 for comparing mock immunization with immunization with 5 × 104 PFU gE2-del, P < 0.01 for comparing mock immunization with 5 × 105 PFU gE2-del, and the P value was not significant for all other comparisons) (Fig. 5B). DRG were harvested 4 days after infection with wild-type virus. Approximately 4 log10 virus was recovered from the DRG of mock-immunized mice, compared with 1 log10 from gE2-del virus-immunized mice (P < 0.01) (Fig. 5C). The same DRG were evaluated for HSV-2 Us9 DNA by qPCR. Approximately 5 log10 HSV-2 Us9 DNA copies were detected in DRG of mock-immunized mice, compared with 2 log10 copies in DRG of mice immunized with gE2-del virus (P < 0.01) (Fig. 5D, left graph). In an effort to distinguish wild-type virus DNA from gE2-del virus DNA, the samples were examined for gE2 and GFP DNAs. All samples were positive for gE2 DNA, indicating infection by wild-type virus (P < 0.01 for comparing mock with gE2-del immunization) (Fig. 5D, middle graph). One sample from a gE2-del virus-immunized mouse was also positive for GFP DNA, suggesting that both wild-type (challenge) and gE2-del virus (immunogen) DNAs were present in the DRG (P < 0.05 for comparing gE2-del with mock immunization) (Fig. 5D, right graph). These findings indicate significant protection of DRG by gE2-del virus immunization and support the results shown in Fig. 1 suggesting that some gE2-del virus DNA can be detected in DRG.
Fig 5.
Protection after gE2-del virus immunization at various doses. Mice were mock immunized or immunized i.m. twice at 3-week intervals with 5 × 103, 5 × 104, or 5 × 105 PFU gE2-del virus. Twenty-eight days later, mice were infected IVAG with 5 × 104 PFU HSV-2 MS and monitored for survival kinetics (A) and vaginal disease scores (B). For panels A and B, 9 mice were in the mock group and the gE2-del virus group immunized with 5 × 105 PFU gE2-del virus, while 5 mice were in each of the other groups. Error bars in panel B represent SEM. (C) Mock-immunized mice or those immunized with 5 × 105 PFU gE2-del virus were evaluated for HSV-2 infectious titers in DRG at 4 days postinfection by plaque assay. (D) The same DRG were evaluated for HSV-2 Us9 DNA copy number by qPCR at 4 days postinfection (left graph), as well as for gE2 DNA (middle graph) and GFP DNA (right graph). The symbols used link results for individual mice in panel D. Results in panels C and D represent geometric means ± SEM. The dotted lines in panel D represent the copy numbers required to consider a sample positive, which are >23 for gE2 DNA and >8 for GFP DNA.
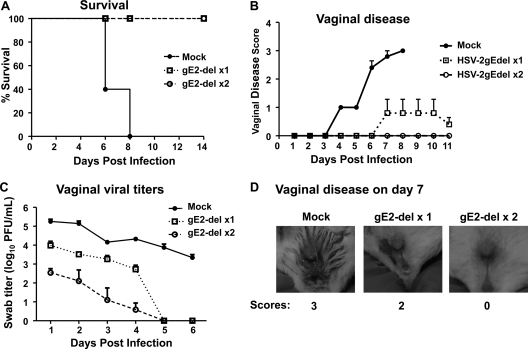
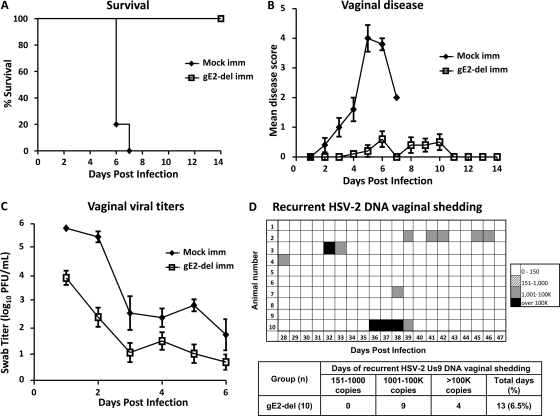
gE2-del virus protects guinea pigs against HSV-2 vaginal infection.
The guinea pig vaginal infection model differs from the murine model in that guinea pigs undergo spontaneous recurrent HSV-2 lesions and recurrent vaginal shedding of HSV-2 DNA that mimic the disease in humans (4, 35, 45). Guinea pigs were mock immunized or immunized i.m. twice at 3-week intervals with 5 × 105 PFU gE2-del virus. Guinea pigs were challenged IVAG 28 days later with 5 × 105 PFU HSV-2 MS (≥500 LD50). Guinea pigs vaccinated with gE2-del virus all survived, while mock-vaccinated animals all died or were euthanized (P < 0.001) (Fig. 6A). Mock-immunized guinea pigs had severe vaginal disease, while animals immunized with gE2-del virus had very mild disease (P < 0.001). Five of 10 guinea pigs in the gE2-del virus group had no disease. Guinea pigs immunized with gE2-del virus had vaginal HSV-2 titers that were lower than those of the mock-immunized animals (P < 0.05) (Fig. 6C). After recovering from acute infection, guinea pigs were monitored for recurrent lesions from 15 to 60 days postinfection. None of the mock-immunized animals survived long enough to be evaluated for recurrences. One of 10 guinea pigs immunized with gE2-del virus had a recurrent genital lesion that lasted 2 days. Vaginal shedding of HSV-2 Us9 DNA was evaluated from days 28 to 47 (20 days) postinfection in gE2-del virus-immunized animals (Fig. 6D). Five animals had no vaginal shedding, while 5 had occasional episodes. The vaginal shedding results are summarized in the table in Fig. 6D and indicate that animals shed HSV-2 Us9 DNA on 6.5% of days. The positive samples were amplified using GFP primers and probes to determine if the gE2-del virus caused recurrent vaginal shedding. None of the samples were positive for GFP DNA.
Fig 6.
gE2-del immunization protects guinea pigs against HSV-2 vaginal challenge. Guinea pigs were mock immunized or immunized i.m. twice with 5 × 105 PFU gE2-del virus and were challenged with 5 × 105 PFU HSV-2 MS. Five guinea pigs were in the mock group, and 10 were in the gE2-del group. (A) Survival kinetics. (B) Vaginal disease scores for surviving animals only. (C) Vaginal viral titers. Results in panel B represent means ± SEM, while those in panel C represent geometric mean titers ± SEM. (D) Recurrent vaginal shedding of HSV-2 DNA was evaluated for 20 days, from days 28 to 47 postinfection. The table summarizes the number of episodes of shedding of 151 to 1,000 copies, 1,001 to 100,000 copies, or >100,000 copies of HSV-2 Us9 DNA.
Dual-modality immunization with live virus and subunit glycoprotein antigens.
The DRG were not totally protected in gE2-del virus-immunized mice after IVAG challenge (Fig. 5C and D). In addition, recurrent genital lesions and vaginal shedding of HSV-2 DNA developed in some guinea pigs. In separate studies, immunization of mice and guinea pigs with a bivalent subunit glycoprotein vaccine containing gC2 and gD2 antigens provided better protection against vaginal challenge than immunization with either antigen alone; however, the bivalent vaccine also did not totally prevent HSV-2 DNA from reaching the DRG of mice during acute infection or prevent recurrent vaginal shedding of HSV-2 DNA (4). Therefore, we evaluated a dual-modality approach using both gE2-del virus and gC2 and gD2 subunit antigens in an effort to improve protection.
Guinea pigs were mock immunized or immunized i.m. with 5 × 105 PFU of gE2-del virus on days 0 and 21 and with gC2 and gD2, given with CpG and alum, on days 7 and 28. Two weeks after the final immunization, sera were obtained for measurements of ELISA titers to gC2 and gD2 and neutralizing antibody titers and compared with those in sera obtained from guinea pigs immunized with gE2-del virus alone (Fig. 6). Guinea pigs immunized with gE2-del virus had a mean titer to gC2 of 1:400 and a mean titer to gD2 of 1:3,200 (Fig. 7A and B). In contrast, animals immunized with gE2-del plus gC2 and gD2 had a mean titer of approximately 1:25,600 to gC2 and gD2 (P < 0.001 for comparing the combined vaccine with gE2-del virus; P < 0.001 for comparing gE2-del with mock immunization). Guinea pigs were not immunized with gC2 and gD2 alone here, although in a recent study from our laboratory, ELISA titers after gC2 and gD2 immunization were similar to those obtained using gE2-del plus gC2 and gD2 (4). Neutralizing antibody titers, determined in the absence of complement, were also significantly higher in the gE2-del–gC2–gD2-immunized animals (P < 0.05 for comparing mock immunization and gE2-del virus, P < 0.001 for comparing mock immunization and gE2-del–gC2–gD2 immunization, and P < 0.05 for comparing gE2-del virus immunization and gE2-del–gC2–gD2 immunization) (Fig. 7C).
Fig 7.
ELISA and neutralizing antibody titers. Guinea pigs were mock immunized or immunized i.m. with gE2-del virus or gE2-del virus plus gC2 and gD2 subunit antigens. Serum was collected 14 days after the last immunization and assessed for ELISA titers to gC2 (A), ELISA titers to gD2 (B), and neutralizing antibodies (C). Results in panels A and B represent mean titers ± SEM for 13 mock-immunized animals, 3 gE2-del-immunized animals, and 10 gE2-del–gC2–gD2-immunized animals. Results in panel C are means and SEM for three serum samples, each run in duplicate.
Guinea pigs immunized using the dual-modality immunogens all survived IVAG challenge with 5 × 105 PFU HSV-2 MS, while none of the mock-immunized guinea pigs survived (P < 0.001) (Fig. 8A). Vaginal disease was severe in the mock-immunized animals, while animals in the dual-modality group were highly protected, with only 1 of 10 animals developing lesions (P < 0.001) (Fig. 8B). HSV-2 vaginal titers were 2 log10 lower in the dual-modality immunization group than in the mock-immunized group on day 1 and remained lower for 6 days (P < 0.001) (Fig. 8C). Three of 10 animals immunized using the dual-modality approach had recurrent genital lesions from days 15 to 60 postinfection, with 1 animal having two episodes. Each episode lasted 1 day, except for one episode that lasted 2 days. Five of 10 animals in the dual-modality group had recurrent vaginal shedding of HSV-2 Us9 DNA from days 28 to 48 postinfection (Fig. 8D). None of the samples were positive for GFP DNA. Therefore, although the dual-modality immunization was highly protective, it did not totally prevent acute vaginal disease, recurrent genital lesions, or recurrent vaginal shedding of HSV-2 DNA.
Fig 8.
Dual-modality immunization with gE2-del virus and gC2 and gD2 subunit antigens. Guinea pigs were either mock immunized or immunized i.m. twice with 5 × 105 PFU gE2-del virus plus gC2 and gD2 subunit antigens and were challenged IVAG with 5 × 105 PFU HSV-2 MS. (A) Survival kinetics. (B) Vaginal disease scores for surviving animals only. (C) Vaginal viral titers. Results in panel B represent means ± SEM, while those in panel C represent geometric means ± SEM for 5 animals in the mock-immunized group and 10 animals in the gE2-del–gC2–gD2-immunized group. (D) Recurrent vaginal shedding of HSV-2 DNA at 28 to 48 days postinfection. The table summarizes the number of episodes of shedding of 151 to 1,000 copies, 1,001 to 100,000 copies, or >100,000 copies of HSV-2 Us9 DNA.
gE2-del virus as a therapeutic vaccine.
Thirty guinea pigs were infected IVAG with 5 × 104 PFU of a less virulent, plaque-purified strain of HSV-2 MS, with the intent of having animals survive the acute infection. All animals were infected, based on vaginal titers determined on days 1 and 2 postinfection (result not shown). However, only 17 guinea pigs survived. These animals were assigned to receive mock or gE2-del virus immunization 51 and 81 days after IVAG infection (Fig. 9A). Animals were assigned to either the mock or vaccine group based on the severity of the acute vaginal disease. Four animals in each group had no acute vaginal disease, two in each group had mild acute vaginal disease (cumulative scores of ≤4), and two in each group had severe acute vaginal disease (cumulative scores of ≥10). One additional animal with moderate acute vaginal disease (cumulative score of 6) was assigned to the gE2-del virus group. ELISA titers to gD2 were distributed comparably between the mock and gE2-del immunization groups. Four animals in each group had negative titers to gD2, two animals in the mock group and three in the gE2-del group had titers of 100 to 400, and two animals in each group had titers of ≥800. ELISA titers to gD2 increased over time in the gE2-del virus immunization group (P < 0.01 for comparing titers after the second immunization with titers prior to immunization [17 days postinfection]; P < 0.02 for comparing titers after the first immunization with titers after the second immunization) (Fig. 9B). The rise in titers after the second mock immunization was not statistically significant and likely represented a response to recurrent infection, while the higher boost in titer in the gE2-del virus group after the second immunization may reflect immunization and recurrent infection.
Fig 9.
Therapeutic treatment of HSV-2-infected guinea pigs with gE2-del virus. (A) Schedule of initial vaginal infection, therapeutic immunizations, and bleeds. (B) gD2 antibody responses in mock-immunized and gE2-del virus-immunized guinea pigs. Results represent mean titers ± SEM. (C) Seven weeks of cumulative lesion recurrences per animal are plotted, beginning 1 week after the first immunization.
Animals were scored for 7 weeks for recurrences, starting 8 days after the first immunization. Guinea pigs in the mock immunization group averaged 13.1 genital lesion days per animal, while animals in the gE2-del virus group averaged 3.7 lesion days per animal (P < 0.001) (Fig. 9C). The individual recurrent lesion day scores for each animal were 0, 0, 1, 1, 1, 3, 6, 7, and 14 in the gE2-del virus group and 0, 0, 2, 3, 5, 17, 28, and 50 (a lesion was present every day) in the mock-immunized group. Even if the animal with 50 days of recurrences was omitted as an outlier from the statistical analysis, the differences between the two groups remained significant (P < 0.001).
DISCUSSION
An HSV-1 gE deletion mutant was shown by our laboratory to be safe and effective in protecting against HSV-1 challenge in the murine flank infection model and against HSV-2 in the murine vaginal infection model (9). The HSV-1 gE deletion strain is impaired in neuronal spread, making it a novel vaccine candidate (25, 26). A similar deletion was made in the gene encoding HSV-2 gE, and that mutant strain was also shown to be defective in neuronal spread (46). The current study evaluated whether the HSV-2 gE deletion strain, gE2-del virus, is safe and effective in animal models as a prophylactic and therapeutic vaccine.
BALB/c and SCID mice were inoculated i.m., i.v., and IVAG to evaluate gE2-del virus safety. Somewhat surprisingly, only minor differences were detected in comparing the LD50 of wild-type HSV-2 2.12 for BALB/c and SCID mice. The gE2-del virus did not cause illness or death in BALB/c or SCID mice inoculated i.m., i.v., or IVAG at the highest dose tested, which was 5 × 106 PFU. One of 5 mice died when gE2-del virus was inoculated i.c. at 5 × 105 PFU, and 9 of 10 mice died at 5 × 106 PFU. In contrast, all mice died when HSV-2 2.12 was inoculated i.c. at 5 PFU, which was the lowest dose evaluated. The defect in gE2-del virus is in neuroinvasion, that is, in its ability to reach the brain from peripheral sites (46). The mutant virus does not have a defect in its ability to replicate in neurons; therefore, death following direct i.c. inoculation is not surprising (46). The reduction in mortality after i.c. inoculation of gE2-del virus likely reflects the marked defect of the mutant strain in cell-to-cell spread (46). Importantly, no deaths were recorded following high-dose i.v. inoculation, which suggests that even if high titers of gE2-del virus are inadvertently injected i.v. during vaccination, no adverse outcome is likely.
The safety of gE2-del virus was further evaluated by determining whether gE2-del virus was detected in the DRG or spinal cord 4 days after immunization. No gE2-del virus was isolated from DRG or spinal cord tissue, while wild-type virus was readily detected, despite being inoculated at titers that were 102 to 105 PFU lower than the dose used for the gE2-del virus. When HSV-2 DNA copy number was evaluated, DRG from some mice inoculated with gE2-del virus by the i.m. route had low copy numbers of GFP DNA detected, suggesting that gE2-del virus DNA is capable of reaching the DRG after i.m. inoculation, although gE2-del virus was not reactivated from explant cultures of DRG. From a safety perspective, an ideal live virus vaccine candidate should not infect the DRG. The gE2-del virus has a profound defect in spread from the neuron cell body into the axon (anterograde spread), including a lack of spread from the retina to the brain after virus inoculation into the mouse retina (46). The anterograde spread defect suggests that even if very low titers of gE2-del virus reach the DRG after i.m. immunization, the virus is not likely to cause recurrences (46). In support of this postulate, no recurrent vaginal shedding of gE2-del virus DNA was detected in guinea pigs immunized with the gE2-del virus.
Two immunizations were more effective than one at inducing ELISA and neutralizing antibody titers and protecting mice after vaginal infection. Booster doses are generally required for optimum protection for other replication-competent live virus vaccines, such as measles, mumps, rubella, rotavirus, and varicella vaccines. In the case of gE2-del virus, perhaps the impaired cell-to-cell spread phenotype accounts for the need for a second immunization to enhance protection.
Immunization of mice with 5 × 105 PFU provided better protection against challenge than immunization with 10- and 100-fold lower doses and provided excellent protection against challenge with 104 LD50, based on survival, vaginal disease, vaginal titers, and DRG titers at 4 days postinfection. Note that the HSV-2 DNA copy number was reduced by 2 to 3 log10 in DRG of gE2-del virus-immunized mice compared with mock-immunized mice. The gE2-del virus was also protective in guinea pigs after IVAG challenge. Five of 10 guinea pigs were totally protected against acute infection, and 9 of 10 had no recurrent lesions. It is possible that higher gE2-del virus doses may have further improved protection, as recently shown for a replication-defective, attenuated live virus vaccine candidate that compared protection provided by 5 × 105 PFU and 2 × 106 PFU of CJ2-gD2 (1).
Recent studies from our laboratory reported that gC2 and gD2 subunit immunization was highly protective against IVAG infection in mice and guinea pigs (4). However, the live virus vaccine reported here and the subunit glycoprotein vaccine reported previously did not totally protect against acute vaginal infection or recurrences (4). Therefore, experiments were performed to combine live virus and subunit antigens. Neutralizing antibody responses were increased significantly in the animals that received both immunogens compared with those in gE2-del virus-immunized animals. The dual-modality vaccine approach resulted in less acute vaginal disease and lower acute vaginal titers than those in the group receiving gE2-del virus alone, suggesting a possible role for neutralizing antibodies in modifying acute disease; however, both groups had comparable frequencies of recurrent lesions and vaginal shedding, suggesting that neutralizing antibody titers may not correlate with recurrences. Overall, combined immunization with live and subunit antigens offered little benefit over immunization with live virus alone.
From a safety perspective, administering the subunit antigen first may be preferred so that the live virus vaccine is administered in the setting of preexisting immunity. However, we chose to deliver the live virus vaccine first because of the concern that subunit immunization may blunt responses to the live virus vaccine. Our concern about prior immunity blunting live virus vaccine immune responses may have been unfounded based on the results using gE2-del virus as a therapeutic vaccine. Despite an immune response elicited by infection, the live virus vaccine administered therapeutically significantly boosted the gD2 antibody response and protected the animals against recurrent lesions. This result suggests that the gE2-del virus boosts immunity in previously infected animals.
Other live virus vaccine candidates are in preclinical testing. HSV-2 0ΔNLS is an ICP0 deletion mutant that is interferon sensitive and attenuated for virulence in mice. It induces sufficient protective immunity to prevent death in mice after corneal challenge with the parental virus used to derive the vaccine strain (19). However, whether the vaccine strain protects against vaginal infection, whether protection extends to strains other than the parental virus used to derive the vaccine candidate, and whether the protection is comparable to or better than that provided by other vaccine candidates remain to be determined.
HSV-2 dl5-29 is another live virus vaccine candidate. This mutant virus is defective in genes encoding the helicase-primase complex (UL5) and the single-stranded DNA binding protein (UL29). In two studies, the HSV-2 dl5-29 vaccine strain performed comparably to or perhaps slightly better than a gD2 subunit antigen in protecting guinea pigs against acute disease, in lowering vaginal titers during acute infection, in reducing the number of recurrent infections, and in lowering HSV-2 DNA copy levels in DRG (20, 21). CJ2-gD2 is another live virus vaccine candidate, in which the ICP0 gene has been replaced by gD2 under the control of the HSV-1 ICP4 promoter and a dominant-negative version of the UL9 gene has been inserted into the ICP0 locus to inhibit HSV-2 DNA replication (1). The vaccine strain protected mice against vaginal disease and provided partial protection of DRG after vaginal challenge. Whether gE2-del virus provides comparable or better protection than these other live virus vaccine candidates will require head-to-head comparisons performed in the same laboratory and using the same challenge viruses.
We previously reported that the gC2 and gD2 subunit antigens are effective immunogens in mice and guinea pigs when used to prevent IVAG HSV-2 infection (4). The experiments using gE2-del virus or gC2 and gD2 subunit antigens in mice and guinea pigs were performed in animals immunized, challenged, and evaluated at the same time, enabling us to compare those results. Both vaccine approaches were highly protective, although the subunit antigen immunogens performed better in protecting DRG of mice at 4 days postinfection. The DRG infectious virus titers were 1.3 ± 0.3 log10 in the gE2-del virus-immunized animals (Fig. 5), while titers were undetectable in gC2-gD2-immunized animals (4). Similarly, qPCR DNA levels were lower in the subunit antigen group (1.9 ± 0.1 for gE2-del virus [Fig. 5D] versus 0.66 ± 0.3 for gC2-gD2) (4). In guinea pigs, both vaccine candidates were highly effective; however, neither approach eliminated recurrent vaginal shedding, with 6.5% vaginal shedding days for gE2-del virus immunization (Fig. 6), compared with 7.1% for gC2-gD2 immunization (4). Ideally, a prophylactic HSV-2 vaccine would prevent acute and recurrent genital disease and vaginal shedding of HSV-2 DNA. No candidate vaccine has achieved this goal to date.
More than 2 billion people are likely infected with HSV-2, based on seroprevalence rates (33). An effective therapeutic vaccine may help to control symptomatic recurrences and perhaps reduce asymptomatic HSV-2 shedding. Studies of guinea pigs reported fewer recurrences after therapeutic immunization with HSV-2 glycoprotein antigens or a glycoprotein H-deleted replication-defective live virus vaccine as immunotherapy (8, 34). In humans, immunization with 100 μg gD2 subunit antigen administered with alum resulted in fewer recurrences per month than placebo immunization (37). When gD2 was combined with gB2 and each antigen was administered at a considerably lower dose (10 μg) and with a different adjuvant (MF59), subjects had less impressive responses; however, the duration and severity of first recurrences were reduced (38). The gH-deleted replication-defective vaccine that was effective in guinea pigs was not effective in reducing recurrences in humans (16). Our results demonstrate the therapeutic potential of the gE2-del vaccine in guinea pigs, suggesting that additional studies comparing the therapeutic potentials of gE2-del virus and other vaccine candidates are warranted.
ACKNOWLEDGMENTS
We thank Gary Cohen and Roselyn Eisenberg, University of Pennsylvania, for providing gC2 and gD2 purified proteins, Sarah Ratcliffe (Center for AIDS Research Epidemiology and Biostatistics Core) for advice on statistics, and Farida Shaheen (Center for AIDS Research Viral and Molecular Core) for advice on qPCR.
This work was supported by NIH grants RO1 HL028220 and RO1 AI033063 and by a grant from Merck & Co.
H.M.F., E.E.Z., and F.W. have a patent application pending on the gE-2 deletion mutant strain (H. M. Friedman, E. E. Zumbrun, and F. Wang, U.S. patent application 13/260,835).
Footnotes
Published ahead of print 8 February 2012
REFERENCES
- 1. Akhrameyeva NV, Zhang P, Sugiyama N, Behar SM, Yao F. 2011. Development of a glycoprotein D-expressing dominant-negative and replication-defective herpes simplex virus 2 (HSV-2) recombinant viral vaccine against HSV-2 infection in mice. J. Virol. 85:5036–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Awasthi S, Lubinski JM, Eisenberg RJ, Cohen GH, Friedman HM. 2008. An HSV-1 gD mutant virus as an entry-impaired live virus vaccine. Vaccine 26:1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Awasthi S, Lubinski JM, Friedman HM. 2009. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine 27:6845–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awasthi S, et al. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J. Virol. 85:10472–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belshe RB, et al. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourne N, et al. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Infect. Dis. 187:542–549 [DOI] [PubMed] [Google Scholar]
- 7. Bourne N, Stanberry LR, Bernstein DI, Lew D. 1996. DNA immunization against experimental genital herpes simplex virus infection. J. Infect. Dis. 173:800–807 [DOI] [PubMed] [Google Scholar]
- 8. Boursnell ME, et al. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brittle EE, Wang F, Lubinski JM, Bunte RM, Friedman HM. 2008. A replication-competent, neuronal spread-defective, live attenuated herpes simplex virus type 1 vaccine. J. Virol. 82:8431–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown ZA, et al. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337:509–515 [DOI] [PubMed] [Google Scholar]
- 11. Brown ZA, et al. 1987. Effects on infants of a first episode of genital herpes during pregnancy. N. Engl. J. Med. 317:1246–1251 [DOI] [PubMed] [Google Scholar]
- 12. Brown ZA, et al. 2003. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 289:203–209 [DOI] [PubMed] [Google Scholar]
- 13. Canziani G, et al. 1999. Exploring biomolecular recognition using optical biosensors. Methods 19:253–269 [DOI] [PubMed] [Google Scholar]
- 14. Corey L, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340 [DOI] [PubMed] [Google Scholar]
- 15. Corey L, Wald A, Celum CL, Quinn TC. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435–445 [DOI] [PubMed] [Google Scholar]
- 16. de Bruyn G, et al. 2006. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 24:914–920 [DOI] [PubMed] [Google Scholar]
- 17. Dix RD, McKendall RR, Baringer JR. 1983. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect. Immun. 40:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halford WP, et al. 2011. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One 6:e17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halford WP, Puschel R, Rakowski B. 2010. Herpes simplex virus 2 ICP0 mutant viruses are avirulent and immunogenic: implications for a genital herpes vaccine. PLoS One 5:e12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoshino Y, et al. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoshino Y, et al. 2009. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J. Infect. Dis. 200:1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoshino Y, et al. 2008. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine 26:4034–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 316:1444–1449 [DOI] [PubMed] [Google Scholar]
- 24. Lubinski JM, et al. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. 2009. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J. Virol. 83:8315–8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGraw HM, Friedman HM. 2009. Herpes simplex virus type 1 glycoprotein E mediates retrograde spread from epithelial cells to neurites. J. Virol. 83:4791–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meignier B, Jourdier TM, Norrild B, Pereira L, Roizman B. 1987. Immunization of experimental animals with reconstituted glycoprotein mixtures of herpes simplex virus 1 and 2: protection against challenge with virulent virus. J. Infect. Dis. 155:921–930 [DOI] [PubMed] [Google Scholar]
- 28. Meignier B, Martin B, Whitley RJ, Roizman B. 1990. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J. Infect. Dis. 162:313–321 [DOI] [PubMed] [Google Scholar]
- 29. Morello CS, Levinson MS, Kraynyak KA, Spector DH. 2011. Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease. J. Virol. 85:3461–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagashunmugam T, et al. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prichard MN, et al. 2005. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 23:5424–5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 33. Smith JS, Robinson NJ. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186:S3–S28 [DOI] [PubMed] [Google Scholar]
- 34. Stanberry LR, Burke R, Myers MG. 1988. Herpes simplex virus glycoprotein treatment of recurrent genital herpes. J. Infect. Dis. 157:156–163 [DOI] [PubMed] [Google Scholar]
- 35. Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC., Jr 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J. Infect. Dis. 146:397–404 [DOI] [PubMed] [Google Scholar]
- 36. Stanberry LR, et al. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661 [DOI] [PubMed] [Google Scholar]
- 37. Straus SE, et al. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460–1463 [DOI] [PubMed] [Google Scholar]
- 38. Straus SE, et al. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129–1134 [DOI] [PubMed] [Google Scholar]
- 39. Tal-Singer R, et al. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. 2006. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J. Virol. 80:5283–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson RL, Sawtell NM. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 75:6660–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tirabassi RS, et al. 2011. A mucosal vaccination approach for herpes simplex virus type 2. Vaccine 29:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wald A. 2006. Genital HSV-1 infections. Sex. Transm. Infect. 82:189–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45–52 [DOI] [PubMed] [Google Scholar]
- 45. Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770–775 [DOI] [PubMed] [Google Scholar]
- 46. Wang F, et al. 2010. Herpes simplex virus type 2 glycoprotein E is required for efficient virus spread from epithelial cells to neurons and for targeting viral proteins from the neuron cell body into axons. Virology 405:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu F, et al. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 48. Yamamura J, et al. 2000. Long-term gene expression in the anterior horn motor neurons after intramuscular inoculation of a live herpes simplex virus vector. Gene Ther. 7:934–941 [DOI] [PubMed] [Google Scholar]