Abstract
We have shown that sequential replicating adenovirus type 5 host range mutant human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) recombinant priming delivered first intranasally (i.n.) plus orally and then intratracheally (i.t.), followed by envelope protein boosting, elicits broad cellular immunity and functional, envelope-specific serum and mucosal antibodies that correlate with protection from high-dose SIV and simian/human immunodeficiency virus (SHIV) challenges in rhesus macaques. Here we extended these studies to compare the standard i.n./i.t. regimen with additional mucosal administration routes, including sublingual, rectal, and vaginal routes. Similar systemic cellular and humoral immunity was elicited by all immunization routes. Central and effector memory T cell responses were also elicited by the four immunization routes in bronchoalveolar lavage fluid and jejunal, rectal, and vaginal tissue samples. Cellular responses in vaginal tissue were more compartmentalized, being induced primarily by intravaginal administration. In contrast, all immunization routes elicited secretory IgA (sIgA) responses at multiple mucosal sites. Following a repeated low-dose intrarectal (i.r.) challenge with SIVmac251 at a dose transmitting one or two variants, protection against acquisition was not achieved except in one macaque in the i.r. immunized group. All immunized macaques exhibited reduced peak viremia compared to that of controls, correlated inversely with prechallenge serum antienvelope avidity, antibody-dependent cellular cytotoxicity (ADCC) titers, and percent antibody-dependent cell-mediated viral inhibition. Both antibody avidity and ADCC titers were correlated with the number of exposures required for infection. Notably, we show for the first time a significant correlation of vaccine-induced sIgA titers in rectal secretions with delayed acquisition. Further investigation of the characteristics and properties of the sIgA should elucidate the mechanism leading to this protective effect.
INTRODUCTION
As the number of worldwide cases of human immunodeficiency virus (HIV)/AIDS continues to rise, the generation of an effective HIV/AIDS vaccine remains a global priority. Recent results in Thailand with the recombinant canarypox (ALVAC-HIV) prime-gp120 (AIDSVAX B/E) protein boost vaccine approach showed evidence of a modest protective effect and gave hope that an AIDS vaccine is ultimately achievable (45). Nevertheless, the results from this trial, as well as the disappointing outcome of the Step Study trial (6, 46), highlight the need to better understand the immune correlates of vaccine-induced protection and develop more efficacious HIV vaccines. One area for improved design is elicitation of mucosal immunity. The mucosal lining of the gastrointestinal and genital tracts is a primary area of HIV transmission, with the draining lymph nodes associated with these sites providing a reservoir of CD4+ CCR5+ cells susceptible to HIV infection and viral replication. The replication of HIV at mucosal sites results in the rapid systemic destruction of CD4+ T cells, an early marker of progressive HIV infection (33, 44). Therefore, in addition to systemic immunity, a successful HIV vaccine should induce both cellular and humoral immunity at mucosal sites of transmission.
Adenovirus (Ad)-based vaccine vectors are one of the most promising platforms for AIDS vaccine development. We have been pursuing a replicating Ad-HIV/simian immunodeficiency virus (SIV) recombinant prime/envelope protein boost approach (16, 41) which elicits broad cellular immunity and functional, envelope-specific serum and mucosal antibodies that correlate with protection from HIV, SIV, and simian/human immunodeficiency virus (SHIV) challenges in rhesus macaque and chimpanzee models (3, 9, 18, 30, 31, 40, 47, 53, 55). Vaccine-induced, SIV-specific IgG and IgA memory B cells have also been shown to correlate with functional antibody responses and reduced viremia (4). Our previous studies with macaques have used primarily immunization to the upper respiratory tract (URT), including intranasal (i.n.), followed by intratracheal (i.t.), administration. Due to the biology of the Ad vector and its propensity to replicate in the URT, the i.n./i.t. route of administration can provide strong immunogenicity and broadly target mucosal effector sites. In our nonhuman primate studies, we have also incorporated oral immunization into the preclinical vaccine regimen, directly administering a recombinant Ad in phosphate-buffered saline (PBS) to the stomach following infusion of sodium bicarbonate. In fact, the use of this oral administration technique together with i.n. priming followed by i.t. administration of Ad recombinants was particularly effective compared to a regimen in which two sequential oral and i.n. administrations were used (43). Subsequent studies showed that administration of Ad recombinants solely by the oral route using readily deliverable enteric-coated tablets induced cellular mucosal responses comparable to those achieved with an i.n./oral priming regimen and similar protective efficacy against a mucosal intrarectal (i.r.) SIVmac251 challenge (54). However, systemic immunity following the oral/oral enteric tablet immunizations, as measured by immune responses in peripheral blood, was poor, suggesting that such a vaccine regimen might be too anatomically restrictive.
The sublingual (s.l.) route of oral administration has historically been used as a means to induce tolerance to allergens (13). However, as a vaccine strategy, s.l. immunization has recently been shown to be comparable to i.n. immunization and superior to oral immunization in eliciting systemic and mucosal immune responses against a cholera toxin-coupled protein antigen in the murine model (8); perhaps this is attributable to the dense network of dendritic cells in the lamina propria and epithelial compartment of the s.l. mucosa. The s.l. immunization of mice with inactivated influenza vaccine has also induced both systemic and mucosal immunity and protection against a lethal influenza challenge (49). In the HIV vaccine field, s.l. administration of an HIV gp41 envelope vaccine together with a reverse transcriptase polypeptide coupled to the cholera toxin B subunit induced specific IgA antibodies and antibody-secreting cells in the genital mucosa (17). Moreover, s.l. immunization of mice with a replication-defective Ad5-HIVgag vaccine elicited cytotoxic T lymphocyte responses in both the systemic and mucosal compartments at levels comparable to those achieved by oral-gastric immunization (1).
With this background, it was of interest to examine s.l. immunization of rhesus macaques with our replication-competent Ad recombinants as an alternative to the URT. We conducted a comprehensive investigation of cellular and humoral, systemic and mucosal immune responses induced following immunization by the s.l. and i.n./i.t. routes. In view of the known compartmentalization of the mucosal immune system (21, 35), we also immunized a small number of macaques by the intravaginal (i.vag.) and i.r. routes in order to compare immune responses elicited locally with those induced at the same rectal/genital sites following administration of the replicating Ad recombinants at the distant i.n./i.t. and s.l. sites. Subsequently, protective efficacy against a repetitive low-dose SIVmac251 mucosal challenge of all of the immunized macaques was assessed.
MATERIALS AND METHODS
Animals, immunization, and challenge.
Twenty-seven Indian rhesus macaques (Macaca mulatta), 20 male and 7 female, were housed and maintained at Bioqual Inc. (Rockville, MD) according to the standards of the American Association for Accreditation of Laboratory Animal Care. All were negative for prior exposure to SIV, simian retrovirus type D, and simian T cell leukemia virus. Rhesus macaques were immunized at weeks 0 and 12 with the replication-competent Ad5 host range SIVsmH4env/rev (Ad5hr-SIVsmH4env/rev) mutant plus Ad5hr-SIV239gag (5 × 108 PFU/recombinant) as outlined in Table 1, six via the s.l. route, six via the intranasal/intratracheal (i.n./i.t.) routes (first administration i.n. and second i.t.), three via the i.vag. route, and three via the i.r. route. The priming immunizations also included 5 × 108 PFU of an Ad5hr-green fluorescent protein (Ad5hr-GFP) recombinant in order to investigate the biodistribution of the replicating vector as described elsewhere (L. J. Patterson et al., submitted for publication). The macaques were boosted intramuscularly (i.m.) with native SIVmac251 gp120 protein in a monophosphoryl lipid A-stable emulsion (MPL-SE) adjuvant at weeks 24 and 36. Control macaques received the empty Ad5hr vector (a total dose of 1.5 × 109 PFU) and adjuvant alone. Four Mamu A*01-positive macaques and one Mamu B*08-positive macaque were distributed among the five groups as shown in Table 1. An additional three naïve Mamu A*01-positive macaques were added to the control group at the time of challenge. All macaques were challenged intrarectally at week 45 with a low dose (a 1:500 dilution equivalent to 130 50% tissue culture infective doses [TCID50]) of the highly pathogenic SIVmac251 challenge stock originally provided by Ronald C. Desrosiers and obtained from Nancy Miller, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH. Doses were administered weekly until plasma viral RNA was positively detected by quantitative nucleic acid sequence-based amplification (NASBA) assay.
Table 1.
Immunization regimen
| Route (no. of animals) | Primed | Booste | Macaque(s)a |
|---|---|---|---|
| s.l. (6) | Ad5 h-SIV env/rev + Ad5hr-SIV gag | SIV gp120 + MPL-SE | G403 G409, G414, G416, G418,b G433 (F) |
| Ad5 h + GFP | |||
| i.n./i.t. (6) | Ad5hr-SIV env/rev + Ad5hr-SIV gag | SIV gp120 + MPL-SE | G400 G405, G407, G408, G432,b G434 (F) |
| Ad5hr + GFP | |||
| i.vag. (3) | Ad5hr-SIV env/rev + Ad5hr-SIV gag | SIV gp120 + MPL-SE | G415 (F) G430c (F), G431 (F) |
| Ad5hr + GFP | |||
| i.r. (3) | Ad5hr-SIV env/rev + Ad5hr-SIV gag | SIV gp120 + MPL-SE | G401b G404, G412 (F) |
| Ad5hr + GFP | |||
| Control (6) | Ad5hr-vector | MPL-SE | G402b G411, G417, G420, G422, G428 (F) |
| Ad5hr + GFP | |||
| Control (3) | Naive | G419,b G421,b G423b |
(F), female macaque.
Mamu A*01-positive macaque.
Mamu B*08-positive macaque.
At 0 and 12 weeks, various routes.
At 24 and 36 weeks, i.m.
Sample collection.
Peripheral blood mononuclear cells (PBMC) obtained throughout the immunization course and postchallenge were Ficoll treated and used immediately for intracellular cytokine staining assays. Bronchoalveolar lavage (BAL) fluid samples, jejunal strips, rectal pinch biopsy specimens, and vaginal biopsy samples were collected and processed as previously described (39). Lymphocytes were isolated from BAL fluid and jejunal, rectal, and vaginal biopsy specimens using Percoll gradients of 35% and 65% layered solutions. Serum samples were collected, aliquoted, and stored at −70°C until use. Saliva samples were collected using Sarstedt Salivette devices. Nasal, rectal, and vaginal secretions were collected by using Weck-Cel sponges, as described previously (25), and stored at −70°C until analyzed.
Intracellular cytokine staining.
Freshly isolated PBMC (2 × 106) were stimulated with peptide pools of SIVmac239 Gag or SIVsmH4 Env at a 1-μg/ml final concentration in the presence of anti-CD28–phycoerythrin (PE)-Cy7 (1 μg/ml) and anti-CD49d (5 μg/ml) antibodies and brefeldin A (10 μg/ml). For each assay day, an unstimulated control and a positive control (Staphylococcus enterotoxin B) were included in the experiment. Tubes were incubated at 37°C for 6 h. Cells were washed twice with PBS, resuspended in 95 μl of PBS plus 5 μl of AquaBlue viability dye (a 1:50 dilution of a dimethyl sulfoxide stock; Invitrogen) and incubated at room temperature (RT) for 10 min. Cells were washed once with PBS and surface stained for 30 min at RT with CD4-Qdot605 (Invitrogen), CD8-Qdot655 (Invitrogen), and CD95-PE-Cy5 (BD Biosciences) antibodies at concentrations determined by dilution of stock or according to the manufacturer's instructions. Cells were washed with PBS containing 2% fetal bovine serum (FBS), fixed with Cytofix/Cytoperm solution (BD Biosciences), permeabilized with 1× Perm/Wash, and incubated with CD3-Alexa 700 (BD Biosciences), gamma interferon (IFN-γ)-allophycocyanin (APC) (BD Biosciences), tumor necrosis factor alpha (TNF-α)-perdinin chlorophyll protein-Cy5.5 (BioLegend), and interleukin-2 (IL-2)–APC-Cy7 (Invitrogen) antibodies. After 30 min of incubation at 4°C in the dark, cells were washed once with 1× Perm/Wash and once with PBS containing 2% FBS and resuspended in 1% paraformaldehyde in PBS. Approximately 500,000 lymphocytes were acquired for analysis using an LSRII Flow Cytometer. A singlet, followed by live/dead and then lymphocytic gates, was first applied. CD3+ T cells were divided into CD4+ and CD8+ populations, and each population was further subdivided into CD28+ CD95+ central memory (CM) and CD28− CD95+ effector memory (EM) cells. The percentage of cytokine-secreting cells in each memory cell subset was then determined following subtraction of the values obtained with nonstimulated samples. Data were analyzed using FlowJo software (TreeStar Inc.) and Pestle and Spice (version 4.2.2) programs (Mario Roederer, Vaccine Research Center, NIAID).
Systemic binding, neutralizing, and nonneutralizing antibodies.
Serum binding antibodies to SIVmac251 gp120 Env protein were assessed by enzyme-linked immunosorbent assay (ELISA) as described previously (7). The antibody titer was defined as the reciprocal of the serum dilution at which the optical density (OD) of the test serum was two times greater than that of the negative-control serum diluted 1:50.
Neutralizing antibody titers against the primary SIVmac251 CS.41 pseudovirus were determined using the TZM-bl Luc cell line and those against T cell line-adapted (TCLA) SIVmac251 (H9 grown) were determined using M7-Luc cells as previously described (39). The titer was defined as the reciprocal serum dilution at which there was a 50% reduction in relative luminescence units compared to virus control wells which contained no test sample.
Antibody-dependent cellular cytotoxicity (ADCC) titers were determined by the RFADCC assay as described previously (15) using human PBMC effector cells and CEM-NKr target cells coated with SIVmac251 gp120 at an effector-to-target cell ratio of 50:1. Tenfold serial dilutions of serum starting at 1:10 were evaluated. The ADCC titer was defined as the reciprocal of the serum dilution at which percent killing was greater than the mean plus 3 standard deviations of all negative-control samples.
Antibody-dependent cell-mediated viral inhibition (ADCVI) was assessed as previously described (11, 53). Rhesus PBMC targets were stimulated with 2 μg/ml phytohemagglutinin (Sigma-Aldrich) and 0.5 ng/ml recombinant IL-2 (Invitrogen) for 72 h, washed, and infected with SIVmac251 (200 TCID50). After adsorption for 1 h, cells were washed and incubated in R-10 medium at 37°C in 5% CO2 for 48 h. Infected target cells (5 × 104/50 μl/well) were plated in 96-well round-bottom microtiter plates, and a 1/200 dilution of test serum (100 μl) was added to the target cells, along with 50 μl of rhesus PBMC effector cells at an effector-to-target cell ratio of 20:1. A serum control in the absence of effector cells was not needed, as all sera were negative for neutralization of primary SIVmac251 pseudovirus at a 1:20 dilution (data not shown). After 7 days of incubation, supernatant fluids were collected and assayed for p27 by antigen capture ELISA (ABL). All samples were tested in triplicate, and percent virus inhibition was calculated as the decrease in the p27 concentration of the test sample relative to that of the matched prebleed sample.
Serum antibody avidity.
The avidity of Env-specific antibody was evaluated by parallel ELISA as previously described (53). Briefly, serum samples were serially diluted and applied in duplicate to a 96-well plate coated with 1 μg/ml SIVmac251 gp120 protein (ABL). After 2 h of incubation, the plate was washed and half of the samples were treated with 100 μl of PBS while the paired samples were treated with 1.5 M sodium thiocyanate (NaSCN; Sigma-Aldrich) for 10 min at room temperature. Horseradish peroxidase (HRP)-conjugated goat anti-monkey IgG (AlphaDiagnostic) and the substrate, 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich), were used in sequential steps, and then the OD at 450 nm was read. The avidity index was calculated by multiplying by 100 the ratio of the NaSCN-treated serum dilution giving an OD of 0.5 to the PBS-treated serum dilution giving an OD of 0.5. Preimmune sera served as negative controls. A standard serum with known avidity was included on every 96-well plate.
Env-specific secretory IgA (sIgA) in mucosal samples.
Nasal, salivary, rectal, and vaginal secretions were tested for blood contamination using Chemstrips 5 (Boehringer Mannheim). Due to significant blood contamination in many of the samples, Env-specific sIgA in the secretions was determined by ELISA using anti-monkey secretory component. Briefly, mucosal samples were 2-fold serially diluted and applied to a half-area 96-well plate (Greiner Bio-One) coated with 1 μg/ml SIVmac251 gp120 protein and incubated at 4°C overnight. HRP-conjugated goat anti-monkey secretory component (GAMon/SC/PO; Nordic) and the TMB substrate were used in sequential steps, followed by reading of the OD at 450 nm. High-titer sera positive for reactivity against SIV gp120 were negative in this assay at a serum dilution of 1:10. Endpoint titers were defined as the reciprocal of the dilution at which the OD of the test sample was equal to twice the mean background OD.
Quantitation of viral RNA.
SIVmac251 RNA in plasma was quantified by the NASBA assay (26, 48). The threshold of detection was 50 SIV RNA copies/ml plasma.
TRIM5α polymorphisms.
TRIM5α genotypes were determined as previously described (10). Briefly, genomic DNA was isolated from PBMC using the QIAamp DNA Blood mini kit (Qiagen) by following the manufacturer's protocol. A 526-nucleotide portion of the B30′2/SPRY domain of TRIM5α was amplified by PCR using AmpliTaq gold 360 master mix (Applied Biosystems); the forward (CAGTGCTGACTCCTTTGCTTG) and reverse (GCTTCCCTGATGTGATAC) primers both at 0.2 μM, and 200 to 400 ng DNA. Following initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 30 s, and extension at 72.5°C for 1 min were carried out, followed by a final extension at 72.5°C for 7 min. TRIM5α amplicons were isolated on 1% agarose gels and purified using the Invitrogen PureLink Quick Gel Extraction Kit according to the manufacturer's instructions. Sequencing was conducted using the same forward and reverse primers by the NCI Sequencing and Gene Expression Core Facility. Sequences were then aligned with the genomic DNA sequence of the rhesus macaque (accession number DQ842021.1) using Sequence Massager (Attotron Biotechnologies Corp.) and Clustal W2 multiple-sequence alignment (European Bioinformatics Institute) to characterize polymorphisms at nucleic acid positions 997, 1015 to 1020, and 1022 of TRIM5α.
Viral RNA extraction and cDNA synthesis.
From each plasma specimen and the viral inoculum stock, 20,000 viral RNA copies were extracted using the QIAamp Viral RNA mini kit (Qiagen). RNA was eluted and immediately subjected to cDNA synthesis. Reverse transcription of RNA to single-stranded cDNA was performed using SuperScript III reverse transcriptase according to the manufacturer's recommendations (Invitrogen). In brief, a cDNA reaction mixture of 1× reverse transcription buffer, 0.5 mM each deoxynucleoside triphosphate, 5 mM dithiothreitol, 2 U/ml RNaseOUT (RNase inhibitor), 10 U/ml SuperScript III reverse transcriptase, and 0.25 mM antisense primer SIVEnvR1 (5′-TGT AAT AAA TCC CTT CCA GTC CCC CC-3′) was incubated at 50°C for 60 min and 55°C for 60 min, heat inactivated at 70°C for 15 min, and then treated with 2 U of RNase H at 37°C for 20 min. The newly synthesized cDNA was used immediately or frozen at −80°C.
Single-genome amplification of SIVmac251 env.
The entire env gene from each animal at peak viremia was sequenced using a limiting dilution PCR so only one amplifiable molecule was present in each reaction mixture. Single-genome amplification was performed by serially diluting cDNA distributed among independent PCRs to identify a dilution where amplification occurred in <30% of the total number of reactions. PCR amplification was performed with 1× PCR buffer, 2 mM MgSO4, 0.2 mM each deoxynucleoside triphosphate, 0.2 μM each primer, and 0.025 U/μl Platinum Taq polymerase (Invitrogen) in a 20-μl reaction mixture. The first-round PCR was performed with sense primer SIVEnvF1 (5′-CCT CCC CCT CCA GGA CTA GC-3′) and antisense primer SIVEnvR1 (5′-TGT AAT AAA TCC CTT CCA GTC CCC CC-3′) under the following conditions: 1 cycle of 94°C for 2 min and 35 cycles at 94°C for 15 s, 55°C for 30 s, and 68°C for 4 min, followed by a final extension of 68°C for 10 min. Next, 1 μl of the first-round PCR product was added to a second-round PCR mixture that included sense primer SIVEnvF2 (5′-TAT AAT AGA CAT GGA GAC ACC CTT GAG GGA GC-3′) and antisense primer SIVEnvR2 (5′-ATG AGA CAT RTC TAT TGC CAA TTT GTA-3′); the conditions were the same as those used for the first-round PCR but with a total of 45 cycles. Correct-size amplicons were identified by agarose gel electrophoresis and directly sequenced with second-round PCR primers and six SIV-specific primers using BigDye Terminator technology. To confirm PCR amplification from a single template, chromatograms were manually examined for multiple peaks, indicative of the presence of amplicons resulting from PCR-generated recombination events, Taq polymerase errors, or multiple variant templates. Sequences containing two or more ambiguous sites were excluded from analysis.
Statistical analysis.
Analyses of intracellular cytokine responses, antibody titers, and viral loads used the exact Wilcoxon rank sum test for two-group comparisons, and the exact Kruskal-Wallis test for comparisons across the four immunization groups or all five groups at once. The Spearman rank correlation test was used to assess relationships between immune responses and virologic parameters. For associations with delayed acquisition and peak viremia, prechallenge antibody responses were analyzed. For associations with decreased chronic viremia in the s.l. group, prechallenge antibody parameters and pre- and postchallenge cellular responses were analyzed. The number of challenges required for infection was modeled as a function of log-transformed antibody titers in mucosal secretions using proportional hazards regression and Monte Carlo estimation of Wald test P values. Results of statistical analyses were considered significant if they produced P values of ≤0.05.
Nucleotide sequence accession numbers.
All 241 sequences determined in this study were deposited in GenBank under accession numbers JQ085996 to JQ086220.
RESULTS
Induction of systemic SIV-specific memory T cell responses.
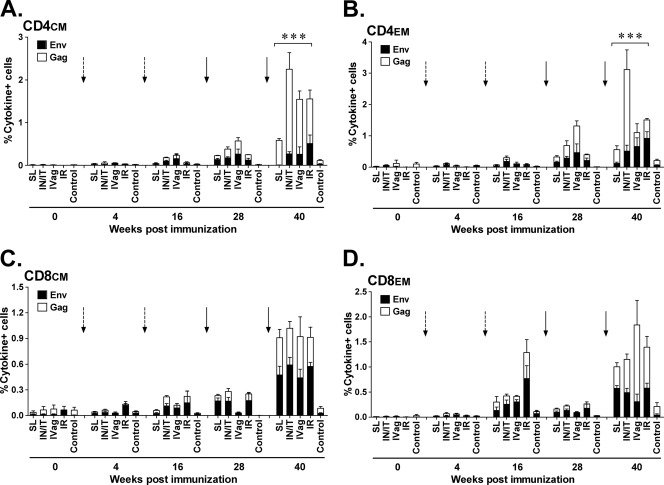
As reported elsewhere in a paper focused on the biodistribution of the replicating Ad5hr vector, no significant differences between immunization groups in cellular immune responses as measured by IFN-γ enzyme-linked immunosorbent spot assay or T cell proliferation assays were observed (Patterson et al., submitted). Here we investigated the induction of SIV Env- and Gag-specific CM and EM CD4+ and CD8+ T cell responses in PBMC over the course of immunization by intracellular cytokine staining for the production of IFN-γ, IL-2, and TNF-α. Both CD4+ and CD8+ memory T cells specific for SIV Env and Gag were expanded and detectable at week 16 after two Ad priming immunizations and were markedly elevated by week 40 after two envelope protein boosts in all immunized groups (Fig. 1A to D). The increased Gag responses presumably result from replication-competent Ad5hr recombinant expression, which persists at least 25 weeks following the last Ad recombinant immunization (Patterson et al., submitted), and the use of MPL-SE adjuvant in the Env boost, which can enhance both antibody and T cell responses (19). For CD4+ CM T cells (Fig. 1A), the low-level responses to Env and Gag seen in the s.l. group in comparison to those of the other three groups resulted in significant differences over all four immunization groups for Env (P = 0.0006)- and Gag (P = 0.0023)-specific responses separately, as well as for the sum of the Env and Gag responses (P = 0.0005).
Fig 1.
Intracellular cytokine staining of PBMC for SIV-specific CD4+ and CD8+ memory T cells secreting IFN-γ, IL-2, and TNF-α. (A to D) Stacked responses to SIV Env and Gag peptide pools by CD4+ and CD8+ CM and EM cells over the course of immunization. Dashed arrows mark the Ad recombinant administrations at weeks 0 and 12. Solid arrows mark the gp120 protein boosts at weeks 24 and 36. In panel A, *** signifies P values across the four immunization groups of 0.0006 for Env, 0.0023 for Gag, and 0.0005 for the sum of both. In panel B, *** signifies P values across the four immunization groups of 0.0092 for Env, 0.0006 for Gag, and 0.0006 for the sum of both.
Env- and Gag-specific CD4+ EM responses were also observed in all immunization groups at 40 weeks postimmunization (Fig. 1B). Again, significant differences were obtained for Env-specific responses across all immunization groups (P = 0.0092), mainly due to the low-level response in the s.l. group, and for Gag-specific responses (P = 0.0006), due to the elevated response in the i.n./i.t. group. Overall, a significant difference in the sum of the Env and Gag responses across all four groups was also obtained (P = 0.0006).
In contrast to the CD4+ memory T cells, by week 40 postimmunization, Env- and Gag-specific CD8+ CM and CD8+ EM T cell levels were remarkably similar in all four immunization groups (Fig. 1C and D), with no significant differences observed. Thus, overall, mucosal immunization by any of the tested routes with the replicating Ad recombinants was able to elicit comparable levels of systemic memory T cells, although with some variability seen in the CD4+ memory T cell responses in the s.l. and i.n./i.t. groups.
Induction of cellular immunity at mucosal sites.
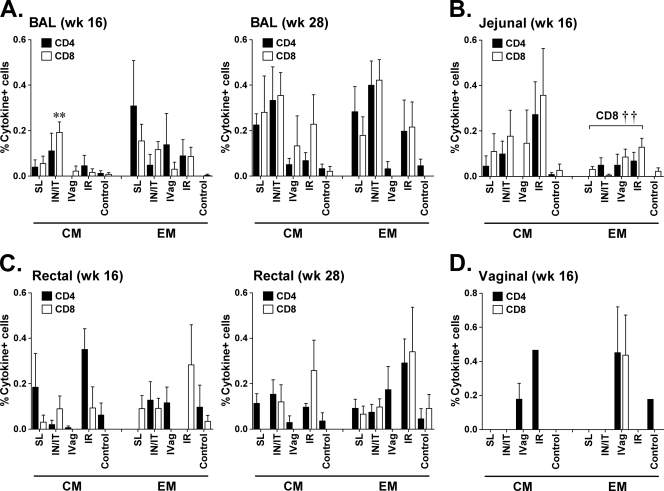
To evaluate the effect of priming by different routes on the induction of local mucosal cellular immunity, which is believed to be crucial for HIV vaccine efficacy, we collected mucosal biopsy specimens at weeks 16 (4 weeks after the second Ad recombinant administration) and 28 (4 weeks after the first envelope boost) and analyzed Env-specific T cell memory responses. Representative staining of BAL cells and rectal tissue is shown in Fig. S1 in the supplemental material. Gag-specific responses were not evaluated due to the limited number of available cells. Initially, we examined memory T cell responses in BAL cells (Fig. 2A). The lung is a mucosal effector site that is thought to provide a “window” to events occurring in the intestine (42). Not surprisingly, therefore, proportionately more CD4+ and CD8+ EM responses than CM responses were seen early in the course of immunization at week 16. As the s.l. and i.n./i.t. routes are proximal to the lung, responses in both of these groups tended to be higher than in the others. Among the CM responses, the CD8+ CM Env-specific response in the i.n./i.t. group was significantly higher at this time point than in the other groups (P = 0.003). By week 28, Env-specific CD4+ and CD8+ CM-positive cells became more apparent. In general, although Env-specific T cell responses developed in all immunization groups, compared to the i.vag. and i.r. groups, stronger responses tended to remain in the s.l. and i.n./i.t. groups, although significant differences were not obtained.
Fig 2.
Intracellular cytokine staining for SIV-specific CD4+ and CD8+ memory T cells secreting IFN-γ, IL-2, and TNF-α in mucosal tissues. Env-specific responses of CD4+ and CD8+ CM and EM cells in BAL fluid (A), jejunal biopsy specimens (B), rectal pinch biopsy specimens (C), and vaginal biopsy specimens (D) at week 16 (after two Ad recombinant immunizations) and/or at week 28 (after the first envelope boost). **, P = 0.003 for CD8+ CM cells in BAL fluid of the i.n./i.t. group compared to the other groups; ††, P = 0.0053 for the difference in jejunal CD8+ EM cells across all four immunization groups.
In both jejunal (Fig. 2B) and rectal (Fig. 2C) biopsy specimens, the i.r. group in general exhibited the highest Env-specific responses, perhaps reflecting a compartmentalization effect of the immunization route. In the jejunum, CD4+ and CD8+ CM responses tended to be higher than EM responses; however, a significant difference across all four immunization groups was observed only among CD8+ EM cells (P = 0.0053), where the difference was due mostly to elevated responses in the i.r. and i.vag. groups. In rectal biopsy specimens, responses were sporadic at week 16 but more consistently observed at week 28. No significant differences across immunization groups were observed at either time point.
As expected, i.vag. immunization induced the greatest proportion of memory T cells in vaginal biopsy specimens (Fig. 2D), although this result stems from only three females in the i.vag. group and one female in each of the other groups. The other routes elicited no responses at this site, with the exception of CD4+ CM T cells seen in i.r.-immunized macaques (Fig. 2D). It should be pointed out that the jejunal and vaginal biopsy specimens were obtained only after the second Ad immunization, whereas BAL fluid and rectal biopsy specimens were also examined following the first envelope boost, where responses tended to be stronger. This may have resulted in the detection of lower-level Env-specific responses in the jejunal and vaginal tissues.
Taking all of the cellular immune data together, there was an overall trend indicating a diverse and rapidly expanding population of memory cells in both blood and multiple tissue compartments following immunization by multiple mucosal routes with the replication-competent Ad recombinants.
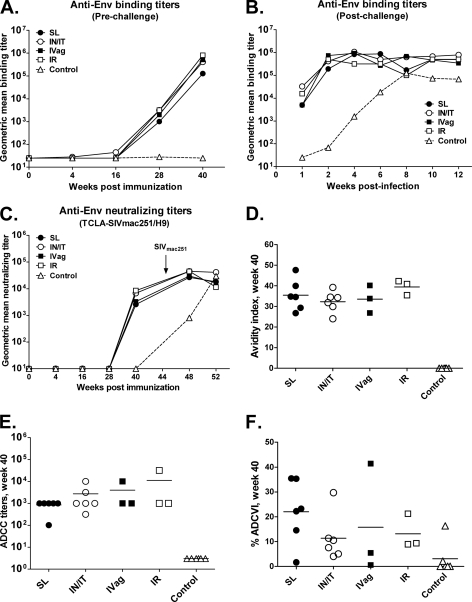
Systemic Env-specific antibody responses.
SIV Env-specific binding antibody titers in serum were detected only in the i.n./i.t. group at week 16 following the second Ad priming immunization but were significantly boosted to titers of over 105 in all immunization groups following the second envelope protein immunizations (Fig. 3A). One week postchallenge, binding antibody titers had slightly declined but quickly rebounded to comparable values in all immunization groups by week 2 postchallenge (Fig. 3B). Serum neutralizing antibodies against TCLA SIVmac251 were induced by week 40 following the second envelope protein boost. Although the i.n./i.t. and i.r. groups exhibited somewhat higher neutralizing antibody titers than the s.l. and i.vag. groups, the values overall were comparable and remained so postchallenge, with no significant differences detected (Fig. 3C). None of the immunized macaques developed antibodies at any time point able to neutralize primary SIVmac251 pseudovirus (data not shown). Furthermore, none of the control macaques developed binding or neutralizing antibodies prior to challenge.
Fig 3.
Vaccine-induced antibody responses in serum. Shown are prechallenge (A) and postchallenge (B) geometric mean antibody binding titers against SIV gp120 protein and geometric mean neutralizing antibody titers against TCLA-SIVmac251 (H9 grown) over time (C). The arrow indicates the time of SIVmac251 challenge. Also shown are the avidity of anti-Env antibody at week 40 (D), serum ADCC titers at week 40 (E), and percent ADCVI at week 40 (F).
Antibody maturation following vaccination was evaluated by assessing avidity, a measure of the strength of the binding interaction between an antigen with multiple antigenic determinants and multivalent antibodies. As shown in Fig. 3D, all immunization groups developed similar levels of Env-specific antibody avidity, higher than the controls at week 40 following the second protein immunization. Thus, mucosal priming by the four routes tested was able to prime similar levels of mature, systemic antibody.
Recent studies have shown significant correlations between nonneutralizing antibody activities and protective efficacy against both HIV and SIV (see references 5 and 52 for reviews). Therefore, we evaluated the ability of vaccine-elicited antibodies to mediate both ADCC and ADCVI. Sera of all immunized macaques exhibited ADCC activity, with comparable mean titers across all immunization groups (Fig. 3E). Similarly, the majority of the immunized macaques developed antibodies able to mediate low levels of ADCVI (Fig. 3F). Again, no significant differences between immunization groups were observed. Thus, the route of Ad recombinant administration did not affect the development of serum antibodies able to mediate nonneutralizing antibody activities.
Mucosal Env-specific antibody responses.
We further investigated whether the immunization regimens elicited virus-specific antibody at mucosal sites by examining saliva and nasal, rectal, and vaginal secretions at week 40 following the second envelope protein boost. Many of the secretions exhibited significant blood contamination, precluding an assessment of whether the antibody originated locally or systemically. Therefore, the secretions were evaluated by ELISA as described in Materials and Methods using HRP-conjugated anti-monkey secretory component to detect sIgA bound to SIV gp120. As shown in Table 2, Env-specific sIgA was readily induced in all immunization groups and regularly appeared in all secretory fluids except saliva. Not surprisingly, given the propensity of the Ad5hr vector to replicate in the URT, sIgA titers were highest in nasal secretions regardless of the immunization route. Titers in rectal and vaginal secretions across the four immunization groups were comparable. Immunization via the rectal route elicited the highest sIgA titers overall, followed by the i.n./i.t. and i.vag. routes.
Table 2.
Env-specific sIgA titers after second gp120 boost
| Secretion | Immunization group (Mean log2-transformed sIgA titerb ± SEM) |
||||
|---|---|---|---|---|---|
| s.l. | i.n./i.t. | i.v. | i.r. | Control | |
| Rectal | 1.3 ± 0.7 | 2.2 ± 0.8 | 1.7 ± 0.3 | 3.0 ± 0.6 | 0.5 ± 0.2 |
| Nasal | 3.2 ± 0.5 | 3.8 ± 0.5 | 3.0 ± 1.0 | 4.0 ± 1.0 | 0.5 ± 0.2 |
| Vaginala | 1.0 | 2.0 | 2.0 ± 0.6 | 7.0 | 0 |
| Saliva | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.7 ± 0.3 | 0.2 ± 0.2 |
One female macaque per group except three in i.v. group.
Titers of <2 were scored as 1 (log2 transformed = 0).
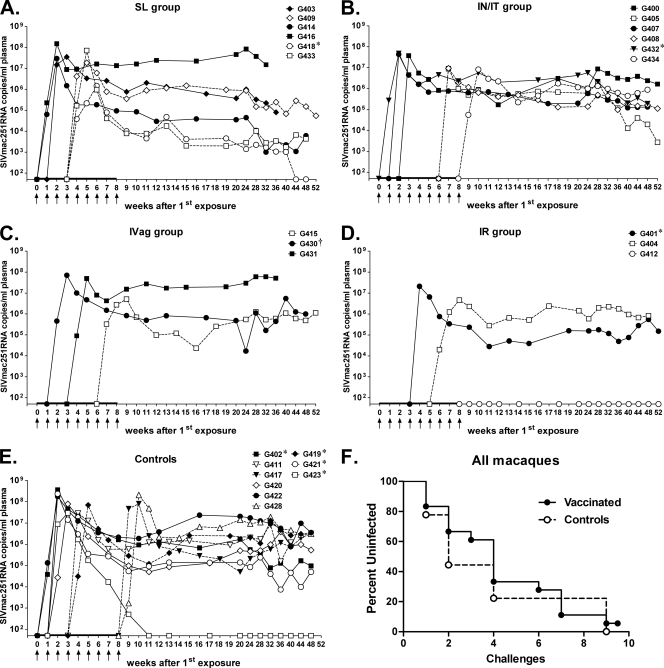
Low-dose challenge with SIVmac251.
At week 45, vaccinated and control macaques were challenged intrarectally with weekly low doses of SIVmac251, a highly virulent and pathogenic SIV strain, to assess the protective efficacy of the vaccine regimens primed via different mucosal immunization routes. The low dose used (a 1:500 dilution, equivalent to 130 TCID50) was subsequently shown to transmit a single SIV variant in 8 of the 9 control macaques (see Fig. S2 in the supplemental material). Two variants were transmitted to the remaining macaque. Plasma samples were assayed 5 days after each challenge for SIV RNA by NASBA, and uninfected animals were rechallenged 2 days later. Challenges were terminated once an animal tested positive for SIV plasma viremia. All animals became infected after 9 challenges (Fig. 4A to E), except one (G412) in the i.r. group (Fig. 4D). This animal was notable in exhibiting high Env-specific sIgA titers in rectal, nasal, and vaginal fluids (titers of 16, 32, and 128, respectively) and was one of only two macaques to exhibit a positive titer (of 2) in saliva. It also developed the highest Env-specific serum binding antibody titer (2 × 106) shared by only one other macaque (G430) and the highest avidity index of 42.2, compared to the mean among immunized macaques of 34.3 ± 1.5, indicating strong antibody maturation. No correlations can be drawn, however, with only a single macaque completely protected. Overall, none of the immunization groups provided significant protection against acquisition. No significant differences were observed in the number of challenges required for infection of the animals in the five groups (s.l., 2.7 ± 0.6 [mean ± standard error of the mean]; i.n./i.t., 4.8 ± 1.3; i.vag., 4.3 ± 1.5; i.r., 5.0 ± 1.0; control, 4.3 ± 1.5), whether they were analyzed together (Fig. 4F) or separately (see Fig. S3 in the supplemental material).
Fig 4.
Time of infection and plasma viral loads following repeated low-dose intrarectal challenges with SIVmac251. Panels A to E illustrate results for the s.l., i.n./i.t., i.vag., i.r., and control groups, respectively. Arrows mark sequential low-dose challenges. The macaques in each group are listed. * and † denote Mamu A*01- and Mamu B*08-positive macaques, respectively. Three naïve macaques (G419, G421, and G423) were added to the study at the time of challenge. (F) Kaplan-Meier plot of the percentage of uninfected macaques versus the number of challenges.
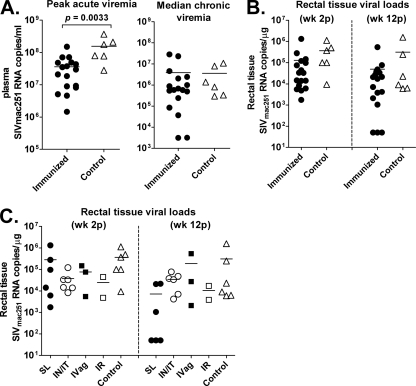
While protection from SIV acquisition was not observed, all vaccinated macaques exhibited a modest but significant reduction in peak viremia compared to controls (P = 0.0033, Fig. 5A), although this did not extend to the chronic phase, as shown by similar median viral loads in the vaccinated and control macaques (Fig. 5A). Although macaques in the s.l. group displayed declining viral loads during the chronic phase of infection (Fig. 4A), a significant difference from vector/adjuvant controls or macaques in the other immunization groups was not reached. Note that the three naïve macaques added at the time of challenge were not included in this chronic viral load analysis, as they were all Mamu A*01 positive. One of these (G423) showed a rapid decline to undetectable viremia during the chronic phase of infection (Fig. 4E). Previous studies have shown that Mamu-A*01-positive macaques control SIV replication better than Mamu-A*01-negative macaques (36, 37). Mamu B*08-positive macaques have also been associated with elite control of SIV viremia (29). Here, among the immunized and mock-vaccinated macaques, a single Mamu A*01-positive macaque was included in each group (except for the i.vag. group, which had a B*08-positive animal instead) in order to balance this effect.
Fig 5.
Peak acute and median chronic plasma viremia (A) and rectal tissue viral loads (B, C). In panel B, the viral loads of all immunized macaques and controls are shown. In panel C, the viral loads of individual immunization groups are shown.
To evaluate SIV replication at the site of transmission, rectal pinch biopsy specimens were obtained from all macaques at weeks 2 and 12 postinfection for analysis of viral RNA. The uninfected macaque, G412, was again negative at both time points, confirming lack of transmission, and was excluded from further statistical analysis, as were the three naïve control macaques. All other samples were positive at similar levels at the 2-week time point (Fig. 5B). By week 12, 3 macaques in the s.l. group had dropped to undetectable levels, consistent with the lower plasma viremia levels seen in this group during the chronic phase of infection. A comparison of the rectal viral loads across the four immunization groups showed that they approached a significant difference (P = 0.063).
Immunological correlates of viremia control.
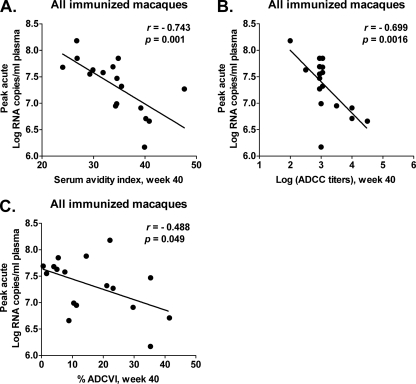
To elucidate the basis for the significantly reduced acute viremia in the immunized macaques, we investigated several immune responses potentially correlated with the outcome. Cellular immune responses prior to challenge were not associated with the reduced viremia, nor were binding or neutralizing antibody titers. However, the avidity of Env-specific serum binding antibodies at week 40 was significantly correlated with reduced peak viremia (r = −0.743, P = 0.001; Fig. 6A).
Fig 6.
Immune responses correlated with reduced peak acute viremia. Significant correlations between reduced peak acute viremia in all immunized macaques and antibody avidity (P = 0.001) (A), ADCC titers (P = 0.0016) (B), and percent ADCVI (P = 0.049) (C). The correlation coefficients (r values) and P values are from Spearman rank analysis.
We have previously shown that antibody avidity is directly correlated with functional antibody activities, such as ADCC and ADCVI. Therefore, we investigated whether either or both activities were also correlated with reduced peak viremia. As shown in Fig. 6B and C, both ADCC and ADCVI activities were inversely correlated with peak viremia, suggesting a role in the modest viremia reduction observed.
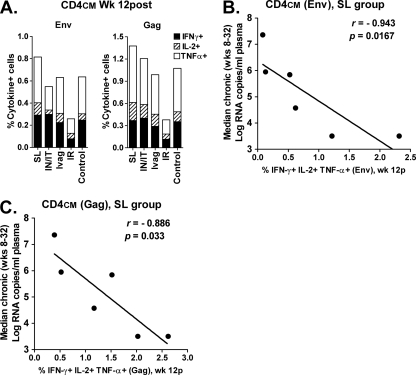
During the chronic phase of infection, the s.l. group exhibited a gradual decline in plasma viremia relative to the other groups of macaques (Fig. 4) and, compared to the macaques overall (Fig. 5B), also exhibited better control of rectal tissue viral loads (Fig. 5C). Although these outcomes were not statistically significantly different from those of the control macaques, it was nevertheless of interest to examine immunologic parameters that might have influenced this result. Twelve weeks postchallenge, CD4+ CM T cells in PBMC of the s.l. group exhibited somewhat higher recall cytokine responses against both Env and Gag peptides (Fig. 7A) compared to other immunization groups. The sum of percentages of CD4+ CM cells secreting IFN-γ, IL-2, and TNF-α in response to Env and Gag stimulation of all immunized macaques taken together was inversely correlated with median chronic viremia (for Gag, r = −0.5607, P = 0.0192; for Env, r = −0.6687, P = 0.0033 [data not shown]). Moreover, this same relationship was seen for macaques in the s.l. group alone (P = 0.0167 and P = 0.033 for Env and Gag, respectively; Fig. 7B and C). Thus, vaccine-induced cellular immunity apparently contributed to control of SIV in the s.l. group. However, it is possible that SIV-specific CD4+ CM cells were simply better preserved in macaques with lower viremia, as described previously (38).
Fig 7.
Postchallenge immune responses contributing to decreased viremia in macaques after s.l. immunization. (A) Env- and Gag-specific CD4+ CM responses in PBMC at 12 weeks postchallenge. (B, C) Inverse correlation of summed percentages of Env- and Gag-specific CD4+ CM cells, respectively, secreting IFN-γ, IL-2, and TNF-α with median chronic viremia.
Factors associated with delayed acquisition.
We also investigated parameters that might have contributed to delayed acquisition. We first tested whether TRIM5α, an innate restriction factor that is polymorphic in rhesus macaques, might have played a role by typing our rhesus macaques for TRIM5α polymorphisms. Overall, 15 of the macaques had restrictive alleles, while 6 had sensitive alleles and 6 were heterozygous. However, no correlation between the number of challenges required for infection and the TRIM5α genotype was observed (data not shown). This is consistent with recent results showing that mucosal infection by SIVmac251 is not influenced by TRIM5α, in contrast to intravenous infection (10).
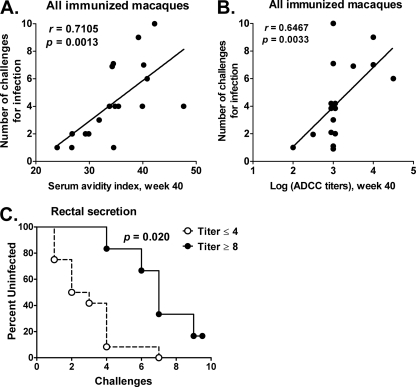
However, the avidity of week 40 binding antibodies (Fig. 8A) and ADCC titers at the same time point (Fig. 8B) were directly correlated with the number of challenges required for infection in all immunized macaques (r = 0.7105 and P = 0.0013 for avidity; r = 0.6467 and P = 0.0033 for ADCC). More strikingly, titers of anti-Env sIgA in rectal secretions were significantly associated with delayed acquisition in the immunized macaques. Higher titers were associated with a greater number of exposures (P = 0.0019 by the score test of the proportional hazards regression model using the log titers). The dichotomization into the two groups in a Kaplan-Meier plot (Fig. 8C) gave a significant difference (P = 0.020), corrected for the number of possible dichotomizations.
Fig 8.
Immune responses correlated with delayed SIV acquisition. Significant correlation of the serum avidity index (A) and the week 40 ADCC titer (B) with the number of challenges needed for infection. (C) Significant correlation between Env-specific sIgA titers in the rectal secretions of immunized macaques and delayed SIV acquisition.
DISCUSSION
Most HIV infections occur via a mucosal route, including the gastrointestinal and genital tracts, suggesting that induction of mucosal immunity will be necessary for an efficacious HIV vaccine. One criterion for the use of Ad as an HIV vaccine vector is its propensity for replication in cells lining the URT and other mucosal epithelia, hence targeting mucosal inductive sites. We have routinely used i.n., i.t., and oral routes of Ad recombinant administration in our preclinical studies and have observed induction of strong systemic and mucosal immunity (3, 9, 30, 31, 39, 40). However, recent descriptions of use of the s.l. route for vaccine administration prompted us to investigate it in comparison to the i.n./i.t. route. While several groups have evaluated oral and tonsillar routes of vaccine administration (12, 32, 50, 51), assessment of the s.l. route for Ad-based SIV vaccine administration in the rhesus macaque model has not been described previously. In order to evaluate immunogenicity and protective efficacy resulting from the i.n./i.t. and s.l. routes relative to local immunization at key sites of potential infection, we also investigated the i.vag. and i.r. routes.
Overall, administration of Ad5hr-SIV recombinants by any of the mucosal routes resulted in highly effective priming of systemic immunity. As reported elsewhere, no differences were seen in the secretion of IFN-γ and T cell proliferative responses by PBMC across the four immunization routes (Patterson et al., submitted). Similarly, here we observed no difference in CM or EM CD8+ T cell responses among groups. However, CD4+ T cell memory responses were not equivalent among the groups, with the s.l. group showing somewhat weaker Env- and Gag-specific responses than the other administration route groups and the i.n./i.t. group showing elevated Gag-specific responses (Fig. 1A and B). With regard to systemic humoral immunity, anti-envelope binding antibody titers were slightly diminished at week 40 in the s.l. group as reported elsewhere (Patterson et al., submitted) but were very comparable among the groups postchallenge (Fig. 3B). Moreover, functional antibody responses, including neutralization, ADCC, and ADCVI, and avidity indices showed no differences among the four groups. The reasons for the slightly lower CD4+ memory responses in PBMC from the s.l. group are not immediately apparent. This administration route was subject to the greatest variability in terms of administration, as the vaccine was simply placed in liquid form under the tongue, and some swallowing or dilution and/or loss of the inoculum in saliva may have occurred. Future applications for s.l. delivery should be formulated in a more stable form.
Cellular responses at mucosal sites were more sporadic and showed greater variability among groups, overall. However, administration of the replicating Ad recombinants at all four mucosal sites resulted in responses in the lung, intestine, and rectum. Nevertheless, responses in the lung tended to be higher in the s.l. and i.n./i.t. groups, while jejunal and rectal responses tended to be higher following i.r. priming. Not surprisingly, vaginal immunization appeared to be optimal for achieving measurable cellular responses at the vaginal site, reflecting greater mucosal compartmentalization. In contrast to the cellular immune responses, greater uniformity was observed with Env-specific sIgA, which was elicited at multiple mucosal sites by all four priming regimens. Vaginal antibody measurements were limited because of the small number of female macaques in the study, and salivary antibody exhibited low or negative titers. Note that because sIgA is a dimer, the overall sIgA titers are lower than titers one would expect to see if IgA were measured at these mucosal sites. However, due to significant blood contamination in many of the samples, the sIgA measurement gave the most accurate assessment of mucosal antibody induction.
Originally, a single high-dose challenge was planned for this study. However, repeated low-dose challenges have convincingly been shown to recapitulate mucosal HIV infection, transmitting, on average, a single viral variant (23, 24, 28, 34). Therefore, although statistical power was limited, we added an additional three naïve macaques to the control group and conducted a repeated low-dose i.r. challenge as a pilot study, using a dilution of SIVmac251 that transmitted a single variant. Protection against SIV acquisition was not observed, as only one macaque in the i.r. group remained uninfected following nine sequential challenges. However, modestly reduced peak viremia was observed in the immunized macaques, compared to that of controls (P = 0.0033), and correlated inversely with prechallenge serum envelope avidity (P = 0.001) and nonneutralizing ADCC and ADCVI activities (P = 0.0016 and P = 0.049, respectively). This result suggests that although lacking the ability to neutralize the challenge virus, vaccine-elicited antibody had a transient effect on early infection events, perhaps delaying systemic spread from initial foci of infected cells in the mucosa.
No overall effect on viremia levels of immunized versus control macaques during the chronic phase of infection was observed; however, the s.l. group of macaques exhibited a much broader spread in chronic viremia levels (Fig. 4A) and a trend, although not significant, toward lower overall median values (data not shown). Rectal tissue viral loads of the s.l. group were also low at 12 weeks postchallenge (Fig. 5C). As shown in Fig. 7, median chronic viremia levels in the s.l. group were significantly correlated with postchallenge CD4+ CM. The marginal improvement in viremia control in the s.l. group was somewhat surprising, as prechallenge CD4+ CM and EM T cell responses in PBMC tended to be lower than those of the other immunized groups. Assessment of mucosal cellular responses in BAL fluid and rectal and jejunal biopsy specimens was not performed at week 40 following the last immunization, and it is possible that these assays would have revealed enhanced cellular memory in the s.l. group. Taken together, these data hint that Ad recombinant administration via the s.l. route may target important antigen-presenting cells, perhaps at sites not assessed here. Investigation of Ad-GFP recombinants administered by these four mucosal routes has shown no differences in the levels of GFP expression in macrophages and myeloid dendritic cells in the lung and rectum among the four groups (Patterson et al., submitted). Future studies should examine the elicitation of cellular responses at additional sites throughout the gastrointestinal tract following s.l. immunization.
Although protection from SIV acquisition was achieved in only a single immunized macaque in the i.r. group, the significant correlation of the prechallenge secretory antibody titer in rectal secretions with delayed acquisition in the immunized macaques overall is notable. This mechanism of protection has not been previously described yet is not unexpected. Mucosal antibody has been expected to be important in the control of SIV infection. Here we show that all four mucosal immunization routes induced sIgA at multiple mucosal sites, suggesting that mucosal humoral immunity may be less compartmentalized than its cellular counterpart, as reflected by restricted induction of i.vag. cellular immunity following s.l. and i.n./i.t. immunization. Studies are ongoing to fully characterize the properties of the sIgA induced and elucidate its functional properties.
Here we have demonstrated that, regardless of the mucosal route of administration, replication-competent Ad5hr-SIV recombinants can effectively elicit systemic, as well as mucosal, cellular immune responses at multiple mucosal sites and prime systemic and mucosal antibody responses following an envelope subunit boost. This is in line with their broad dissemination with concomitant transgene expression in vivo (Patterson et al., submitted) and contrasts with nonreplicating Ad vectors whose induction of cellular immunity following mucosal administration has been reported to be more restricted and dependent on the mucosal immunization route in the murine system (22). Recently, i.m. administration of replication-defective Ad recombinants was shown to elicit long-term memory T cell responses in peripheral blood, as well as in lung, intestinal, and vaginal tissues of nonhuman primates (27). Possible mechanisms for this result include the induction of retinoic acid in dendritic cells infected by Ad, leading to their expression of α4β7 on antigen-specific CD8+ T cells (2, 14). Additionally, a recently described mechanism in mice for selecting avid effector memory cells at mucosal sites involves interaction of the major histocompatibility complex class I thymus leukemia antigen (TL) on dendritic cells with CD8αα on activated CD8αβ+ T cells, leading to affinity-based selection of memory precursor cells. Further, expression of TL on epithelial cells can lead to selection of mature CD8αβ memory cells in the intestine (20). Whether the latter mechanism is operative in humans or nonhuman primates is not known. It will be of interest to determine if mucosal immunization with the replicating Ad vector will induce a longevity of cellular memory similar to or greater than that induced by the nonreplicating vector. Moreover, whether the nonreplicating Ad vector administered systemically will elicit similarly potent systemic and mucosal antibody responses as the replication-competent Ad vector will await future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ronald C. Desrosiers, New England National Primate Research Center, for developing the SIVmac251 challenge stock and Nancy Miller, Division of AIDS, NIAID, for making it available and arranging the evaluation of transmitted variants; Katherine M. McKinnon, Vaccine Branch Flow Cytometry Core Facility (NCI/NIH), for technical support of the LSR II flow cytometer; and Anthony Cook, Steve Harbaugh, and Jeff Harbaugh, Bioqual, Inc., for excellent care of the rhesus macaques and performance of all animal technical procedures.
This study was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Published ahead of print 15 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. 2011. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin. Vaccine Immunol. 18:150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belyakov IM, Ahlers JD. 2011. Simultaneous approach using systemic, mucosal and transcutaneous routes of immunization for development of protective HIV-1 vaccines. Curr. Med. Chem. 18:3953–3962 [DOI] [PubMed] [Google Scholar]
- 3. Bogers WM, et al. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology 382:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brocca-Cofano E, et al. 2011. Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine 29:3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brocca-Cofano E, Xiao P, Robert-Guroff M. 2011. HIV envelope-specific antibody and vaccine efficacy, p 257–280 In Dumais N. (ed), HIV and AIDS—updates on biology, immunology, epidemiology, and treatment strategies. Intech Open-Access Publisher, Rijeka, Croatia: http://www.intechopen.com/articles/show/title/hiv-envelope-specific-antibody-and-vaccine-efficacy [Google Scholar]
- 6. Buchbinder SP, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buge SL, et al. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 71:8531–8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuburu N, et al. 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25:8598–8610 [DOI] [PubMed] [Google Scholar]
- 9. Demberg T, et al. 2007. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 81:3414–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fenizia C, et al. 2011. TRIM5{alpha} does not affect SIVmac251replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J. Virol. 85:12399–12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forthal DN, et al. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 80:9217–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freissmuth D, et al. 2010. Analysis of humoral immune responses in rhesus macaques vaccinated with attenuated SIVmac239Deltanef and challenged with pathogenic SIVmac251. J. Med. Primatol. 39:97–111 [DOI] [PubMed] [Google Scholar]
- 13. Frew AJ. 2008. Sublingual immunotherapy. N. Engl. J. Med. 358:2259–2264 [DOI] [PubMed] [Google Scholar]
- 14. Ganguly S, Manicassamy S, Blackwell J, Pulendran B, Amara RR. 2011. Adenovirus type 5 induces vitamin A-metabolizing enzymes in dendritic cells and enhances priming of gut-homing CD8 T cells. Mucosal Immunol. 4:528–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gómez-Román VR, et al. 2006. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 308:53–67 [DOI] [PubMed] [Google Scholar]
- 16. Gómez-Román VR, Robert-Guroff M. 2003. Adenoviruses as vectors for HIV vaccines. AIDS Rev. 5:178–185 [PubMed] [Google Scholar]
- 17. Hervouet C, et al. 2010. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine 28:5582–5590 [DOI] [PubMed] [Google Scholar]
- 18. Hidajat R, et al. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J. Virol. 83:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins SC, Mills KHG. 2010. TLR, NLR agonists, and other immune modulators as infectious disease vaccine adjuvants. Curr. Infect. Dis. Rep. 12:4–12 [DOI] [PubMed] [Google Scholar]
- 20. Huang Y, et al. 2011. Mucosal memory CD8(+) T cells are selected in the periphery by an MHC class I molecule. Nat. Immunol. 12:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kantele A, et al. 1998. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 66:5630–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. 2010. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J. Virol. 84:5986–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keele BF, et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keele BF, et al. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozlowski PA, et al. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immune Defic. Syndr. 24:297–309 [DOI] [PubMed] [Google Scholar]
- 26. Lee EM, et al. 2010. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J. Virol. Methods 163:287–294 [DOI] [PubMed] [Google Scholar]
- 27. Li H, et al. 2011. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J. Virol. 85:11007–11015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, et al. 2010. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J. Virol. 84:10406–10412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loffredo JT, et al. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81:8827–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lubeck MD, et al. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3:651–658 [DOI] [PubMed] [Google Scholar]
- 31. Malkevitch NV, et al. 2006. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology 353:83–98 [DOI] [PubMed] [Google Scholar]
- 32. Marthas ML, et al. 2011. Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine 29:3124–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mattapallil JJ, et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 34. McDermott AB, et al. 2004. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. J. Virol. 78:3140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mestecky J. 1987. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 7:265–276 [DOI] [PubMed] [Google Scholar]
- 36. Mothé BR, et al. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pal R, et al. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palmer BE, Voritz E, Wilson CC. 2004. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 172:3337–3347 [DOI] [PubMed] [Google Scholar]
- 39. Patterson LJ, et al. 2011. Rapid SIV Env-specific mucosal and serum antibody induction augments cellular immunity in protecting immunized, elite-controller macaques against high dose heterologous SIV challenge. Virology 411:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patterson LJ, et al. 2004. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 78:2212–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patterson LJ, Robert-Guroff M. 2008. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin. Biol. Ther. 8:1347–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Picker LJ, et al. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinczewski J, et al. 2005. Enhanced immunity and protective efficacy against SIVmac251 intrarectal challenge following ad-SIV priming by multiple mucosal routes and gp120 boosting in MPL-SE. Viral Immunol. 18:236–243 [DOI] [PubMed] [Google Scholar]
- 44. Pope M, Haase AT. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847–852 [DOI] [PubMed] [Google Scholar]
- 45. Rerks-Ngarm S, et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 46. Robb ML. 2008. Failure of the Merck HIV vaccine: an uncertain step forward. Lancet 372:1857–1858 [DOI] [PubMed] [Google Scholar]
- 47. Robert-Guroff M, et al. 1998. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J. Virol. 72:10275–10280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romano JW, et al. 2000. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J. Virol. Methods 86:61–70 [DOI] [PubMed] [Google Scholar]
- 49. Song JH, et al. 2008. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:1644–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stahl-Hennig C, et al. 2007. A single vaccination with attenuated SIVmac 239 via the tonsillar route confers partial protection against challenge with SIVmac 251 at a distant mucosal site, the rectum. Front. Biosci. 12:2107–2123 [DOI] [PubMed] [Google Scholar]
- 51. Vagenas P, et al. 2009. Tonsillar application of AT-2 SIV affords partial protection against rectal challenge with SIVmac239. J. Acquir. Immune Defic. Syndr. 52:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wren L, Kent SJ. 2011. HIV Vaccine efficacy trial: glimmers of hope and the potential role of antibody-dependent cellular cytotoxicity. Hum. Vaccin. 7:466–473 [DOI] [PubMed] [Google Scholar]
- 53. Xiao P, et al. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 84:7161–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou Q, et al. 2007. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251). Vaccine 25:8021–8035 [DOI] [PubMed] [Google Scholar]
- 55. Zolla-Pazner S, et al. 1998. Induction of neutralizing antibodies to T cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime-boost vaccine regimen in chimpanzees. J. Virol. 72:1052–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.