Abstract
HIV-exposed, uninfected (EUN) babies born to HIV-infected mothers are examples of natural resistance to HIV infection. In this study, we evaluated the titer and neutralizing potential of gp41-specific maternal antibodies and their correlation with HIV transmission in HIV-infected mother-child pairs. Specific gp41-binding and -neutralizing antibodies were determined in a cohort of 74 first-time mother-child pairs, of whom 40 mothers were infected with HIV subtype C. Within the infected mother cohort, 16 babies were born infected and 24 were PCR negative and uninfected at birth (i.e., exposed but uninfected). Thirty-four HIV-uninfected and HIV-unexposed mother-child pairs were included as controls. All HIV-positive mothers and their newborns showed high IgG titers to linear epitopes within the HR1 region and to the membrane-proximal (MPER) domain of gp41; most sera also recognized the disulfide loop immunodominant epitope (IDE). Antibody titers to the gp41 epitopes were significantly lower in nontransmitting mothers (P < 0.01) and in the EUN babies (P < 0.005) than in HIV-positive mother-child pairs. Three domains of gp41, HR1, IDE, and MPER, elicited antibodies that were effectively transmitted to EUN babies. Moreover, in EUN babies, epitopes overlapping the 2F5 epitope (ELDKWAS), but not the 4E10 epitope, were neutralization targets in two out of four viruses tested. Our findings highlight important epitopes in gp41 that appear to be associated with exposure without infection and would be important to consider for vaccine design.
INTRODUCTION
Antibodies (Ab) are known to play a key role in neutralizing HIV infection in vitro. Local immunity relies on antibodies blocking the mucosal entry of the virus, whereas at the systemic level, antibodies can neutralize the virus directly or via Ab-dependent cellular cytotoxicity (ADCC)-dependent virus clearance by effector cells. Several animal models have shown the efficacy of passive antibody transfer in preventing simian immunodeficiency virus (SIV) infection (5, 20, 21, 33, 34, 38, 41, 43, 50). More recently, the immunization of nonhuman primate models with HIV-1 gp41 induced protective antibodies (9).
In the course of natural infection, high titers of HIV-neutralizing antibodies (NAbs) may be elicited that usually target a limited number of regions within the HIV env protein (13, 16, 18, 26, 27, 32, 35, 36, 39, 40, 42, 44). A few monoclonal antibodies (MAbs) display neutralizing properties, e.g., 2F5, 4E10, and Z13, which bind to the membrane-proximal external region (MPER) of gp41. Moreover, some antibodies were associated with HIV resistance in exposed but uninfected subjects; the corresponding targets were an epitope placed within the N-terminal alpha-helical region HR1 and another within MPER (10, 24, 25, 45, 51).
In this study, we compared humoral responses to specific, linear gp41 epitopes that were already known to be the target of broadly neutralizing antibodies (9, 20, 21, 23, 39, 42, 46) in a cohort of sub-Saharan mother-child pairs, a population where the generation of protective antibodies and their passive transmission to newborns is likely to play a key role in preventing mother-to-child HIV infection (MTCT). The sub-Saharan region hosts the core of the pediatric HIV epidemic (46) mostly due to the transmission of subtype C strains, accounting for roughly 50% of all infections worldwide (19). Remarkably, it has been estimated that 60 to 80% of untreated infected mothers do not transmit the infection, suggesting that some yet-to-be-defined viral and/or host factors, including the generation and the transmission of neutralizing and/or blocking antibodies, can play a role in preventing MTCT transmission (6, 47, 48). This study focused on maternal gp41-specific antibodies, with particular reference to the mother-to-child transmission of their neutralizing potential via passive immunity.
MATERIALS AND METHODS
Study population.
Seventy-four South African first-time mothers (mean age, 22 years; range, 18 to 30) attending Coronation Women and Children Hospital, Johannesburg, South Africa, were studied. At enrolment, all underwent HIV-1 testing to assess seropositivity, viral load, and subtype. Forty mothers were infected with HIV subtype C, and 34 were uninfected. Cord blood (CB) was taken from all mothers, and sera were obtained from mothers and newborns. MTCT prevention programs in South Africa currently administer a single dose of nevirapine (NVP) to the mother during labor and to the child within 72 h after birth (17). Maternal viral load ranged from 9,000 to 42,000 RNA copies/ml (mean, 22,000; standard deviations [SD], 3,500), and there was no statistical difference between transmitting (TR) and nontransmitting (NT) infected mothers (data not shown). The maternal CD4 cell count ranged from 309 × 103 to 900 × 103 cells/μl (mean, 766; SD, 197). During labor, each mother received 300 mg NVP every 3 h, and after delivery, heel-prick dried blood spots were collected from each newborn and tested for viral RNA by PCR. Of the babies born from HIV-infected mothers, 16 were HIV positive and 24 were HIV negative. Viral loads from the infected babies ranged from 30,000 to 87,000 RNA copies/ml at the time of birth. The routine serological diagnosis of HIV infection in PCR-positive babies was confirmed at 15 months of age. Cord and maternal blood cells and plasma were sampled simultaneously at delivery, and specimens were aliquoted and stored at −80°C until testing. The study was conducted in accordance with the guidelines of the World Medical Association's Declaration of Helsinki and was approved by the Ethics Committee of the University of the Witwatersrand, Johannesburg, South Africa.
Peptide synthesis.
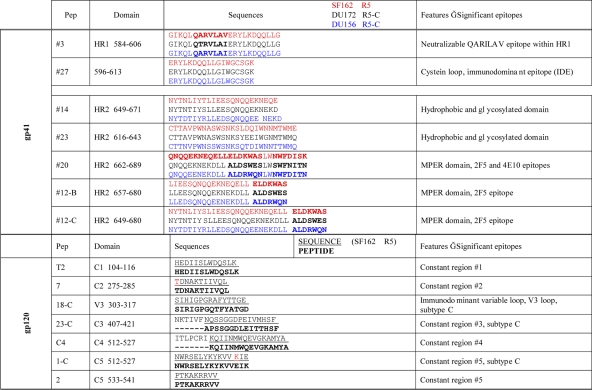
All peptides used in this study are listed in Table 1, where sequences and characteristics are indicated. Peptides were synthesized by the solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) method (14) using an Applied Biosystems model 433 A peptide synthesizer. After peptide assembly, resin-bound peptides were deprotected as previously described (25a) and purified by semipreparative reverse-phase high-performance liquid chromatography. Due to the high homology between subtype B and C consensus sequences of gp41, all peptides corresponded to the subtype B consensus with the exception of peptide 12, which was synthesized in subtype B and subtype C versions (Table 1). All peptides covering the different regions of gp120 were synthesized in a subtype C version.
Table 1.
Peptide sequences
Quantification of serum IgG.
Ninety-six-microwell plates were coated with IgG/IgA fractions (up to 1:128 by 2-fold dilutions) in 50 mM carbonate buffer, pH 9.5, for 1 h at 37°C. A commercial preparation of human IgG or IgA (Sigma-Aldrich, Milan, Italy) was used as the standard. After saturation with phosphate-buffered saline (PBS)–1% milk powder (Humana 3, Germany), the plates were incubated with peroxidase-conjugated rabbit anti-human IgG/IgA (Dako, Denmark) for 30 min at 37°C. The enzymatic reaction was developed with the TMB microwell peroxidase substrate system (KPL, Gaithersburg, MD).
Titration of Env-specific antibodies by ELISA.
Sera from mothers and babies were tested for binding to recombinant gp120/gp41 and to the panel of synthetic gp120/gp41 peptides (Table 1) with standard enzyme-linked immunosorbent assay (ELISA) procedures. Briefly, microplate wells (Nunc, Roskilde, Denmark) were coated with 50 ng/well of each peptide or with 25 ng/well of gp120 or gp41 recombinant protein and incubated overnight at 4°C in 50 mM NaHCO3-Na2CO3 buffer. All plates were saturated with 5% skim milk (Sigma-Aldrich, Italy), 5% fetal bovine serum (FBS), and 0.1% Tween 20 for 1 h at 37°C. Seventeen serial 2-fold serum dilutions, starting from 1:20 to more than 1:1,300,000, were added in duplicate and incubated for 1 h at 37°C. Plates were incubated with biotinylated goat anti-human IgG diluted 1:5,000 (SBA, Birmingham, AL) for 1 h at room temperature and then with horseradish peroxidase streptavidin (Vector Laboratories, Burlingame, CA) diluted 1:3,000 for 1 h at room temperature. The enzymatic reaction was developed and read at 450 nm by a microplate reader (Bio-Rad, Italy). Titers were defined as sample dilutions giving an optical density (OD) higher than the cutoff value. The cutoff was evaluated and defined as the mean OD plus 3 SD per sample dilution from all negative controls. Each OD obtained with a specific sample dilution was compared to the cutoff value and expressed as the titer.
Pseudovirus production for HIV-1 neutralization assay.
Stocks of single-round-infection HIV-1 Env pseudoviruses were produced by cotransfecting 293T/17 cells with 2 μg of an HIV-1 rev/env expression plasmid and 12 μg of an env-deficient HIV-1 backbone plasmid (pSG3DEnv) using Lipofectamine transfection reagent (Invitrogen-Life Technologies, Monza, Italy). Pseudovirus-containing supernatant was harvested 24 h after transfection, clarified by centrifugation, and filtered through 0.45-mm filters, and single-use 1-ml aliquots were stored at −80°C. The 50% tissue culture infectious dose (TCID50) for each pseudovirus preparation was determined by the infection of TZM.bl cells as previously described (29).
Virus neutralization assay.
Neutralization was measured in TZM-bl cells with Env-pseudotyped viruses (29). The virus panel included two low-sensitive pseudoviruses from primary infected subtype C subjects (strains DU172 and DU156) (28) and two very sensitive pseudoviruses from laboratory strains SF162 and IIIB. A virus unrelated to HIV (vesicular stomatitis virus G [VSV-G], strain SVA.MLV#922) was also included. Briefly, 200 TCID50 of pseudoviruses in 50 μl culture media was incubated with 100 μl of all serially diluted sera or TriMab (a 50:50:50 mixture of 2F5, 2G12, and b12 monoclonal antibodies) (NISBC), which was used as a positive control, in a 96-well plate in triplicate for 1 h at 37°C. A 100-μl suspension of TZM-bl cells (1 × 104 cells/well) containing 75 μg/ml DEAE dextran was added, and the cultures then were incubated at 37°C in 5% CO2 for 48 h. Infection was monitored by evaluating the luciferase activity. Titers were calculated as the ID50, the sample dilution at which relative luminescence units (RLU) were reduced 50% compared to those in virus control wells (wells with no inhibitor) after the subtraction of background RLU in control cell wells (wells without virus infection). Specimens showing neutralization titers higher than 1:100 were designated high neutralizing sera.
Preadsorption assay.
The specificity of antibody-mediated virus neutralization was assessed by single or pooled cord blood sera preadsorbed with peptides 3, 12-C, and 27, i.e., peptides showing the highest binding values in ELISAs. Briefly, a saturating concentration of each peptide was allowed to react for 1 h at 37°C with antibodies present in serum before performing the neutralization assay with the virus strain DU156. As a positive control, the TriMab mix was used at concentrations ranging from 66 to 0.1 μg/ml. As a negative control, a scrambled peptide was used at the same molar dilutions adopted for the specific ones.
Transcytosis assay.
HIV-1 transcytosis across epithelial cells and neutralization by Abs was assessed as previously described (8). Briefly, the endometrial cell line HEC-1 (3) was grown as a tight polarized monolayer (106 cell/12-mm-diameter filter unit in Dulbecco's modified Eagle medium [DMEM] with glutamax plus 10% fetal calf serum [FCS]) for 8 to 10 days on a permeable filter support (0.45-μm pore size). This allowed the formation of an interface between two independent chambers, an upper one, including the apical surface of the epithelial monolayer, and a lower one, including the basolateral surface. One ml of medium per well was exchanged at the basolateral side, and 0.5 ml/well was exchanged at the apical side every 72 h. The tightness of the epithelial cells was measured as electrical resistance and was 0.400 Ω/cm2 (range, 420 to 630) at the beginning of analysis with apical HIV-1 transmission or basolateral IgA addition or sampling as described previously (12). Transepithelial electrical resistance was measured with a Millicell ERS resistance apparatus (Millipore, Bedford, MA). To further control the tightness of the epithelial cell barrier, the passage of recombinant p24 antigen was measured. Samples (at 1:10 dilution) then were added to the apical chamber and incubated for 20 min at 37°C. 2F5 (10 μg/ml) was used as a positive control. To initiate virus transcytosis, 5 × 105 peripheral blood mononuclear cells (PBMC) infected with 50 TCID50 of strain SF162 were added to the apical chamber. PBMC were isolated by Ficoll-Paque centrifugation and stimulated with 5 μg/ml phytohemagglutin (PHA) (Sigma) and 10 U/ml of recombinant interleukin-2 (IL-2) (R&D Systems). Contact between HIV-infected PBMC and the epithelial cell monolayer resulted in the rapid budding of the HIV virions, followed by their transcytosis from the apical to the basolateral pole of the epithelial cells. After 2 h, the inhibition of transcytosis by serum samples was determined by the detection of p24 in the basolateral medium by ELISA as previously described (30). p24 levels in the absence or in the presence of negative sera taken from HIV-unexposed, seronegative cord blood was 1,500 pg/ml. This value was taken as 100% transcytosis and used to express the results.
Statistical analysis.
A descriptive analysis of neutralization for each virus was performed by means of whisker plots representing the quartiles of the distribution of neutralization. Outlier values were represented by observations greater than the third quartile plus 3/2 of the interquartile range (IQR) or less than the first quartile minus 3/2 of the IQR. To determine the association between anti-HIV IgG epitope gp120 or gp41 and virus neutralization, a cutoff of 1:20 was established. Accordingly, sera were classified as low/high titer or neutralizing/nonneutralizing according to this cutoff. The hypothesis of independence between antibody titers and virus neutralization was assessed by nonparametric tests, since the expected frequency in any cell was less than 5%. The problem of multiple comparisons was avoided by applying the Kruskal-Wallis test with the Bonferroni adjustment; this method controls the family-wise error rate (FWER; indicated by α) by adjusting the significance level of each individual test belonging to the family. For k tests, testing the individual null hypothesis at the α/k level ensures that FWER remains unchanged (49). For all of the hypotheses tested, two-tailed P values of less than 0.05 were considered to be significant. Statistical analysis was performed using SAS version 9.1 (SAS Institute, Cary, NC), and whisker plots were obtained by using STATA 9.1 (StataCorp LP, College Station, TX).
RESULTS
Quantification of total IgG and IgA in serum samples from mother-child pairs.
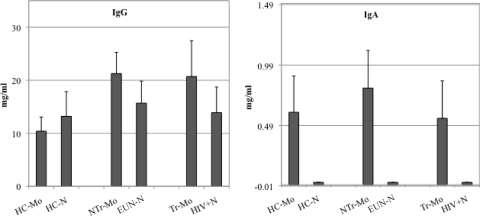
ELISAs were used to quantify total IgG and IgA in all serum samples from mothers and their babies. The mean IgG was 10.39 mg/ml for healthy control uninfected mothers and 21.26 and 20.70 mg/ml for nontransmitting mothers and transmitting mothers, respectively, as shown in the left panel of Fig. 1. The difference between healthy control uninfected mothers and either nontransmitting or transmitting mothers was statistically significant (P < 0.03) and was probably due to the typical hypergammaglobulinemia observed during HIV infection. The differences among babies were not significant (Fig. 1, left). IgA titrations were performed and no significant differences were identified for all mothers shown in Fig. 1, right. As expected, no IgA titers were found in cord blood sera (Fig. 1, right).
Fig 1.
Quantification of IgG and IgA in serum samples from all mother-baby pairs. The left panel shows serum IgG quantification. The right panel shows serum IgA quantification. Bars represent immunoglobulin concentrations in HIV healthy control unexposed mothers and in their babies (HC-Mo and HC-N, respectively), nontransmitter mothers and their babies (NTr-Mo and EUN-N, respectively), and transmitter mothers and their babies (Tr-Mo and HIV+N).
Binding antibodies to gp120 in serum samples from mother-child pairs.
ELISAs were performed with a panel of seven synthetic peptides, covering constant regions of gp120 and the V3 loop from clade B and C HIV strains (Table 1). No immunoreactivity to the constant regions of gp120 was found in transmitting (TR; n = 16/16; 100%) and in most nontransmitting women (NT; n = 20/24; 83%) and their babies. HIV-negative mother-child pairs were also nonreactive to the peptides (data not shown). Of note, six NT women and their babies (n = 6/24; 25%) showed low IgG titers to peptide 18 (mean values of 1:100 in both groups; cutoff value, 1:20). When samples were tested against the complete subtype C gp120, IgG immunoreactivity was found in 88% of sera (n = 21/24) of TR and NT women as well as in their babies. Binding to the whole Env protein (subtype B) and to a peptide corresponding to the V3 loop (subtype B) were assessed and consistently demonstrated in seropositive samples (data not shown). There was no reactivity to gp120 in the HIV-negative control pairs.
Binding antibodies to gp41 epitopes in serum samples from mother-child pairs.
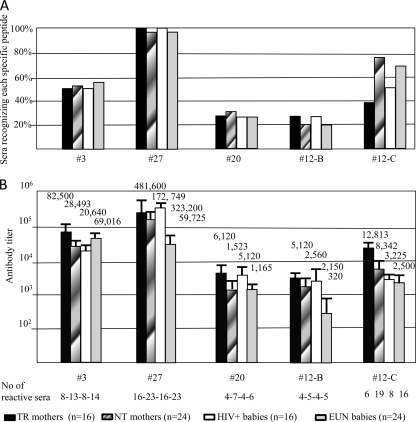
All maternal sera and cord blood samples were assayed on the panel of synthetic peptides covering different gp41 regions (Table 1); their reactivity is summarized in Fig. 2, where it is expressed as relative percentages (Fig. 2A) and specific titers (Fig. 2B). Specimens from HIV-uninfected control women and their babies were negative for anti-HIV antibodies (all titers were below the cutoff value; data not shown). Thirteen sera from NT mothers (n = 13/24; 54%) recognized an HR1 region containing the QARILAV epitope (peptide 3); sera from TR mothers also recognized this gp41 region. Importantly, sera from exposed uninfected (EUN) babies recognized the same HR1 domain, suggesting that this IgG specificity was derived by maternal transmission at birth (Fig. 2A). Ninety-six percent of NT sera (23/24) and 100% of TR sera (16/16) recognized the immunodominant epitopes in gp41, which is found in the disulfide loop domain (peptide 27). This reactivity was confirmed in corresponding children from these mothers (Fig. 2A). Overall, IgG titers against all gp41 regions tested ranged from 1:172,749 to 1:1,523 in NT mothers (Fig. 2B) and from 1:481,600 to 1:5,120 in TR mothers, and the cumulative difference was statistically significant (P < 0.01). Mean IgG titers from EUN babies ranged from 1:69,016 to 1:2,150 (Fig. 2B), whereas in HIV-infected babies titers ranged from 1:323,000 to 1:2,560; also in newborns, the cumulative difference between titers was statistically significant (P < 0.005). No binding antibodies were found in any group against peptides 23 and 14, both corresponding to the hydrophobic regions of gp41 (data not shown).
Fig 2.
Binding antibodies to gp41 in HIV-infected women and their children. The graphs show the percentage of maternal and cord blood serum specimens that were found to contain high-titer antibodies to gp41 peptides (A) and the mean values (1/n) plus standard errors of the means found in mothers and in their children (B). All specimens were tested in duplicate. TR, transmitting mothers; NT, nontransmitting mothers.
Approximately 25% of sera recognized the MPER consensus peptide 20, with NT mother sera being more reactive (29%) than those of TR mothers and EUN and HIV+ children (25%) (Fig. 2A). Antibodies directed to the MPER 2F5 epitope were assayed with two different, subtype-specific peptides (12-B and 12-C) (Table 1). About 20% of the sera from all groups recognized the subtype B peptide 12. In contrast, 19 NT mother sera were found to contain antibodies to the subtype C-specific peptide 12 (n = 19/24; 79%), while only 16 samples from their EUN babies (n = 16/24; 67%) showed antibodies to the subtype C peptide. Six TR maternal sera (n = 6/16; 37.5%) bound the subtype C peptide 12 with high titers, while sera from eight HIV-positive babies (n = 8/16; 50%) showed type-specific anti-MPER antibodies (12-C peptide) (Fig. 2B).
HIV neutralization in sera from mother-child pairs.
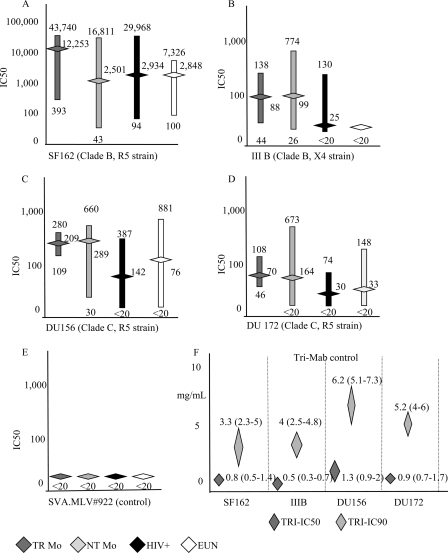
All maternal and newborn specimens were assayed in neutralization assays on four HIV-1 strains, namely, the high-sensitivity strains, the subtype B laboratory strains SF162 (R5-B) and IIIB (X4-B), and the low-sensitivity strains, primary isolates DU172 and DU156 (both R5-C). The latter were subtype C viruses, i.e., similar to the autologous strains which infected mothers enrolled in the study. The limited amount of available serum specimens prevented the isolation of autologous viral strains. Specimens showing neutralization titers higher than 1:100 were considered to be high neutralizing sera. Sera neutralized SF162 virus strain with higher titers than subtype C viruses (Fig. 3A to D); low levels of neutralization were obtained with IIIB (Fig. 3B). Higher neutralizing titers were shown in NT than TR mothers with both subtype C viruses (P < 0.05) and in EUN than HIV-infected babies (P < 0.01). Samples from the uninfected controls did not neutralize any tested virus (mean IC50, <1:20; data not shown). All sera were negative against the unrelated virus subtype SVA.MLV#922 (Fig. 3E). TriMab positive-control antibodies neutralized all viruses tested, as expected (Fig. 3F).
Fig 3.
HIV neutralization assays of either babies or mothers. Neutralization was calculated as the IC50 (range and means) corresponding to the sample dilution leading to 50% neutralization. (A) SF162 (R5); (B) IIIB (X4); (C) DU156 (C-R5); (D) DU172 (C-R5); (E) SVA.MLV#922 (control). (F) IC50 and IC90 (values and ranges) obtained with TriMab. The y axis reports the concentration (μg/ml) of antibodies instead of the neutralizing titer (1/n). All samples were tested in duplicate.
In detail, all sera from NT mothers showed neutralizing activity against SF162 and DU156, and 20/24 (83%) of these also neutralized DU172. Specimens from EUN babies neutralized both SF162 and DU156 strains, and 15/24 (62%) also neutralized DU172. TR control sera showed neutralizing activity against SF162, and 10/16 neutralized both subtype C viruses (DU156 and DU172) (Fig. 3A to D). HIV-1-infected cord blood specimens neutralized SF162 (13/16), 4 out of 16 of them did not neutralize any virus strain, 6 neutralized both subtype C viruses, and 6 neutralized strain DU156 only (Fig. 3A to D).
Neutralizing antibody absorption by gp41 peptides.
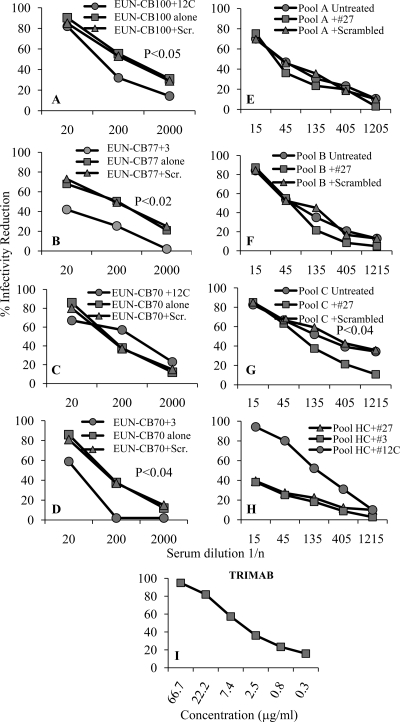
The specificity of virus neutralization was verified through preadsorption assays performed in triplicate on serum from a single cord blood (CB) specimen or on randomly pooled specimens from EUN babies, depending on the availability of the samples; all specimens used in the test contained high-titer neutralizing antibodies. When antibodies were preincubated with the specific N-terminal peptide 3 and C-terminal peptide 12-C, their ability to neutralize HIV infection was greatly reduced, showing that anti-HIV activity was mostly due to antibodies directed against epitopes of HR1 and MPER domains of gp41. Preadsorption experiments performed with peptide 27 employed pooled cord blood specimens with different binding profiles: pool A did not bind peptide 27, while pools B and C were reactive. Pool C showed a significant reduction of neutralization only upon preadsorption with peptide 27 (P < 0.04), suggesting that immunodominant epitope (IDE)-specific neutralizing antibodies could contribute to neutralization. No infectivity modulation was observed when sera were preincubated with scrambled peptides, while the TriMab control inhibited strain DU156 (Fig. 4). To evaluate the effect of gp41 peptides on HIV infection, a pool of 5 HIV-uninfected cord blood sera from seronegative mothers were preincubated with peptides 3, 27, and 12-C before performing neutralization (Fig. 4).
Fig 4.
Neutralization specificity of anti-HIV antibodies from single or pooled cord blood serum samples. All neutralization assays were performed on the DU156 HIV isolate (C-R5) with cord blood specimens from EUN babies; statistical significance (two-tailed t test) is indicated in each panel. Both controls and cord blood samples were tested in three replicates. (A) CB100 preadsorbed with scrambled and 12-C peptides; (B) CB77 preadsorbed with scrambled peptide and peptide 3; (C) CB70 preadsorbed with scrambled peptide and peptide 12-C; (D) CB70 preadsorbed with scrambled peptide and peptide 3; (E) pool A (five random, ELISA-negative samples) preadsorbed with scrambled peptides and peptide 27; (F) pool B (five random, ELISA-positive samples) preadsorbed with scrambled peptides and peptide 27; (G) pool C (five random, ELISA-positive samples) preadsorbed with scrambled peptide and peptide 27; (H) pool of CB serum from 5 HIV-uninfected babies preadsorbed with scrambled peptide and peptides 3, 12-C, and 27; (I) TriMab neutralization control. The x axis from panels A to H report serum dilution as 1/n, panel I reports concentrations of TriMab. Peptide sequences are given in Table 1. Scr., scrambled.
Cord blood antibodies inhibit HIV transcytosis across epithelia.
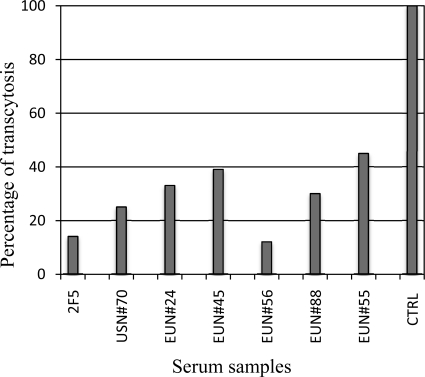
The inhibition of HIV entry across mucosal epithelia has been shown to be an effective mechanism to prevent horizontal HIV infection, acting independently or in synergy with systemic immunity (9). All cord blood samples from EUN newborns therefore were tested in transcytosis assays to evaluate the ability to block HIV entry through a mechanism other than antibody neutralization. A significantly higher blocking activity was found in 6 out of 24 EUN cord blood specimens than in negative controls (P < 0.003), as shown in Fig. 5. The 16 HIV-positive controls did not show any HIV-blocking activity (P < 0.01). Blocking activity was not associated with any gp41 specificity corresponding to peptides under study, although they were reactive against gp41, suggesting it is due to other types of gp41-specific antibodies.
Fig 5.
Inhibition of HIV transcytosis across HEC-1 epithelial cells, forming a model of human epithelium. Cord blood specimens from EUN newborns were tested in transcytosis inhibition assays using the HIV-1 SF162 strain (two replicas). Results are expressed in percentages compared to results with 2F5 MAb (>85% inhibition; positive control) and a pool of HIV-negative cord blood samples (100% transcytosis; negative control [CTRL]).
Correlation between HIV neutralization and fine specificity of gp41 antibodies.
Results observed in neutralization and in preadsorption assays were further investigated using a statistical model of transmitting and nontransmitting groups (TR-HIV+ and NT-EUN pairs, respectively) (Table 2) to establish associations between binding antibodies to each peptide and neutralization activity for each subset of sera. TR pairs showed a lower number of significant values than NT ones, and antibodies to the HR1 domain were not significant at all in TR mothers and in their babies. In the NT mothers, IgGs to the IDE domain (peptide 27) were significantly associated with the neutralization of all viruses except DU156; antibodies to HR1 (peptide 3) and to the MPER domain (subtype C peptide 12) were associated with the neutralization of the two laboratory strains and with the DU172 isolate but not with DU156. Conversely, peptides 12-B and 20 were associated with the neutralization of laboratory strains but not of subtype C isolates (Table 2).
Table 2.
P values of the test for association between high-titer antibodies and virus neutralizationa
| Peptide no. (domain/region) |
P value for: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Very sensitive laboratory strain |
Low-sensitive primary subtype C strain |
|||||||||||||||
| SF162 (R5) |
IIIB (X4) |
DU172 (R5) |
DU156 (R5) |
|||||||||||||
| NTr-Mo | EUN-N | TR-Mo | HIV+N | NTr-M | EUN-N | TR-Mo | HIV+N | NTr-M | EUN-N | TR-Mo | HIV+N | NTr-M | EUN-N | TR-Mo | HIV+N | |
| 3 (HR1, QARILAV) | <0.001 | 0.001 | NS | NS | <0.001 | NS | NS | NS | 0.008 | NS | NS | NS | NS | NS | NS | NS |
| 27 (IDE) | 0.001 | 0.001 | 0.001 | NS | 0.001 | 0.001 | NS | <0.001 | ||||||||
| 20 (HR2, MPER, 2F5, 4E10) | 0.037 | 0.044 | 0.01 | 0.01 | 0.004 | NS | NS | NS | NS | NS | 0.05 | 0.05 | NS | NS | NS | NS |
| 12-B (HR2, MPER, 2F5) | 0.028 | 0.007 | 0.028 | NS | NS | NS | NS | NS | ||||||||
| 12-C (HR2, MPER, 2F5) | 0.001 | 0.001 | 0.001 | 0.02 | 0.001 | NS | 0.001 | 0.05 | <0.001 | <0.001 | NS | NS | NS | NS | 0.05 | 0.03 |
The analysis includes five gp41 peptides that elicited antibodies (titer, >1:20). NS, not significant. NTr-Mo, HIV-1-infected, nontransmitting mothers; EUN-N, HIV-1-exposed, uninfected newborns; TR-Mo, HIV-1-infected, transmitting mothers; HIV+N, HIV-1-infected newborns.
In the EUN babies, IgGs to the IDE domain (peptide 27) were significantly associated with the neutralization of SF162, DU172, and DU156. Antibodies to MPER (peptide 12-C) were associated with the neutralization of SF162 and DU172 strains only, while antibodies to other domains, such as HR1 and HR2 (3, 20, and 12-B), were associated with the neutralization of the SF162 strain only. None of the antibody specificities were significantly associated with neutralizing activity for the IIIB strain (Table 2). In the HIV+ mothers, IgGs to the IDE domain (peptide 27) were significantly associated with the neutralization of two of the four virus strains; antibodies to HR1 (peptide 3) did not show significant associations with the neutralization of any of the tested viruses, while the MPER domain (12-C) was associated with the neutralization of the two laboratory strains and with DU172, but not with DU156. Conversely, reactivity with 12-B and 20 peptides was associated with the neutralization of two laboratory strains and of one of the two subtype C isolates (Table 2). In the HIV+ newborns, IgGs to the IDE and MPER domains (peptides 27 and 12-C) were significantly associated with the neutralization of SF162 and DU172 only. Antibodies to the HR1 domain (peptide 3) failed to show any association with virus neutralization; the HR2 domain (12-B) was associated with the neutralization of strain SF162 only; antibodies to peptide 20 showed the greatest extent of association, being associated with two laboratory strains and with DU156 (Table 2).
DISCUSSION
The study of an African mother-child population offers a unique chance to evaluate the transmission of specific humoral responses from the mother to the child and to assess their protective potential, with the aim of designing a vaccine specific to this population. Although many environmental and host factors could affect immune status and therefore HIV susceptibility (2, 16b, 48), antigenic properties of env proteins have been studied by the use of multiple peptides, covering all constant and V3 regions of gp120 and many N- and C-terminal regions within gp41. Specific IgG antibodies targeting some epitopes within Env proteins had been extensively characterized for their broad neutralizing activity, and their titers and neutralizing activity were compared in maternal sera and in their newborn cord blood specimens in this study.
Successful prevention measures have considerably reduced the perinatal transmission of HIV, and important insights into newborn immune protection can assist in vaccine design. The small size of the cohort in our study (74 mother-child pairs, including 24 HIV+/EUN pairs, 16 HIV+/HIV+ pairs, and 34 HIV−/HIV− control pairs) might have limited the use of more refined statistical modeling, and the scarceness of available samples might have limited the choice of assays performed, thus preventing any sequence analysis; therefore, the preliminary results that we report here should be extended and validated, when possible, in larger populations.
Since all mothers received the same preventive antiretroviral therapy during delivery in a single dose and the babies were born within 12 to 18 h of their mothers receiving drug, it was unlikely that NVP was present in cord blood specimens at the time of harvest. To support this, there appeared to be fine specificity in whether samples could or could not neutralize HIV in vitro. In addition, when we used an unrelated virus (murine leukemia virus-env pseudotype) containing an irrelevant env sequence, which was produced with a reverse transcriptase (RT)-resistant backbone vector, none of the samples were reactive to the sequence (4). Consequently, any interference in the assays due to the presence of NVP was minimal, and we regard the presence of virus neutralization to be specific to the IgG identified.
Antibody reactivity to whole gp120 supported the key role of conformational epitopes, which were recognized more efficiently than the linear ones; other studies also reported that broadly neutralizing antibodies to gp120 preferentially recognized conformation epitopes that were shaped and displayed on trimeric env proteins but not on the corresponding monomers; variable V2 and V3 loops played an important role in neutralization breadth and potency (48). Only clade C-specific sequences from the V3 loop (i.e., peptide 18-C) were recognized by antibodies, possibly due to the R310Q and other mutations introducing significant changes in the charge and hydrophobic pattern of the loop crown (Table 1). According to experiments with alanine mapping, single mutations within the V2 or V3 loop could affect the neutralizing activity of anti-gp120 antibodies dramatically (48). Neither maternal sera nor cord blood specimens showed any significant association between gp120-specific antibodies and virus neutralization, further suggesting that antibodies to linear epitopes of gp120 did not reduce the infectivity of different virus isolates.
Our results show that all HIV-positive mothers and their newborns displayed high IgG titers to three epitopes within gp41, namely, MPER, IDE, and QARILAV (HR1 region). Antibody responses to these gp41 epitopes were quantitatively lower in nontransmitting mothers (P < 0.01) and in their EUN babies (P < 0.005) than in the HIV-positive mother-child pairs, while the proportion of samples reactive against the different epitopes of gp41 was very similar for all studied subgroups (Fig. 1A and B). The finding could be explained by the existence of different pools of antibodies endowed with protective, neutral, or even enhancing effects on HIV, each recognizing similar, contiguous epitopes, as already reported for the MPER domain (1, 7, 9, 11, 27, 37).
Other studies observed no significant differences in potency and breadth of neutralizing antibodies in breastfed newborns who became infected and in those free from HIV, and therefore they concluded that NAbs could be ineffective in preventing HIV transmission unless higher titers or different specificities could be elicited by passive transfer or vaccination. Moreover, the role and extent of other antibody-mediated antiviral activities, such as ADCC, were not addressed in those studies (31). In our study, not only different antibody titers but also different pools of antibodies may exist, with pools being reactive to the MPER or the IDE domains and some of the IgGs displaying virus-neutralizing properties (Fig. 4E to G and Table 2). These data support the hypothesis of an antibody pool containing immunoglobulins endowed with different specificities and different properties.
Specific gp41 antibodies from most mothers and newborns neutralized the SF162 strain; more than 50% of the specimens under study neutralized the two subtype C isolates, DU172 and DU156 (Fig. 3A to D). The latter isolates were genetically very similar to viruses infecting the mothers. However, the paucity of available samples prevented the direct isolation of viral strains from both mothers and their newborns, and this prevented any sequence analysis. Antibodies to MPER (12-C, 12-B, and 20 peptides) were found in a high proportion of maternal specimens; antibodies to 12-C peptide were especially significantly associated with the neutralization of three isolates in all of the mothers; in all newborns, this neutralizing activity was associated with SF162 and DU172 strains and significantly confirmed in preadsorption assays (Fig. 4A and C and Table 2). Conversely, antibodies to peptide 12-B showed a lower association with significant neutralization in NT-EUN than in TR-HIV+ pairs; antibodies to peptide 20 were significant for three virus isolates in both TR mothers and their HIV-infected children. Taken together, these results suggest that epitope 2F5 provides a stronger neutralization target than 4E10, since peptide 20 spans both 2F5 and 4E10, while peptides 12-B and 12-C cover the 2F5 epitope only (Table 1). Previous studies showed that 2F5 is usually ineffective against clade C isolates due to the presence of mutations within the MPER domain with respect to the model clade B sequence (16, 16b, 48). However, neutralizing activity targeting the MPER domain could be due to antibodies recognizing epitopes overlapping with or different from ELDKWAS (i.e., the 2F5 target epitope), therefore explaining why 2F5 did not neutralize subtype C virus (16, 16a, 34). In fact, the generation of at least two subsets of anti-MPER antibodies during HIV infection was already reported in other studies, which observed the rare presence of neutralizing, anti-2F5 antibodies and the more common generation of nonneutralizing antibodies within MPER (1). Another reason for such discrepancy could be due to the superior length of the 12-C peptide used in the study compared to the 12-B one (eight amino acids were added to the N terminus), and this fact might explain why the 2F5 antibody failed to neutralize subtype C virus in other studies (16, 34). It is known that the length of a synthetic MPER domain is a key factor to achieve a more natural conformation of target epitopes, and this factor could be crucial in eliciting protective antibodies and also could explain why peptides 3 and 27 showed low levels of neutralization (9, 11, 44). Moreover, the substantial similarity in terms of charge and hydrophobic profile shared by the three sequences from SF162, DU172, and DU156 strains (Table 2) suggests that antibodies able to bind the subtype C epitope could, in addition, bind similar epitopes from different virus subtypes, provided that the overall conformation and/or other chemical features were conserved (11, 44).
Other studies, where primary isolates were characterized with monoclonal antibodies, adopted a different system and therefore achieved different results, since polyclonal sera contain many different antibody specificities and could provide neutralization through one or more antibodies, e.g., by targeting overlapping or contiguous epitopes to 2F5 within MPER or by taking advantage of synergic interactions among different antibodies (31). One or more of these hypotheses could provide explanations for the significant neutralization activity associated with the MPER domain that was observed in this cohort, although similar results were not reported in other studies.
In our study of MTCT, not only were different types of anti-MPER antibodies generated but different types of IDE-specific antibodies also were observed (1). In fact, different pools of antibodies showed a marked, even significant reduction in neutralization activity when sera were specifically preadsorbed with IDE peptide (Fig. 4F to H). The IDE domain derives its definition from the widespread presence of specific antibodies in nearly all HIV-infected subjects (9a, 15a). Most studies about the IDE domain failed to find neutralizing antibodies with this specificity; however, IDE-specific, neutralizing responses were sometimes reported (10a, 13). Interestingly, the IDE domain was also the target of antibodies enhancing HIV infection (38a). Therefore, the IDE domain may behave similarly to the MPER domain, i.e., it could induce the generation of different pools of antibodies endowed with different effector functions that could be protective, neutral, or even detrimental for the host. In this study, IDE-specific, protective antibodies were transmitted from mothers to newborns, and they were significantly associated with the neutralization of two and three virus strains in TR mothers and in EUN babies, respectively (Table 2). The higher reductions in neutralization were observed at higher dilutions of both pools B and C, while in pool A, which was devoid of neutralizing antibodies, both curves coincided point by point, suggesting that in pool A the neutralizing antibodies were directed to other epitopes. These findings imply that such protective antibodies are seldom elicited or, alternatively, greatly diluted within nonneutralizing, albeit HIV-specific, antibody pools.
Antibodies recognizing the QARILAV epitope were associated with significant neutralization in NT mothers but not in EUN newborns. No significant association between binding to peptide 3 and neutralization were found in TR mothers and in their HIV+ newborns (Table 2). Antibodies can prevent HIV infection at the systemic and local levels through mechanisms other than direct virus neutralization. Our study assessed whether cord blood specimens were able to block HIV infectivity in an in vitro model of mucosal transmission. Some (25%) cord blood specimens from EUN newborns, but none from HIV-infected or control babies, inhibited HIV transmission across an epithelium model, suggesting that the blocking of virus transcytosis takes part in protection from MTCT. This activity was not directly associated with any gp41 peptide tested in the study, implying that anti-gp41 antibodies recognizing other linear or conformational epitopes could be involved, as reported by other studies (9). The inhibition of transcytosis was possibly mediated by IgG antibodies, since cord blood specimens lacked IgA. We, along with others, have previously reported that either serum or mucosal IgG can block HIV-mediated transcytosis; indeed, a classical example of transcytosis inhibition was mediated by 2F5, an IgG1 monoclonal antibody commonly used as a positive control in such assays (8, 9, 22, 45). As HIV-specific antibodies can inhibit infection and replication via multiple mechanisms, such as ADCC (Ab-dependent cellular cytotoxicity), ADCVI (Ab-dependent cell-mediated virus inhibition), neutralization, and transcytosis (9, 23, 50), we suggest that transcytosis plays a role in the protection against HIV transmission in MTCT, although we were not able to define which region was involved in the env-mediated block of transcytosis. Further studies will be required to establish what epitopes are involved in such mechanism(s).
The analysis of this cohort supports the hypothesis that gp41 is important in mitigating HIV transmission, and that domains on the molecule should be considered in vaccine design. Other recent MTCT studies focused on postnatal transmission and the role of maternal antibodies (18, 31). The former one compared Env-specific IgG and IgA in serum and breast milk specimens from 41 HIV-positive women; the IgG fraction from breast milk showed neutralizing and ADCC activities that were 100-fold higher than the IgA ones, and similar differences were observed in serum (15). The latter study enrolled 100 HIV-positive, lactating mothers and their children, and it evaluated serum-neutralizing properties against a panel of viral isolates from different subtypes for 2 years after birth. During the follow-up period, one-third of newborns became infected, but IC50 values from HIV-infected and -exposed babies were not significantly different. This result was not observed in our cohort, where antibody titers from HIV-positive mothers with infected babies were significantly higher than those found in infected mothers with EUN newborns. The risk of HIV transmission was not associated with the overall neutralizing activity or with activity toward any specific virus isolate (31). Neither of the MTCT studies summarized here evaluated the presence and reactivity of specific neutralizing antibodies in their cohorts, a strategy that allowed us to observe different pools of antibodies (neutralizing or not) sharing the same specificity to a target domain but possibly endowed with different effector functions and committed to spread in different compartments, such as mucosal districts.
In conclusion, our study shows that IgG antibodies specific to domains on gp41 exist at various titers, can neutralize HIV in vitro, and inhibit transcytosis, and they can be found in HIV-uninfected babies born to HIV-infected mothers. These data offer a tantalizing glimpse of the types of antibodies that are likely transmitted between mother and child that may confer protection in the EUN baby. Correlates of protection in MTCT are still largely unknown, and our small study underscores that further investigations can extend and confirm our observations and could further improve our understanding of this complex problem.
ACKNOWLEDGMENTS
This study was supported by grant no. 201433 from the European Commission/Seventh Framework Programme, GCE grant no. 53030, and grant no. PP1008144 from the Bill and Melinda Gates Foundation.
We thank Silvia Russo for her editorial help and David Montefiori and his laboratory team for help with the neutralization assays.
Footnotes
Published ahead of print 1 February 2012
REFERENCES
- 1. Alam SM, et al. 2008. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 82:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aldrovandi GM, Kuhn L. 2010. What infants and breasts can teach us about natural protection from HIV infection. J. Infect. Dis. 202(Suppl. 3):S366–S370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alfsen A, Iniguez P, Bouguyon E, Bomsel M. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257–6265 [DOI] [PubMed] [Google Scholar]
- 4. Anderson JA, et al. 2008. Genotypic susceptibility scores and HIV type 1 RNA responses in treatment-experienced subjects with HIV type 1 infection. AIDS Res. Hum. Retrovir. 24:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baba TW, et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 [DOI] [PubMed] [Google Scholar]
- 6. Barin F, et al. 2006. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J. Infect. Dis. 193:1504–1511 [DOI] [PubMed] [Google Scholar]
- 7. Beck Z, Prohaszka Z, Fust G. 2008. Traitors of the immune system-enhancing antibodies in HIV infection: their possible implication in HIV vaccine development. Vaccine 26:3078–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bomsel M, et al. 2007. Natural mucosal antibodies reactive with first extracellular loop of CCR5 inhibit HIV-1 transport across human epithelial cells. AIDS 21:13–22 [DOI] [PubMed] [Google Scholar]
- 9. Bomsel M, et al. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280 [DOI] [PubMed] [Google Scholar]
- 9a. Chiodi F, et al. 1987. Site-directed ELISA with synthetic peptides representing the HIV transmembrane glycoprotein. J. Med. Virol. 23:1–9 [DOI] [PubMed] [Google Scholar]
- 10. Clerici M, et al. 2002. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. AIDS 16:1731–1741 [DOI] [PubMed] [Google Scholar]
- 10a. Cotropia J, et al. 1996. A human monoclonal antibody to HIV-1 gp41 with neutralizing activity against laboratory isolates. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:221–232 [DOI] [PubMed] [Google Scholar]
- 11. Coutant J, et al. 2008. Both lipid environment and pH are critical for determining physiological solution structure of 3-D-conserved epitopes of the HIV-1 gp41-MPER peptide P1. FASEB J. 22:4338–4351 [DOI] [PubMed] [Google Scholar]
- 12. Devito C, et al. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170–5176 [DOI] [PubMed] [Google Scholar]
- 13. Ferrantelli F, et al. 2004. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J. Infect. Dis. 189:2167–2173 [DOI] [PubMed] [Google Scholar]
- 14. Fields GB, Noble RL. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161–214 [DOI] [PubMed] [Google Scholar]
- 15. Fouda GG, et al. 2011. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J. Virol. 85:9555–9567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a. Gnann JW, Jr, Schwimmbeck PL, Nelson JA, Truax AB, Oldstone MB. 1987. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J. Infect. Dis. 156:261–267 [DOI] [PubMed] [Google Scholar]
- 16. Gray ES, et al. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a. Gray ES, et al. 2009. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J. Virol. 83:11265–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Gray ES, Meyers T, Gray G, Montefiori DC, Morris L. 2006. Insensitivity of paediatric HIV-1 subtype C viruses to broadly neutralising monoclonal antibodies raised against subtype B. PLoS Med. 3:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guay LA, et al. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795–802 [DOI] [PubMed] [Google Scholar]
- 18. Guevara H, et al. 2002. Maternal HIV-1 antibody and vertical transmission in subtype C virus infection. J. Acquir. Immune Defic. Syndr. 29:435–440 [DOI] [PubMed] [Google Scholar]
- 19. Hemelaar J, Gouws E, Ghys PD, Osmanov S. 2006. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20:W13–W23 [DOI] [PubMed] [Google Scholar]
- 20. Hessell AJ, et al. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hessell AJ, et al. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84:1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hocini H, et al. 1997. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res. Hum. Retrovir. 13:1179–1185 [DOI] [PubMed] [Google Scholar]
- 23. Jain S, Patrick AJ, Rosenthal KL. 2010. Multiple tandem copies of conserved gp41 epitopes incorporated in gag virus-like particles elicit systemic and mucosal antibodies in an optimized heterologous vector delivery regimen. Vaccine 28:7070–7080 [DOI] [PubMed] [Google Scholar]
- 24. Jain S, Rosenthal KL. 2011. The gp41 epitope, QARVLAVERY, is highly conserved and a potent inducer of IgA that neutralizes HIV-1 and inhibits viral transcytosis. Mucosal Immunol. 4:539–553 [DOI] [PubMed] [Google Scholar]
- 25. Kaul R, et al. 2001. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS 15:431–432 [DOI] [PubMed] [Google Scholar]
- 25a. King DS, Fields CG, Fields GB. 1990. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Protein Res. 36:255–266 [DOI] [PubMed] [Google Scholar]
- 26. Kirchherr JL, et al. 2011. Identification of amino acid substitutions associated with neutralization phenotype in the human immunodeficiency virus type-1 subtype C gp120. Virology 409:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lallemant M, et al. 1994. Maternal antibody response at delivery and perinatal transmission of human immunodeficiency virus type 1 in African women. Lancet 343:1001–1005 [DOI] [PubMed] [Google Scholar]
- 28. Li B, et al. 2006. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J. Virol. 80:5211–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li M, et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopalco L, et al. 2000. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 In vitro. J. Immunol. 164:3426–3433 [DOI] [PubMed] [Google Scholar]
- 31. Lynch JB, et al. 2011. The breadth and potency of passively acquired human immunodeficiency virus type 1-specific neutralizing antibodies do not correlate with the risk of infant infection. J. Virol. 85:5252–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lynch RM, et al. 2011. The B cell response is redundant and highly focused on V1V2 during early subtype C infection in a Zambian seroconverter. J. Virol. 85:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mascola JR, et al. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mascola JR, et al. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 35. Moore PL, Gray ES, Morris L. 2009. Specificity of the autologous neutralizing antibody response. Curr. Opin. HIV AIDS 4:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore PL, et al. 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 5:e1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pancino G, et al. 1998. Apparent enhancement of perinatal transmission of human immunodeficiency virus type 1 by high maternal anti-gp160 antibody titer. J. Infect. Dis. 177:1737–1741 [DOI] [PubMed] [Google Scholar]
- 38. Prince AM, et al. 1991. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res. Hum. Retrovir. 7:971–973 [DOI] [PubMed] [Google Scholar]
- 38a. Robinson WE, Jr, et al. 1990. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 87:3185–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rong R, et al. 2007. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J. Virol. 81:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rong R, et al. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruprecht RM, Ferrantelli F, Kitabwalla M, Xu W, McClure HM. 2003. Antibody protection: passive immunization of neonates against oral AIDS virus challenge. Vaccine 21:3370–3373 [DOI] [PubMed] [Google Scholar]
- 42. Ruprecht RM, et al. 2001. Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies. Transfus. Clin. Biol. 8:350–358 [DOI] [PubMed] [Google Scholar]
- 43. Shibata R, et al. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204–210 [DOI] [PubMed] [Google Scholar]
- 44. Sun ZY, et al. 2008. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 28:52–63 [DOI] [PubMed] [Google Scholar]
- 45. Tudor D, et al. 2009. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2:412–426 [DOI] [PubMed] [Google Scholar]
- 46. UNICEF 22 August 2011, posting date Preventing mother-to-child transmission of HIV. http://www.unicef.org/aids/index_preventionyoung.html
- 47. Van de Perre P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374–3376 [DOI] [PubMed] [Google Scholar]
- 48. Walker LM, et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Westfall P. 1997. Multiple testing of general contrasts using logical constraints and correlations. J. Am. Stat. Assoc. 92:299–306 [Google Scholar]
- 50. Xiao P, et al. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 84:7161–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zwick MB, et al. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905 [DOI] [PMC free article] [PubMed] [Google Scholar]