Abstract
We have investigated the previously uncharacterized human cytomegalovirus (HCMV) UL1 open reading frame (ORF), a member of the rapidly evolving HCMV RL11 family. UL1 is HCMV specific; the absence of UL1 in chimpanzee cytomegalovirus (CCMV) and sequence analysis studies suggest that UL1 may have originated by the duplication of an ancestor gene from the RL11-TRL cluster (TRL11, TRL12, and TRL13). Sequence similarity searches against human immunoglobulin (Ig)-containing proteins revealed that HCMV pUL1 shows significant similarity to the cellular carcinoembryonic antigen-related (CEA) protein family N-terminal Ig domain, which is responsible for CEA ligand recognition. Northern blot analysis revealed that UL1 is transcribed during the late phase of the viral replication cycle in both fibroblast-adapted and endotheliotropic strains of HCMV. We characterized the protein encoded by hemagglutinin (HA)-tagged UL1 in the AD169-derived HB5 background. UL1 is expressed as a 224-amino-acid type I transmembrane glycoprotein which becomes detectable at 48 h postinfection. In infected human fibroblasts, pUL1 colocalized at the cytoplasmic site of virion assembly and secondary envelopment together with TGN-46, a marker for the trans-Golgi network, and viral structural proteins, including the envelope glycoprotein gB and the tegument phosphoprotein pp28. Furthermore, analyses of highly purified AD169 UL1-HA epitope-tagged virions revealed that pUL1 is a novel constituent of the HCMV envelope. Importantly, the deletion of UL1 in HCMV TB40/E resulted in reduced growth in a cell type-specific manner, suggesting that pUL1 may be implicated in regulating HCMV cell tropism.
INTRODUCTION
Human cytomegalovirus (CMV) (HCMV) belongs to the Herpesviridae family and has the largest genome of any characterized human virus, comprised of around 200 predicted open reading frames (ORFs). The 230-kb linear, double-stranded DNA genome consists of long unique (UL) and short unique (US) sequences, each flanked by the inverted repeats referred to as TRL/IRL and IRS/TRS, resulting in the overall genomic configuration TRL-UL-IRL-IRS-US-TRS (35). In spite of the high levels of sequence variability, the gene content is relatively well conserved across related herpesviruses, and all family members share a set of core genes involved in basic metabolic and structural functions (1). Genes that are specific to a given virus or group of viruses are typically nonessential for replication in cell culture and are frequently involved in immune evasion. Certain immunoevasive genes show significant similarity to genes found in host genomes, possibly dating from events of molecular piracy (27).
Sequence analyses of the HCMV genome showed that as many as 70 putative glycoprotein-encoding ORFs are found in clinical isolates (11) and that 57 are found in the AD169 strain (13). HCMV structural glycoproteins can be divided into two broad classes, those conserved between members of the family Herpesviridae (including gB, gH, gL, gM, and gN) and subgenus-specific glycoproteins without homology to other herpesviruses, comprised of, among others, gpTRL10 (51), gpRL13 (53), gpUL132 (52), UL74-encoded gO (29), UL4-encoded gp48 (12), US27 (24), and UL33 (34). Many of the HCMV-specific glycoproteins, in contrast to the conserved glycoproteins, are not essential for in vitro replication in fibroblasts and presumably participate in cell and tissue tropism or pathogenicity (17).
The CMV-specific glycoproteins gpRL13 and gpUL4 are members of the primate CMV RL11 gene family, located on the extremity of the CMV genome. This RL11 gene family is comprised of 11 members conserved in both HCMV and chimpanzee CMV (CCMV) (RL11, RL12, RL13, UL4, UL5, UL6, UL7, UL8, UL9, UL10, and UL11), and HCMV-specific genes (UL1, RL5A, and RL6) presumably originated by gene duplication in the last 6 to 5 million years, which separates both CMV species (13, 14). The members of the RL11 gene family were initially assembled due to the presence of a defined motif in their sequence resembling cellular Thy-1 within a region conserved between other immunoglobulin (Ig) superfamily (IgSF) members (13). Subsequently, in a more detailed analysis, this shared core motif was further examined in the context of the neighboring sequences, allowing the characterization of an RL11 domain (RL11D) (14). The RL11D consists of a region of variable length (65 to 82 amino acids) containing three characteristic conserved residues (a tryptophan and two cysteines) and several potential N-linked glycosylation sites. Regarding the distribution and functions of the RL11 family members, UL4-encoded gp48 and gpRL13 have been described to be virion envelope glycoproteins (12, 53). UL10 and RL13 are so-called temperance genes, with cell type-specific virus growth inhibition functions (17, 53). TRL11 encodes an IgG-Fc binding glycoprotein (3, 33). UL11 encodes a hypervariable protein expressed on the cell surface of infected cells (26). UL7 is a glycoprotein with structural homology to the signaling lymphocyte activation molecule (SLAM) family receptor CD229, with the capacity to mediate adhesion to leukocytes and interfere with cytokine production (21). Of note, RL11 genes, dispensable for virus growth in cell culture, are among the most variable HCMV genes (16, 36), and RL5A, RL13, and UL9 are members of a restricted set of HCMV hypervariable genes (16).
Although there is no information on the expression and function of pUL1, sequence similarity was found with members of the human carcinoembryonic antigen (CEA) family (27). The CEA family, a structural subgroup of the Ig superfamily, is composed of 29 members located on the human chromosome 19q13.2 (32). In general, these molecules possess a leader peptide, one N-terminal Ig variable (IgV)-like domain, and a variable number of two different types of Ig constant (IgC)-like domains. Members of this family are broadly divided into the CEA-related cell adhesion molecule (CEACAM) and the pregnancy-specific glycoprotein (PSG) subgroups, involved in immune responses and reproduction. CEACAMs primarily mediate intracellular adhesion through homophilic and/or heterophilic interactions. Interestingly, members of the CEA family have been reported to function as receptors for bacterial or viral pathogens (7, 32).
In the present study, we have approached the molecular characterization of HCMV-specific UL1. To this end, an HCMV (AD169-derived HB5 background) recombinant with hemagglutinin (HA) epitope-tagged UL1 and a mutant with a full UL1 deletion in the endotheliotropic HCMV TB40/E strain were generated. Our data reveal that the UL1 ORF is transcribed with late kinetics. pUL1 is glycosylated and localizes at the site of virus assembly and secondary envelopment in infected cells, forming part of the envelope of HCMV virions. An HCMV mutant with a targeted deletion of UL1 exhibits a growth defect phenotype in retinal pigment epithelium cells but not in fibroblasts, indicating that this ORF encodes a cell type-specific tropism factor.
MATERIALS AND METHODS
Cell lines and viruses.
The MRC-5 human fibroblast cell line (ATCC CCL-171, passages 5 to 15) and the U373-MG cell line derived from glioblastomas (ECACC 89081403) were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Spain) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (10 μg/ml). The telomerase-immortalized human retina pigment epithelial (RPE) cell line (generously provided by Martin Messerle, University of Hannover, Germany) was grown in DMEM-Ham's F-12 (1:1) medium (Gibco) supplemented with 5% (vol/vol) FBS, penicillin (50 U/ml), streptomycin (50 μg/ml), 0.25% (vol/vol) sodium bicarbonate, and 2 mM glutamine. The following HCMV strains were used: Towne (ATCC VR-977), Toledo (41), endotheliotropic strain TB40/E (49), TB40/E reconstituted from the respective bacterial artificial chromosome (BAC) clone TB40/E BAC4 (48), and AD169 reconstituted from the respective BAC clone pHB5 (6). Infectious viral stocks were propagated as follows: MRC-5 cells were infected at a low multiplicity of infection (MOI), and the supernatants of infected cells that exhibited 100% cytopathic effects were cleared of cellular debris by centrifugation at 6,000 × g for 10 min. The cell-free supernatant was centrifuged at 20,000 × g for 3 h to pellet the virus particles. The amount of infectious virus was determined by counting MRC-5 cells positive for the HCMV immediate-early (IE) protein IE1 using monoclonal antibody (MAb) B810R (Chemicon) against this protein. Briefly, MRC-5 cells were incubated for 2 h with graded volumes of virus-containing supernatants, the inoculums were washed away with a phosphate-buffered saline (PBS) rinse after the adsorption period, and the cells were incubated with DMEM supplemented with 3% FBS. After 20 h, cells were fixed for 20 min at room temperature (RT) with 4% paraformaldehyde (PFA). Samples were blocked with PBS containing 4% bovine serum albumin (BSA), permeabilized in 0.5% Triton X-100, and incubated with the IE1 MAb and Alexa 488-conjugated rabbit anti-mouse (Fab′)2 fragments. Nuclei were counterstained with DAPI (4′-6′-diamidine-2-phenylindole; Sigma-Aldrich) for 5 min at room temperature. Slides were examined with a Leica DM6000B fluorescence microscope. Images were analyzed with Leica fluorescence workstation software (Leica FW4000).
Construction and generation of TB40/E ΔUL1 and AD169 UL1-HA viruses.
HCMV BAC mutagenesis was carried out by homologous recombination between a linear DNA PCR fragment and endotheliotropic HCMV-TB40/E-derived BAC4 (TB40/E BAC4) or the laboratory-adapted AD169 BAC (pHB5) in Escherichia coli. Mutagenesis was performed as previously described by Wagner et al. (58). TB40/E BAC4 (48) was used for the generation of UL1 deletion viruses, removing the complete UL1 gene, and pHB5 was used to generate recombinant AD169 with the UL1 HA epitope-tagged virus. To delete the UL1 sequence in TB40/E BAC4 and generate TB40/E BAC4 ΔUL1, the following oligonucleotides were used: 5′-GTG GTT TTT ATA GGT TAA CCA CTT ATG GTG TAA AGT AGG ATA TTC ATA GTT ATT GAA AAC CGG CCA GTG AAT TCG AGC TCG GTA CCC-3′ and 5′-TGA GAG CTA CAC AAA GTA GCA CAA ATT TAT CCT GAA TAA ACT AGC GTC GAT ACT TTG TAA GAC CAT GAT TAC GCC AAG CTC CC-3′. For the construction of the UL1 HA-tagged virus in pHB5, a forward primer containing the HA tag epitope sequence followed by a stop codon (5′-CGT TCC GGC AAC TCT GAG ACA CAA ACT ACG AAC TAG AAA CAA CGT AAA TCG CAT AGC GGC CTA CCC ATA CGA TGT TCC AGA TTA CGC TTA ACC AGT GAA TTC GAG CTC GGT AC-3′) and a reverse primer containing the homology region downstream of UL1 (without the stop codon) (5′-GAG AAC CGC ACG GAG TAA TCA AAT TTT ATC TTG GAT AAA TTA GTG TCG ATA CTT TGT AAG ACC ATG ATT ACG CCA AGC TCC-3′) were used. PCR fragments using plasmid pSLFRTKn as a template containing a kanamycin resistance (Kanr) gene (flanked by minimal FLP recombination target [FRT] sites) were inserted into TB40/E BAC4 or pHB5 by homologous recombination in E. coli (58). The TB40/E BAC4 ΔUL1 and pHB5 UL1-HA mutants were digested with XbaI, and the pattern of the restriction enzyme was compared to that of wild-type TB40/E BAC4 or wild-type pHB5. The presence of the Kanr gene was further confirmed by probing after Southern blotting with a kanamycin cassette-specific probe. The Kanr gene was excised from both viruses by FLP-mediated recombination (58), and correct mutagenesis was verified by restriction pattern analysis followed by Southern blotting and PCR analysis. Recombinant HCMV was reconstituted from HCMV BAC mutants by the Superfect transfection method as described previously (6). Sequencing to confirm that the appropriate mutagenesis process took place and that TB40/E ΔUL1 and the parental virus had not acquired additional mutations in the UL128 gene locus was performed with DNA extracted from infected cells after the reconstitution of the viruses.
Purification of HCMV particles and analysis of virion protease sensitivity.
HCMV particles were further purified by negative-positive glycerol-tartrate gradient centrifugation as previously described (54). The isolated tartrate gradient-purified AD169 virions were treated with proteinase K. Protease digestion was performed with 10 μg of proteinase K (Roche) per ml for 20 min at 37°C. Some virion samples were solubilized with sodium dodecyl sulfate (SDS), added to a final concentration of 0.5% (wt/vol). Virion preparations were subjected to SDS-PAGE and immunoblot analysis.
UL1 RNA analysis.
For Northern blot analysis, whole-cell RNA was isolated from mock-infected or infected MRC-5 cells at 8, 24, 48, and 72 h postinfection (p.i.) using QIAshredder columns and an RNeasy minikit (Qiagen, Hilden, Germany). For gel electrophoresis, 10 μg of each sample was used, and the preparations were separated on 1% agarose gels containing 2% formaldehyde. Northern blot analysis was carried out according to standard procedures. Hybridization and detection were performed by using the digoxigenin (DIG) system (Roche) according to the manufacturer's instructions. Briefly, UL1-specific DNA hybridization probes were labeled with digoxigenin-dUTPs using a DIG DNA labeling kit (Roche). Probes were prepared by PCR with UL1-specific primers (5′-GCG AAC TTT GTG GAT GGA ACG G-3′ and 5′-ACG TTA TTT CTA GTT CGT AGT-3′), using pHB5 as a template. Late-phase gene expression was inhibited by the use of phosphonoacetic acid (PAA; Sigma-Aldrich). A total of 250 μg/m1 of PAA was added to the medium at the time of infection and maintained throughout infection.
Antibodies.
Commercial MAbs specific for IE1 (B810R; Chemicon) and pp28 (ab6502; Abcam) were employed. gB-specific MAb 27-78 and MAb 58-15 (generously provided by William J. Britt, University of Alabama, Birmingham) were used for immunofluorescence microscopy and immunoblotting, respectively. Reagents specific for calnexin (catalog number 610524; BD Bioscience), GM-130 (catalog number 558712; BD Bioscience), TGN-46 (catalog number AHP500G; AbDseroTec), and ERGIC-53 (catalog number E6782; Sigma-Aldrich) were generously provided by Julia V. Blume (Center for Genomic Regulation, Barcelona, Spain). Rabbit anti-HA (catalog number H6908; Sigma-Aldrich) antibody was used to detect UL1-HA. Secondary antibodies used for immunofluorescence microscopy were Alexa Fluor 555 goat anti-mouse IgG(H+L) (catalog number A21422; Invitrogen) and Alexa Fluor 488 goat anti-rabbit IgG(H+L) (catalog number A11008; Invitrogen). Anti-mouse or anti-rabbit IgG horseradish peroxidase-labeled antibodies (Amersham Bioscience) were used for immunoblotting.
Immunofluorescence microscopy.
MRC-5 cells grown to 90% confluence in 13-mm-diameter coverslips were mock infected or infected with AD169 UL1-HA for 72 h. Coverslips were rinsed with PBS, and cells were then fixed for 20 min at RT in 4% paraformaldehyde freshly prepared in PBS. Cells were blocked with PBS containing 2% BSA for 20 min at RT and permeabilized with 0.5% Triton X-100 in PBS for 5 min. Coverslips were incubated for 1 h at RT with a primary antibody diluted in PBS containing 2% BSA, followed by 45 min of incubation with a fluorochrome-conjugated secondary antibody in PBS containing 2% BSA. Coverslips were mounted in Mowiol solution and analyzed under a confocal laser scanning microscope (Confocal SP2; Leica) at a magnification of ×40.
SDS-PAGE and immunoblotting.
Virus-infected cell proteins were obtained from AD169 UL1-HA-infected MRC-5 cells grown in 6-well tissue culture dishes. Cell lysates of mock-infected MRC-5 cells were used as controls. Cells were lysed under reducing conditions and heated to 100°C. Proteins were separated by gradient SDS-polyacrylamide gel electrophoresis (10 to 15% SDS-PAGE) and transferred onto nitrocellulose membranes that were blocked with PBS containing 0.05% Tween 20 and 3% BSA (or 5% milk powder). Antibodies were diluted in PBS containing 0.05% Tween 20. For the detection of primary antibody binding, horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG followed by enhanced chemiluminescence detection (West Pico Supersignal kit; Pierce, Rockford, IL) was used according to the manufacturer's instructions. The removal of N-linked oligosaccharides from cell lysates was carried out by using N-glycosidase F (PNGase F; Roche), according to the manufacturer's specifications. Briefly, the samples were denatured in glycoprotein-denaturing buffer at 100°C for 10 min and cooled to 4°C for 5 min. The samples were then digested for 4 h at 37°C with PNGase F before being analyzed by immunoblotting.
Viral growth kinetics.
MRC-5, RPE, and U373-MG cells seeded into 24-well plates were infected with TB40/E or TB40/E ΔUL1 at different MOIs (0.025 for MRC-5 cells, 0.1 and 5 for RPE cells, and 0.3 for U373-MG cells). After a 2-h adsorption period, the inoculums were removed, and infected monolayers were rinsed with PBS and incubated with DMEM supplemented with 3% FBS. At various times postinfection, the supernatants of the infected cells were harvested, cleared of cellular debris, and frozen at −70°C. In the case of U373-MG cells, the cell-associated viral progeny was measured. The quantification of infectious virus was determined by a fluorescence focus unit (FFU) assay, counting HCMV IE1-positive cells as indicated above. The number of infected cells (fluorescence focus) was determined by microscopy.
Sequence analysis and evolutionary studies.
Primate CMV genes and proteins were retrieved from the NCBI database (40), and human proteins were retrieved from the Ensembl Human database (28). NCBI accession numbers for the HCMV AD169 strain (13, 15) and chimpanzee cytomegalovirus (CCMV) are NC001347 and NC003521.1, respectively. Hidden Markov models (HMMs) corresponding to members of the Ig domain-containing family, including the V-set (variable), C1-set (constant-1), C2-set (constant-2), and I-set (intermediate) subsets, were downloaded from PFAM (23). A total of 1,314 Ig domain-containing human and HCMV proteins were obtained by using the program HMMsearch (19) with an E value cutoff of 10−3. The BLASTP program was used to search for putative human homologues of RL11 proteins using an E value cutoff of 0.1 (2). Sequence alignments of homologous Ig domain-containing HCMV proteins and cellular proteins were generated with the help of the CLUSTALW program (56). The number of amino acid substitutions per site (K) was calculated for orthologous pairs of HCMV and CCMV proteins by using the PHYLIP program Protdist with default parameters (22). N-glycosylation sites, signal sequences, and transmembrane domains were predicted by using NetNGlyc 1.0 (25), SignalP 3.0 (37), and TMHMM 2.0 (http://www.cbs.dtu.dk/services/), respectively.
RESULTS
Structure of pUL1.
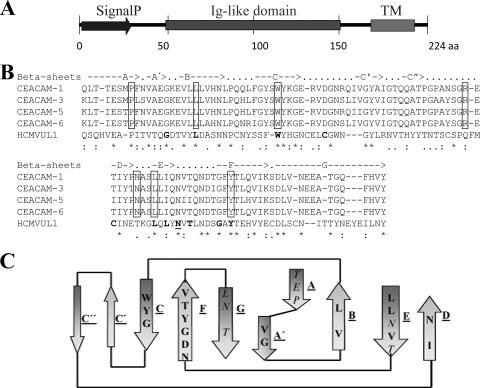
The UL1 gene is predicted to encode a 224-amino-acid type I transmembrane glycoprotein, comprising a signal peptide (amino acids 1 to 26), an Ig-like domain (amino acids 45 to 152) with 8 potential N-linked glycosylation sites, a transmembrane region (amino acids 184 to 206), and a short cytoplasmic tail (amino acids 207 to 224) without apparent signaling motifs (Fig. 1A). Since sequence similarity of pUL1 to pregnancy-specific glycoprotein 5 and other members of the human CEA family (27) was previously noted, we sought to explore this finding in more detail. We performed BLASTP searches of all RL11 gene family members against all human Ig-containing proteins. A significant sequence similarity (E value of <10−3) was found between the Ig-like domain in pUL1 and the N-terminal IgV-like domain in the CEA family members (shown for CEACAM-1, -3, -5, and -6 in Fig. 1B). As an example, the pUL1 Ig-like domain exhibits 61% amino acid similarity and 28% amino acid identity with the human N-terminal CEACAM-1 IgV domain (E value of 0.0004) (Fig. 1B). For the rest of the HCMV RL11 members, significant similarity to CEA proteins was not detected. The alignment of the pUL1 Ig-like domain and the three-dimensional crystal structure of the soluble ectodomain of the murine CEACAM-1a isoform (55) indicated that the formation of Ig-like β-sheets is conserved and that the conserved residues are mostly buried in the hydrophobic core, maintaining the Ig fold. CEACAM positions that appear to be critical for the formation of the typical β-sheets in IgV-like domains are present in pUL1 (Fig. 1C). Furthermore, multiple alignments of the Ig-like domain of pUL1 and the N-terminal IgV domain of several CEA proteins showed that a number of well-conserved residues in members of the CEA family are also present in pUL1, whereas they are absent from other RL11 proteins (Fig. 1B and data not shown).
Fig 1.
pUL1 shares key conserved residues with CEA proteins. (A) Structural predictions for HCMV pUL1. A schematic drawing of the 224-amino-acid UL1 protein is shown, with the signal peptide (SignalP) (amino acids 1 to 26), the Ig-like domain (amino acids 45 to 152), and the transmembrane region (TM) (amino acids 184 to 206) indicated. (B) Multiple-sequence alignment showing the sequence similarity between the HCMV UL1 protein Ig-like domain (amino acids 45 to 152) and the N-terminal IgV domains of four representative members of the CEA family, human CEACAM-1, -3, -5, and -6. Boldface type indicates highly conserved residues in the RL11 family, and the potential N-glycosylated residue in the RL11 family is underlined (14). Highly conserved residues in CEACAM-1 and other IgSF members that appear to be critical for the formation of the β-sheets in IgV-like domains are boxed (30). The arrows above the sequence alignment indicate the β-sheet structure of CEACAM-1a as determined by a previous crystal structure analysis (56). An asterisk indicates identical amino acids, a colon indicates conserved amino acids, and a period indicates less conserved amino acids. (C) Alignment of the pUL1 RL11D and the three-dimensional crystal structure of murine CEACAM-1a. Conserved residues are indicated on the β-sheets, boldface type indicates conserved buried residues, and italic type indicates conserved surface residues.
Origin and evolution of pUL1.
The pULl sequence is found exclusively in HCMV genomes but lacks a counterpart in other CMV lineages and betaherpesviruses; therefore, the origin and evolution of the UL1 gene was investigated. The RL11 gene family is a rapidly evolving and highly variable virus gene family, both in terms of gene members and in terms of sequence divergence across related viruses or strains (16). To better understand the origin and evolution of the RL11 family, we determined the number of amino acid substitutions per site (K) between RL11 HCMV-CCMV orthologous proteins and between pULl and RL11 HCMV paralogous proteins (Table 1). The estimated number of amino acid substitutions per site (K) between RL11 HCMV-CCMV orthologous proteins is, on average, 1.5, about 15 times higher than the average amino acid substitution rate of a prototypical well-conserved herpesvirus gene (i.e., the UL54 gene, encoding DNA polymerase). Among paralogs, the lowest K values are for the alignments of pULl with pTRL11, pTRL12 and pTRL13 (1.9 in the three cases). This, together with the fact that the UL1 gene is located just next to the TRL13 gene, suggests that pUL1 may have originated by duplication from an ancestor gene from the TRL cluster.
Table 1.
Amino acid substitutions per site in viral protein pairwise comparisonsa
| Orthologue of HCMV-CCMV | K (no. of aa substitutions/site) | Paralogue of UL1-HCMV RL11 | K (no. of aa substitutions/site) |
|---|---|---|---|
| RL5a | 3.5 | ||
| RL6 | 3.3 | ||
| TRL11 | 0.7 | TRL11 | 1.9 |
| TRL12 | 1.6 | TRL12 | 1.9 |
| TRL13 | 1.2 | TRL13 | 1.9 |
| UL4 | 2.5 | UL4 | 2.8 |
| UL6 | 1.2 | UL6 | 3.1 |
| UL7 | 1.3 | UL7 | 2.3 |
| UL9 | 2.0 | UL9 | 2.6 |
| UL10 | 2.3 | UL10 | 2.0 |
| UL11 | 1.0 | UL11 | 2.7 |
| UL54 | 0.1 |
aa, amino acid.
UL1 is transcribed with late gene kinetics.
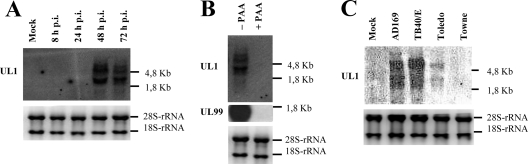
In the HCMV AD169 genome, UL1 extends from nucleotide 11835 to 12509 (675 bp). To analyze potential transcripts arising from the UL1 ORF, total RNA was isolated from MRC-5 cells at different times after infection with HCMV AD169 and subjected to Northern blot analysis using a UL1-specific probe. As shown in Fig. 2A, large UL1 transcripts, of approximately 5.8 kb and 3.8 kb, could be detected no earlier than 48 to 72 h p.i. (Fig. 2A). Interestingly, a similar pattern of large transcripts was also observed previously for other UL1-related genes, such as TRL10 (51) and TRL/IRL13 (61).
Fig 2.
Kinetics of UL1 gene expression. (A) Whole-cell RNA was isolated from uninfected (mock) MRC-5 cells or cells infected at an MOI of 2 with HCMV AD169 at the indicated hours postinfection. Equivalent amounts of RNA were subjected to agarose gel electrophoresis and subsequently analyzed by Northern blotting using an HCMV UL1-specific probe. (B) UL1 transcription is inhibited by PAA. Whole-cell RNA was isolated from HCMV AD169-infected cells at 72 h p.i., incubated with or without PAA, and processed as described above for panel A. In addition to the UL1-specific probe, RNA was hybridized with a probe specific for the late HCMV gene UL99. (C) UL1 transcripts in MRC-5 cells infected with different HCMV strains (AD169, TB40/E, Toledo, and Towne) for 72 h were analyzed as described above for panel A. The bottom panels show ethidium bromide staining of 28S rRNA (4.8-kb) and 18S rRNA (1.8-kb) species.
HCMV lytic gene expression is conventionally divided into three major kinetic classes of viral genes, immediate-early, early, and late. The transcription of UL1 RNA in AD169-infected cells was completely blocked by the viral DNA polymerase inhibitor phosphonoacetic acid (PAA), consistent with the classification of UL1 as a true late-phase gene, as previously described and reproduced here for UL99 (Fig. 2B) (31). The presence of UL1 transcripts in MRC-5 cells infected with a panel of HCMV strains, including laboratory strains AD169 and Toledo and endotheliotropic strain TB40/E, was studied. The Towne strain, previously reported to be UL1 defective due to a deletion in this ORF (47), was used as a negative control. Total RNAs from cells infected with HCMV TB40/E, AD169, Toledo, and Towne were isolated at 72 h p.i. and analyzed by Northern blotting using a UL1 (AD169)-specific probe. As shown in Fig. 2C, the two predominant 5.8-kb and 3.8-kb UL1 transcripts were detected in cells infected by HCMV TB40/E, AD169, and Toledo (Fig. 2C). The signal intensities of these two RNA species were relatively lower in Toledo-infected cells, and as expected, UL1 transcripts were undetectable upon infection with Towne.
Expression of the UL1 glycoprotein during HCMV replication.
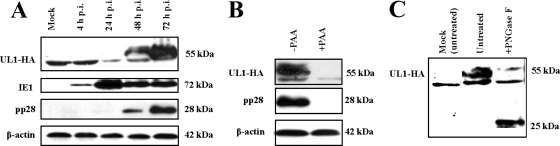
The 224-amino-acid-long UL1 polypeptide (molecular mass of 25.5 kDa) is predicted to be expressed as a type I glycoprotein with a signal peptide and a membrane anchor. We investigated the expression kinetics of the protein product of UL1 during productive HCMV infection. For this purpose, we generated an HCMV (AD169-derived HB5 background) recombinant virus with an HA tag fused to the UL1 C-terminal end. Reconstituted AD169 UL1-HA was used to infect MRC-5 cells, and the kinetics of pUL1 expression were monitored by immunoblot analysis using an anti-HA antibody in cell extracts harvested at different time points ranging from 4 to 72 h p.i. HCMV-specific MAbs that are reactive with the major immediate protein IE1 pp72 (UL123) and the pp28 (UL99) late phosphoprotein were used as controls. As shown in Fig. 3A, the anti-HA antibody showed two protein species, a faster-migrating nonspecific band, which was present in both HCMV-infected and mock-infected MRC-5 cells, and a band of ∼55 kDa, which was weakly detectable at 48 h p.i. but was abundantly found at 72 h p.i, coincident with the expression kinetics of pp28 (31). This 55-kDa HA-tagged UL1 band was absent in immunoblots of wild-type AD169-infected cells (data not shown), further confirming its specific detection. At time points after infection when signals for pUL1 were not observed, IE1 pp72 was present, as expected, already being expressed after 4 h p.i. and remaining for the duration of the HCMV replication cycle. Consistent with the observed kinetic pattern and the results obtained at the transcriptional level, a metabolic blockade with PAA resulted in the disappearance of the 55-kDa band (Fig. 3B), indicating that the UL1 protein is expressed late during HCMV replication.
Fig 3.
Expression of the UL1 glycoprotein in HCMV-infected fibroblasts. (A) Uninfected (mock) and AD169-infected MRC-5 cells at an MOI of 5 were harvested at the indicated times p.i. Equivalent amounts of cell lysates were subjected to SDS-PAGE and analyzed by immunoblotting with antibodies reactive with HA or the HCMV IE1 and pp28 proteins. β-Actin detection was used as a protein loading control. (B) Experiments were performed as described above for panel A to assess the effect of PAA treatment (+PAA) on late-phase protein expression. (C) Whole-cell lysates of AD169 UL1-HA-infected MRC-5 cells (72 h p.i.) were left untreated or digested with PNGase F. The preparations were subjected to immunoblot analysis using an anti-HA antibody.
Many of the HCMV structural glycoproteins form high-molecular-weight disulfide-linked oligomers, such as the gCI complex, composed of multidimers of gB (9, 39); therefore, we analyzed UL1 protein migration patterns under both nonreducing and reducing (with β-mercaptoethanol) conditions. The UL1 protein was β-mercaptoethanol resistant, implying that it is not part of a disulfide-linked oligomer in infected MRC-5 cells (data not shown). The high number of putative N-linked glycan sites (n = 9) present in pUL1 and the difference between the apparent molecular mass (55 kDa) observed for this protein by immunoblotting and the calculated molecular mass (25.5 kDa) of the 224-amino-acid polypeptide backbone suggested that pUL1 undergoes an extensive posttranslational glycosylation process. Indeed, digestion with PNGase F, which removes complex and high-mannose N-linked sugars, enhanced the migration of the specific band from 55 kDa to 25 kDa (Fig. 3C). These results confirmed that pUL1 is expressed as a glycoprotein.
Subcellular localization of pUL1 in HCMV-infected cells.
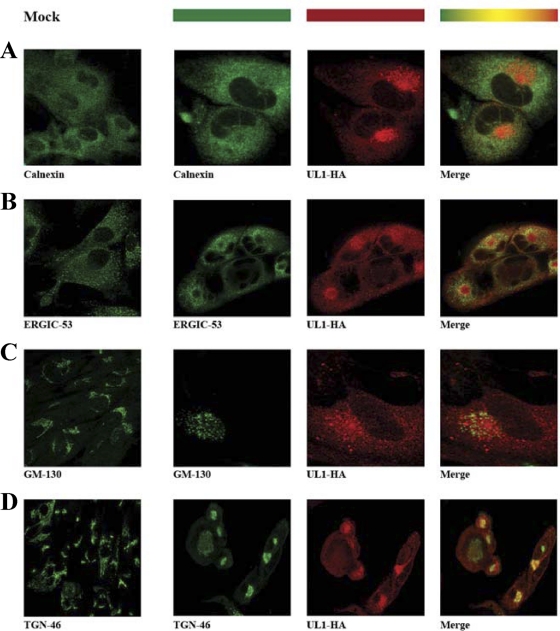
To analyze the localization of pUL1 in HCMV-infected cells, the HA epitope of the AD169 UL1-HA virus was employed to trace its subcellular distribution by confocal microscopy in comparison with a panel of molecular markers of the exocytic and endocytic pathways. In MRC-5 cells following fixation at 72 h p.i., pUL1-HA accumulated in a large cytoplasmic compartment (Fig. 4). This compartment was not detectable in uninfected and wild-type AD169-infected MRC-5 cells, thus confirming the specificity of the observed staining pattern (see Fig. S1 in the supplemental material). pUL1-containing structures failed to colocalize with several intracellular markers, including the endoplasmic reticulum (ER) marker calnexin (Fig. 4A) and ERGIC-53 (Fig. 4B), an integral protein localized in the ER-Golgi-intermediate compartment (ERGIC). The staining of GM-130, a cis-Golgi marker, appeared in a dotted ring forming structures in the proximity of pUL1 but did not exhibit a superimposing pattern indicative of colocalizing proteins (Fig. 4C). As observed previously by other investigators, alterations in the morphologies of the Golgi compartment and the ERGIC were seen during HCMV infection (45). During its final envelopment, HCMV acquires membranes containing endosomal and trans-Golgi network markers, while lysosomes and cis- and medial-Golgi markers are not present and surround the virus envelopment and assembly site (10). Markers for the endocytic pathway, such as the early endosomal marker EEA-1, and recycling endosomes (transferrin receptor) did not colocalize with pUL1 (data not shown). Furthermore, pUL1 colocalization with the lysosomal protein LAMP-1 was also ruled out (data not shown). However, a partial colocalization of pUL1-containing structures and the trans-Golgi marker TGN-46 (Fig. 4D) was observed.
Fig 4.
Subcellular localization of pUL1-HA in a cytoplasmic compartment. MRC-5 cells grown on glass coverslips were infected with AD169 UL1-HA at an MOI of 5 and fixed at 72 h p.i. Uninfected MRC-5 cells were processed similarly (mock) (left panel of each row). The various cellular compartments in the secretory system were stained with the following antibodies: calnexin for the ER (A), ERGIC-53 for the ERGIC (B), GM-130 for the Golgi apparatus (C), and TGN-46 for the trans-Golgi network (D). The cellular markers were detected with Alexa 488 goat anti-mouse IgG, and pUL1-HA was detected with Alexa 555 goat anti-rabbit IgG. Colocalization is indicated by a yellow signal in the merge channel.
During late stages of the infectious cycle, pUL1 did not colocalize with markers of the cis- or medial-Golgi compartment (ERGIC-53 and GM-130). To further confirm these results, infected cells were incubated with brefeldin A (BFA), and the distributions of UL1-HA-, GM-130-, and ERGIC-53-positive membranes were compared. BFA interferes with exocytic cellular transport, and the treatment resulted in the vesiculation of the ring-structured GM-130- and ERGIC-53-positive membranes, whereas such changes were not observed for the pUL1-HA-containing structure (data not shown). The envelopment of the infectious HCMV particles was proposed previously to take place within a juxtanuclear cytoplasmic site (44, 45), resembling the pUL1 distribution pattern. Altogether, these results suggest that pUL1 in infected cells, associated with the trans-Golgi apparatus, accumulates mainly in a juxtanuclear site argued to constitute a virus assembly compartment.
Colocalization of pUL1 with tegument and envelope HCMV proteins.
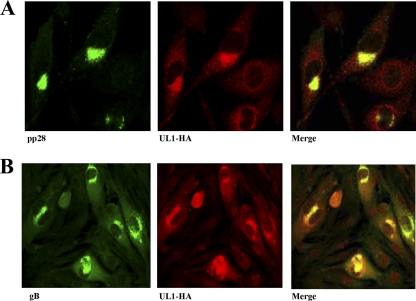
Based on the cytoplasmic compartmentalization of pUL1, we explored whether other structural HCMV proteins gathered at the pUL1-containing site. To this end, antibodies reactive with the tegument phosphoprotein pp28 and the major virion envelope glycoprotein gB (UL55) were employed. HCMV pp28 was reported previously to colocalize with other tegument and viral envelope proteins at the virus assembly site during the late phase of the infectious cycle (45, 46), and it is also acquired by the virion in the cytoplasm. Interestingly, pUL1 colocalized with pp28 (Fig. 5A) and gB (Fig. 5B), consistent with its presence at the site of viral assembly and final envelopment, thus suggesting that it might be integrated into the HCMV virion.
Fig 5.
Colocalization with tegument and envelope HCMV proteins. MRC-5 cells grown on glass coverslips were infected with AD169 UL1-HA at an MOI of 5 and fixed at 72 h p.i. Following fixation, samples were incubated with MAbs specific for the tegument protein pp28 and HA (A) or the envelope glycoprotein gB and HA (B). pp28 and gB were further detected with Alexa 488 goat anti-mouse IgG, and UL1-HA was further detected with Alexa 555 goat anti-rabbit IgG. Colocalization is indicated by a yellow signal in the merge channel.
pUL1 is a component of the HCMV virion.
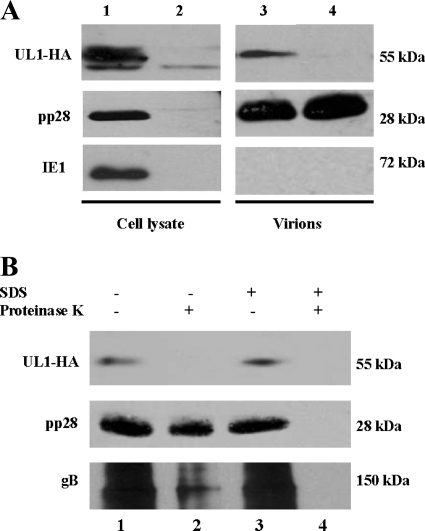
To explore whether pUL1 is a virion structural glycoprotein, we purified AD169 UL1-HA particles by negative-viscosity–positive-glycerol-tartrate gradient centrifugation and analyzed them by immunoblotting with the anti-HA antibody. This purification process facilitates the isolation of intact virions, separated from noninfectious enveloped particles (NIEPs) and dense bodies (DBs), and the removal of contaminating host cell debris. Purified wild-type AD169 virions were used as a negative control due to the absence of the HA epitope. In these experiments, the 55-kDa HA-tagged UL1-specific band observed in lysates of AD169 UL1-HA-infected cells was used as a positive control, the detection of which was reproduced both in lysates and in AD169 UL1-HA virions, in parallel with the pp28 structural protein but not IE1 pp72, a nonstructural protein present only in preparations of infected cells (Fig. 6A, lanes 1 and 3). As expected, the 55-kDa HA-tagged UL1 band was not detected in wild-type AD169 virions, while the tegument phosphoprotein was (Fig. 6A, lane 4). In order to define the virion incorporation of pUL1, additional experiments were performed. Previous studies have shown that extracellular virion envelope glycoproteins are sensitive to protease digestion, while capsid and tegument proteins are protected (4, 60). Thus, we analyzed the sensitivity of virion pUL1 to protease digestion. AD169 UL1-HA virions were treated with detergent (SDS) to disrupt the envelope and then digested with proteinase K (Fig. 6B). When samples were untreated, the tegument protein pp28 and the envelope glycoprotein gB were detectable (Fig. 6B, lane 1). Upon proteinase K digestion and in the absence of detergent, pp28 remained intact, whereas pUL1 was no longer detected, and the highly abundant gB (oligomeric form) was largely digested (Fig. 6B, lane 2). In samples treated with SDS only, both tegument and envelope proteins remained intact (Fig. 6B, lane 3). In contrast, when initially solubilized and then digested with proteinase K, pUL1, gB, and pp28 were no longer detected (Fig. 6B, lane 4). Altogether, these findings provided evidence that pUL1, in a manner similar to that of gB, is sensitive to protease treatment and likely exposed on the HCMV envelope.
Fig 6.
pUL1 is a structural glycoprotein that forms part of the HCMV envelope. (A) Analysis of pUL1 in cell lysates of AD169 UL1-HA-infected MRC-5 cells for 72 h (lane 1) and AD169 UL1-HA virions (lane 3). Mock-infected cells (lane 2) and wild-type AD169 virions (lane 4) were used as negative controls. Western blots were developed by using anti-HA, anti-pp28, or anti-IE1 antibodies. (B) AD169 UL1-HA virions were treated with SDS (lanes 3 and 4) or left untreated (lanes 1 and 2). One-half of each virus preparation was subsequently digested with proteinase K (lanes 2 and 4) or left undigested (lanes 1 and 3). The preparations were subjected to immunoblot analysis using anti-HA, anti-pp28, or anti-gB antibodies.
Deletion of UL1 results in HCMV growth defects in epithelial cells.
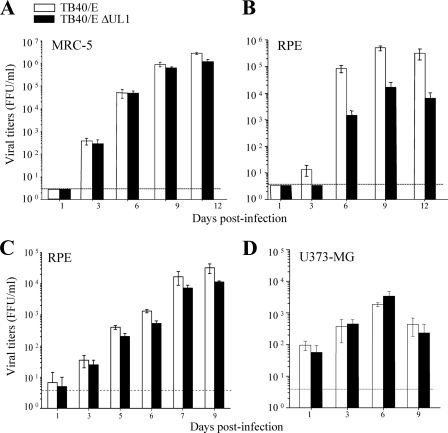
A number of the HCMV envelope glycoproteins have been shown to play a role in critical steps of the viral life cycle, i.e., in processes such as cell entry, assembly, and envelopment of virions or cell-to-cell spread. To assess the impact that the pUL1 envelope glycoprotein may have on the HCMV replication cycle, we constructed an HCMV mutant lacking the UL1 ORF using the TB40/E-derived BAC (48). Unlike the fibroblast-adapted AD169 strain, TB40 virions exhibit an intact tropism for endothelial as well as epithelial cells, the entry of which is mediated by the pentameric gH/gL/UL128/UL130/UL131 envelope complex, which is deficient in AD169 (43). The complete UL1 sequence was removed from the genome of endotheliotropic strain TB40/E, generating the recombinant virus TB40/E ΔUL1. We then infected human MRC-5 cells, retinal pigment epithelial (RPE) cells, and U373-MG cells, derived from glioblastomas, at a low MOI with TB40/E ΔUL1 or parental strain TB40/E. An MOI of 0.025 was used to infect the fully permissive fibroblasts, while RPE and U373-MG cells (which support HCMV replication to a lesser extent) were infected at MOIs of 0.1 and 0.3, respectively. The production of extracellular (MRC-5 and RPE cells) or cell-associated (U373-MG cells) virus was analyzed over the course of the replicative cycle. As shown in Fig. 7B, in RPE cells, the growth levels of the UL1-deficient virus were significantly reduced (up to 100-fold) in comparison to those obtained with the parental virus (P < 0.05 at the different time points). Consistent with this finding, TB40/E ΔUL1 produced smaller plaques than those obtained after TB40/E infection of this cell type (data not shown). In contrast, under these conditions of multistep growth, infections of MRC-5 and U373-MG cells demonstrated that TB40/E ΔUL1 and parental strain TB40/E grew with comparable kinetics (Fig. 7A and D). To examine whether the inefficient growth displayed by TB40/E ΔUL1 in RPE cells could also be observed when high viral doses are used, RPE cells were infected with TB40/E ΔUL1 or parental strain TB40/E at an MOI of 5. As shown in Fig. 7C, under these high-MOI conditions, the TB40/E ΔUL1 growth defect, although apparent, was substantially diminished (see the differences of between 2- and 3-fold obtained during TB40/E and TB40/E ΔUL1 infections at high MOIs versus the 1- to 2-log differences displayed at low MOIs). These results indicate that pUL1 is required for efficient HCMV growth in a cell type-specific manner.
Fig 7.
Growth kinetics of TB40/E ΔUL1 in different cell types. MRC-5 (A), RPE (B and C), and U373-MG (D) cells were infected with TB40/E or TB40/E ΔUL1 at MOIs of 0.025 (A), 0.1 (B), 5 (C), and 0.3 (D). On the designated days p.i., cultures were collected, and yields of extracellular (MRC-5 and RPE cells) or cell-associated (U373-MG cells) infectious virus present were determined by FFU assays. Each data point represents the average and standard deviation of data from three separate cultures. The dashed line indicates the limit of detection. The data are representative of three independent experiments.
DISCUSSION
Growing numbers of viral glycoproteins have been reported to be components of the HCMV envelope, including molecules conserved between members of the Herpesviridae family (gB, gH, gL, gM, and gN) and others that are HCMV specific (57) (e.g., gO [29], gpTRL10 [51], gpRL13 [53], UL4/gp48 [12], gpUL128 [59], gpUL130 [38], gpUL132 [52], US27 [24], and UL33 [34]). It is believed that genus-specific envelope glycoproteins contribute to the broad cell/tissue tropism of HCMV and its pathogenicity. In this study, we have characterized the HCMV UL1-encoded glycoprotein, transcribed in fibroblast-adapted strains AD169 and Toledo as well as in the endotheliotropic strain TB40/E. pUL1 is a PNGase F-sensitive glycoprotein, with a putative C-terminal transmembrane anchor domain, which is synthesized late during productive HCMV infection. In infected human fibroblasts, pUL1 is targeted to a cytoplasmic site together with several HCMV tegument and envelope proteins, consistent with its final destination at the previously described distinct compartment of virion assembly and final envelopment (reviewed in reference 8). Consequently, pUL1 can be incorporated into purified AD169 virions and becomes exposed in the HCMV envelope. Based on these results, we propose that pUL1 constitutes a novel HCMV structural envelope glycoprotein.
UL1 belongs to the highly variable and rapidly evolving RL11 gene family. The fact that UL1 is an HCMV-specific gene lacking a counterpart in other CMV lineages and betaherpesviruses prompted us to surmise that UL1 may have arisen from a gene duplication event, which likely occurred after the speciation of human and chimpanzee. Of note, two other HCMV RL11 family members, RL6 and RL5A, are not found in chimpanzee CMV or in the more distantly related rhesus macaque CMV and are thus likely to have been formed within the RL11 cluster as well in the last 5 to 6 million years. Based on evolutionary estimates of the RL11 family in primate CMVs, we propose that UL1 may have originated from a gene from the RL11-TRL cluster (TRL11, TRL12, or TRL13) and subsequently diverged at a high rate. This is supported by the K values found between UL1 and its closest homologues (other genes in the TRL cluster), which are higher than those typically observed between orthologous HCMV and CCMV genes. Indeed, a substantial heterogeneity of pUL1 among different HCMV strains has been appreciated (16, 47). Phylogenetic analyses of UL1 in a variety of clinical HCMV isolates allowed the differentiation of three distinct genotypes, with the existence of a considerable number (4/31; 12.9%) of isolates containing impaired versions of the UL1 sequence due to internal stop codon mutations (47). In addition, the fibroblast-adapted Towne strain, which has lost virulence and experienced substantial genome alterations during extensive in vitro passaging (41), bears a deletion in UL1 (47). The hypothesis that the UL1 polymorphisms existing in clinical isolates may have an effect on HCMV tissue tropism and pathogenesis remains to be substantiated in the future.
Both the transcriptional analysis of UL1 and the time course of pUL1 synthesis in HCMV-infected MRC-5 cells proved exclusive expression in the late phase of the replication cycle. The absence of UL1 transcription and translation after treatment with the potent viral DNA polymerase inhibitor PAA corroborated that UL1 belongs to the late class of CMV genes. In infected MRC-5 cells, pUL1 does not seem to be organized in higher-order disulfide-linked complexes. We show that gpUL1 acquires a molecular mass of 55 kDa. The glycoprotein is sensitive to PNGase F, producing a 25-kDa polypeptide. Based on these results, one could conclude that most if not all of the 9 potential N-glycosylation sites that pUL1 bears are modified by N-linked carbohydrates, with eight of them residing in the Ig-like domain and the remaining one residing in the 32-amino-acid stretch that connects the Ig-like domain to the transmembrane sequence. It must also be noted that in this pUL1 region expanding from amino acids 153 to 183, several potential O-glycosylation sites are present. After entering the secretory pathway, the UL1 glycoprotein exits the ER to accumulate in close vicinity to other components of the HCMV particle, such as the tegument protein pp28 and the envelope glycoprotein gB, at the juxtanuclear cytoplasmic site of virion assembly. The HCMV envelope is still incompletely defined (8). Based on previous mass spectrometric studies (57), at least 19 structural glycoproteins of AD169 virions could be identified. It is interesting to note that pUL1, among several other bona fide virion glycoproteins (51, 53), was not resolved by this approach. We find that after tartrate gradient viral particle purification, pUL1 can be detected as a virion constituent. Moreover, biochemical analyses of purified virions revealed the same features of pUL1 as those demonstrated for gB, allowing the assignment of pUL1 to the virion envelope.
pUL1 shares a striking similarity to the N-terminal IgV domain of cellular CEA family molecules that are involved in cellular adhesion by homotypic and heterotypic interactions with each other. The CEACAM subfamily includes inhibitory and activating receptors widely expressed in different cell types and tissues, and members of this family have been reported to be critical modulators of several physiological processes (32). Multiple alignments showed that a number of well-conserved residues found in several members of the CEA family are also present in pUL1. CEACAM positions that appear to be critical for the formation of the typical β-sheets in Ig-like domains are conserved as well. This sequence similarity raises the possibility that pUL1 acts as a decoy receptor mimicking host CEA proteins. Molecular mimicry is a strategy frequently employed by viruses to exploit or subvert host protein functions (20). As other members of the RL11 family, despite containing RL11-characteristic features and conserved residues of the IgSF in their Ig-like domains, do not exhibit a significant degree of similarity to CEA proteins (E values below cutoff levels of 0.1 in our BLASTP searches), we hypothesize that pUL1 may have evolved to mimic members of the CEA family by sequence convergence, gaining CEA-like features along the million years of virus-host coevolution. The molecular targets and the biological significance of the sequence similarity between pUL1 and members of the CEA family remain to be explored. Notably, a number of pathogens take advantage of the CEA extracellular domains to bind and infect their target cells; e.g., mouse CEACAM-1a is targeted by mouse hepatitis virus (18). Moreover, neisserial pathogens have been shown to use CEACAM-1 in bronchial epithelial cells to suppress Toll-like receptor 2 (TLR-2) signaling (50). TLR-2 has been described to sense HCMV entry through a physical interaction with gB and gH, which results in the activation of NF-κB and cellular inflammatory cytokine secretion (5). Thus, pUL1 might contribute to HCMV access to host cells by interacting with members of the CEA family.
UL1 is dispensable for HCMV growth in cell culture (42). Our results indicate, however, that pUL1 might be of significant relevance for HCMV to establish efficient infections in certain cell types. As a virion envelope glycoprotein, pUL1 has the potential to modulate HCMV host cell tropism. We demonstrate that an HCMV TB40/E mutant lacking UL1 displayed attenuated growth in RPE cells, which was not observed for MRC-5 or U373-MG cells. This differential growth phenotype is MOI dependent, being measurable although less apparent at high viral doses. Notably, an involvement in cell tropism was reported previously for other members of the RL11 glycoprotein family: a UL10 deletion mutant grew better than the parental virus in an epithelial cell line (17), and the RL13 envelope glycoprotein was shown to repress HCMV replication in certain cell types (53). The nature of the inefficient growth associated with the UL1 deficiency is at present unknown. A possibility is that the UL1 glycoprotein may have a role in entry or egress when replicating in epithelial cells, as represented by RPE cells. As indicated above, and in line with the homology of the UL1 glycoprotein with members of the CEACAM family, an attractive hypothesis to test is whether this viral protein could be interacting with a member of this family at the host cell surface. In addition, the possibility that pUL1 may represent a relevant target of humoral immune responses during HCMV infection should be considered. Future studies will be aimed at clarifying the function(s) of pUL1 during HCMV replication.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Spanish Ministry of Science and Innovation (SAF2010-22153 to M.L.-B. and SAF2008-00382 and SAF2011-25155 to A.A.), the Marie Curie Training Network, European Union (MRTN-CT-2005-019284), and the Carlos III Health Institute/European Regional Development Fund (ERDF) (ISCIII/FEDER; Red HERACLES). M.S. and E.M.-M. were supported by MRTN-CT-2005-019284.
We thank Albert Zimmermann and Vu Thuy Khanh Le for scientific advice, William J. Britt and Julia V. Blume for providing antibodies, C. Cothia for revising the alignment between pUL1 and murine CEACAM-1a, and Xavier Sanjuan and Gemma Heredia for technical support.
Footnotes
Published ahead of print 15 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Alba MM, Das R, Orengo CA, Kellam P. 2001. Genomewide function conservation and phylogeny in the Herpesviridae. Genome Res. 11:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atalay R, et al. 2002. Identification and expression of human cytomegalovirus transcription units coding for two distinct Fcgamma receptor homologs. J. Virol. 76:8596–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baldick CJ, Jr, Shenk T. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boehme KW, Guerrero M, Compton T. 2006. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177:7094–7102 [DOI] [PubMed] [Google Scholar]
- 6. Borst EM, Hahn G, Koszinowski UH, Messerle M. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320–8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulton IC, Gray-Owen SD. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229–236 [DOI] [PubMed] [Google Scholar]
- 8. Britt B. 2007. CMV maturation and egress, p 311–323 In Arvin A, et al. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom: [PubMed] [Google Scholar]
- 9. Britt WJ, Vugler LG. 1992. Oligomerization of the human cytomegalovirus major envelope glycoprotein complex gB (gp55-116). J. Virol. 66:6747–6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cepeda V, Esteban M, Fraile-Ramos A. 2010. Human cytomegalovirus final envelopment on membranes containing both trans-Golgi network and endosomal markers. Cell. Microbiol. 12:386–404 [DOI] [PubMed] [Google Scholar]
- 11. Cha TA, et al. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang CP, Vesole DH, Nelson J, Oldstone MB, Stinski MF. 1989. Identification and expression of a human cytomegalovirus early glycoprotein. J. Virol. 63:3330–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chee MS, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125–169 [DOI] [PubMed] [Google Scholar]
- 14. Davison AJ, et al. 2003. Homology between the human cytomegalovirus RL11 gene family and human adenovirus E3 genes. J. Gen. Virol. 84:657–663 [DOI] [PubMed] [Google Scholar]
- 15. Davison AJ, et al. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17–28 [DOI] [PubMed] [Google Scholar]
- 16. Dolan A, et al. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85:1301–1312 [DOI] [PubMed] [Google Scholar]
- 17. Dunn W, et al. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223–14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dveksler GS, et al. 1991. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J. Virol. 65:6881–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eddy SR. 1998. Profile hidden Markov models. Bioinformatics 14:755–763 [DOI] [PubMed] [Google Scholar]
- 20. Elde NC, Malik HS. 2009. The evolutionary conundrum of pathogen mimicry. Nat. Rev. Microbiol. 7:787–797 [DOI] [PubMed] [Google Scholar]
- 21. Engel P, et al. 2011. Human cytomegalovirus UL7, a homologue of the SLAM-family receptor CD229, impairs cytokine production. Immunol. Cell Biol. 89:753–766 [DOI] [PubMed] [Google Scholar]
- 22. Felsenstein J. 1989. Mathematics vs. evolution: mathematical evolutionary theory. Science 246:941–942 [DOI] [PubMed] [Google Scholar]
- 23. Finn RD, et al. 2010. The Pfam protein families database. Nucleic Acids Res. 38:D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraile-Ramos A, et al. 2002. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 3:218–232 [DOI] [PubMed] [Google Scholar]
- 25. Gupta R, Brunak S. 2002. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 310–322 [PubMed] [Google Scholar]
- 26. Hitomi S, et al. 1997. Human cytomegalovirus open reading frame UL11 encodes a highly polymorphic protein expressed on the infected cell surface. Arch. Virol. 142:1407–1427 [DOI] [PubMed] [Google Scholar]
- 27. Holzerlandt R, Orengo C, Kellam P, Alba MM. 2002. Identification of new herpesvirus gene homologs in the human genome. Genome Res. 12:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hubbard TJ, et al. 2007. Ensembl 2007. Nucleic Acids Res. 35:D610–D617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huber MT, Compton T. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191–8197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kammerer R, Zimmermann W. 2010. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kohler CP, et al. 1994. Use of recombinant virus to assess human cytomegalovirus early and late promoters in the context of the viral genome. J. Virol. 68:6589–6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuespert K, Pils S, Hauck CR. 2006. CEACAMs: their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 18:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lilley BN, Ploegh HL, Tirabassi RS. 2001. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J. Virol. 75:11218–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Margulies BJ, Browne H, Gibson W. 1996. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology 225:111–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mocarski ES, Courcelle CT. 2001. Cytomegaloviruses and their replication, p 2629–2673 In Knipe DM, et al. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 36. Murphy E, et al. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 100:14976–14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen H, Engelbrecht J, Brunak S, von Heijne G. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1–6 [DOI] [PubMed] [Google Scholar]
- 38. Patrone M, et al. 2005. Human cytomegalovirus UL130 protein promotes endothelial cell infection through a producer cell modification of the virion. J. Virol. 79:8361–8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pötzsch S, et al. 2011. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog. 7:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pruitt KD, Tatusova T, Klimke W, Maglott DR. 2009. NCBI reference sequences: current status, policy and new initiatives. Nucleic Acids Res. 37:D32–D36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quinnan GV, Jr, et al. 1984. Comparative virulence and immunogenicity of the Towne strain and a nonattenuated strain of cytomegalovirus. Ann. Intern. Med. 101:478–483 [DOI] [PubMed] [Google Scholar]
- 42. Ripalti A, Mocarski ES. 1991. The products of human cytomegalovirus genes UL1-UL7, including gp48, are dispensable for growth in cell culture, p 57–62 In Landini MP. (ed), Progress in cytomegalovirus research: proceedings of the Third International Cytomegalovirus Workshop. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 43. Ryckman BJ, et al. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 82:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanchez V, Angeletti PC, Engler JA, Britt WJ. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sanchez V, Greis KD, Sztul E, Britt WJ. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanchez V, Sztul E, Britt WJ. 2000. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-Golgi-intermediate compartment. J. Virol. 74:3842–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sekulin K, Gorzer I, Heiss-Czedik D, Puchhammer-Stockl E. 2007. Analysis of the variability of CMV strains in the RL11D domain of the RL11 multigene family. Virus Genes 35:577–583 [DOI] [PubMed] [Google Scholar]
- 48. Sinzger C, et al. 2008. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J. Gen. Virol. 89:359–368 [DOI] [PubMed] [Google Scholar]
- 49. Sinzger C, et al. 1999. Modification of human cytomegalovirus tropism through propagation in vitro is associated with changes in the viral genome. J. Gen. Virol. 80(Pt 11):2867–2877 [DOI] [PubMed] [Google Scholar]
- 50. Slevogt H, et al. 2008. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat. Immunol. 9:1270–1278 [DOI] [PubMed] [Google Scholar]
- 51. Spaderna S, Blessing H, Bogner E, Britt W, Mach M. 2002. Identification of glycoprotein gpTRL10 as a structural component of human cytomegalovirus. J. Virol. 76:1450–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spaderna S, et al. 2005. Deletion of gpUL132, a structural component of human cytomegalovirus, results in impaired virus replication in fibroblasts. J. Virol. 79:11837–11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stanton RJ, et al. 2010. Reconstruction of the complete human cytomegalovirus genome in a BAC reveals RL13 to be a potent inhibitor of replication. J. Clin. Invest. 120:3191–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Talbot P, Almeida JD. 1977. Human cytomegalovirus: purification of enveloped virions and dense bodies. J. Gen. Virol. 36:345–349 [DOI] [PubMed] [Google Scholar]
- 55. Tan K, et al. 2002. Crystal structure of murine sCEACAM1a[1,4]: a coronavirus receptor in the CEA family. EMBO J. 21:2076–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thompson JD, Gibson TJ, Higgins DG. 2002. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinformatics 2:2.3. [DOI] [PubMed] [Google Scholar]
- 57. Varnum SM, et al. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagner M, Gutermann A, Podlech J, Reddehase MJ, Koszinowski UH. 2002. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. U. S. A. 102:18153–18158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao F, Courtney RJ. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 66:2709–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu D, Smith GA, Enquist LW, Shenk T. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.