Abstract
We applied a custom tiled microarray to examine murine gammaherpesvirus 68 (MHV68) polyadenylated transcript expression in a time course of de novo infection of fibroblast cells and following phorbol ester-mediated reactivation from a latently infected B cell line. During de novo infection, all open reading frames (ORFs) were transcribed and clustered into four major temporal groups that were overlapping yet distinct from clusters based on the phorbol ester-stimulated B cell reactivation time course. High-density transcript analysis at 2-h intervals during de novo infection mapped gene boundaries with a 20-nucleotide resolution, including a previously undefined ORF73 transcript and the MHV68 ORF63 homolog of Kaposi's sarcoma-associated herpesvirus vNLRP1. ORF6 transcript initiation was mapped by tiled array and confirmed by 5′ rapid amplification of cDNA ends. The ∼1.3-kb region upstream of ORF6 was responsive to lytic infection and MHV68 RTA, identifying a novel RTA-responsive promoter. Transcription in intergenic regions consistent with the previously defined expressed genomic regions was detected during both types of productive infection. We conclude that the MHV68 transcriptome is dynamic and distinct during de novo fibroblast infection and upon phorbol ester-stimulated B cell reactivation, highlighting the need to evaluate further transcript structure and the context-dependent molecular events that govern viral gene expression during chronic infection.
INTRODUCTION
Murine gammaherpesvirus 68 (MHV68; also known as γHV68 or murid herpesvirus 4) infection of mice is a model pathogenesis system for gammaherpesviruses such as Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) and Epstein-Barr virus (EBV) (5, 20). The life cycles of these lymphotropic and transforming viruses in the host involve the initial transit across a mucosal barrier to gain access to and establish latency in a leukocyte reservoir, followed by intermittent reactivation and dissemination to the mucosal surfaces for spread to new hosts (32, 74, 77, 82).
Replication at the site of primary infection impacts MHV68 dissemination and latency establishment in secondary lymphoid tissues of mice. The absence of proteins essential for lytic replication, such as the viral transactivator ORF50/mRTA (61) and the ORF6/single-stranded DNA-binding protein (ssDBP) (49), or the inhibition of viral DNA replication by the administration of cidofovir (62) impairs the establishment of latency in the splenic B cell compartment. In addition, virus replication at the mucosa triggers the innate and adaptive immune responses critical for host control (6, 45, 72, 73). These responses might play a critical role in the recruitment and activation of target cells such as dendritic cells that precede dissemination to distal reservoirs, such as splenic B cells and peritoneal macrophages (5, 25). Thus, the lytic gene expression program in newly infected cells that are permissive for productive infection is an important aspect of pathogenesis.
Reactivation from latency is another critical mode of productive infection during chronic infection. As with KSHV and EBV, MHV68 relies on the host response to productive infection to maintain the virus-host détente. Dysfunction of immune control mechanisms during MHV68 infection leads to increased reactivation (6), recrudescence (37, 43, 88), and numerous pathologies, including arteritis, pneumonia, fibrosis, lymphoid hyperplasia, and increased mortality (5, 20). MHV68 is detected in multiple cell types during chronic infection, including fibroblasts, epithelial and endothelial cells, macrophages, and multiple B cell types (5, 10, 42, 58, 64, 89). B cells are the predominant latency reservoir; B cell activation and terminal differentiation to plasma cells are in vivo mechanisms for driving MHV68 reactivation from latency (51, 75).
As with KSHV and EBV, MHV68 latency in B cells and processes of reactivation are modeled using cell culture systems whereby reactivation is stimulated by mitogens, sodium butyrate, or phorbol esters. The MHV68 lytic gene cascade is dependent on the lytic transactivator RTA encoded by ORF50 (66, 91). RTA expression, in turn, is regulated by numerous mechanisms and signaling events (28–30, 50, 68). These signals, in combination with viral coactivators and cellular factors, overcome repressive factors and chromatin modification that likely flip the switch to a lytic gene expression cascade in a latent cell (7, 67, 76, 77). It is not known whether MHV68 gene expression during a reactivation event follows a pattern of gene expression similar to that of a newly infected cell that is permissive for productive infection.
MHV68 genes have been classified as immediate early (IE), early (E), or late (L) on the basis of kinetics of expression and sensitivity to drugs that block protein synthesis (early and late genes) and inhibit the viral DNA polymerase (late genes) (2, 18, 59). Traditional molecular approaches have identified alternative spliced gene products (3, 4, 12, 16, 29) and an alternate reading frame (19). More recently, deep sequencing and microarray studies have uncovered over a dozen microRNAs (miRNAs) embedded within the viral tRNA-like transcripts (69, 93) and extensive regions of transcription outside annotated open reading frame (ORF) regions (40). Clearly, further characterization of the MHV68 transcriptome during all the phases of chronic infection is required to define the full coding and regulatory potential of the genome. We designed and applied a tiled viral microarray consisting of ∼12,000 60-mer oligonucleotides to examine the transcriptome of MHV68 upon de novo fibroblast infection and following phorbol ester-stimulated reactivation from a B cell line. A bioinformatic analysis of the time course data revealed distinct transcriptional profiles for the two types of productive infection and also defined new transcript structures.
MATERIALS AND METHODS
Tissue culture and virus preparation.
NIH 3T3 fibroblasts, NIH 3T12 fibroblasts, and HEK 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 U of penicillin per ml, 100 mg of streptomycin per ml, 10% fetal calf serum (FCS), and 2 mM l-glutamine. A20 B cells and A20-HE2 MHV68-positive (22) B cells were maintained in RPMI medium supplemented with 100 U of penicillin per ml, 100 mg of streptomycin per ml, 10% FCS, 50 μM 2-mercaptoethanol, and, for the A20-HE2 cell line, 300 μg/ml hygromycin (InvivoGen, San Diego, CA). The bacterial artificial chromosome (BAC)-excised virus present in the A20-HE2 cells was generated by BAC-mediated recombination and contains a cytomegalovirus (CMV) promoter-driven hygromycin-enhanced green fluorescent protein (eGFP) fusion protein in the transgenic region from ORF27 to ORF29b (1, 22). γHV68/MHV68 WUMS (ATCC VR1465) was the wild-type (WT) virus. Virus passage and titer determination were performed as previously described (87).
Virus infection and reactivation.
For de novo infection gene expression studies, NIH 3T3 cells were infected with WT MHV68 at a multiplicity of infection (MOI) of 10.0 PFU/cell for 1 h in low volume. For reactivation studies, A20-HE2 cells were subcultured 1:3 in the absence of hygromycin and on the following day were resuspended at 1.5 × 106 cells per ml of conditioned medium supplemented with 20 ng 12-O-tetradecanoylphorbol-13-acetate (TPA) per ml.
RNA preparation.
For array analysis, total RNA was prepared using Qiagen RNeasy columns (Qiagen, Valencia, CA) and either left untreated or treated with 1.0 unit DNase per 5 μg RNA per the manufacturer's recommendations (Ambion Turbo DNA free; Applied Biosystems, Austin, TX). DNA contamination was assessed by quantitative PCR of RNA samples without reverse transcription (RT). RNA integrity was verified by an Agilent 2100 bioanalyzer (Agilent, Santa Clara, CA).
Tiled MHV68 microarray construction, cDNA labeling, and hybridization.
The custom MHV68 tiled arrays in both 8 by 15,000 (design 025537) and 4 by 44,000 (design 027295) formats were designed using the Agilent eArray software. Briefly, for both formats, a total of 11,940 60-mer oligonucleotides with 20-nucleotide (nt) spacing were generated on the basis of the MHV68/murid herpesvirus 4 genomic sequence (NCBI accession number NC_001826) (85) to enable triple probe coverage of every nucleotide of both strands of the virus. RNA (1 to 1.5 μg) was labeled with linear amplification using an Agilent Quick Amp labeling kit. Initial reverse transcription used an oligo(dT) promoter-primer that enabled the generation of Cy5- or Cy3-labeled cRNA by T7 RNA polymerase. Adenovirus spike-in controls were added to each labeling reaction to allow normalization per the two-color spike-in kit instructions (Agilent). The Cy5-labeled reference RNA derived from an independent infection of NIH 3T3 fibroblasts at 8 h postinfection (hpi) with WT MHV68 was generated, purified, fragmented, and then hybridized in parallel with the Cy3-labeled sample RNA at 65°C for 17 h. Microarrays were scanned with Agilent Scanner Control software (version 7.0), and hybridization signal intensities were quantified using Agilent Feature Extraction software (version 9.5).
Bioinformatic analysis.
For Integrated Genome Browser (IGB; version 6.4) data visualization, data were normalized on the basis of the data for the Agilent spike-in positive-control probes using a linear scale factor. The expression value of each probe was represented by the linear ratio of normalized sample signal versus normalized reference signal. Further, nonspecific background signals were removed from experiments by subtracting the probe expression values in the corresponding mock sample. The signal ratio for every 20-nucleotide region is the mean ratio of the three probes that contain that region. For the IGB output, the coding sequence (CDS) and mRNA tracks were created on the basis of the sequence with NCBI accession number NC_001826. Additional coordinates for the spliced transcript structures are as previously indicated for M2 (16), ORF50 (29), ORF57 (56), ORF72 (3, 4), ORF73 (4, 12), the noncoding expressed genomic regions (EGRs) (40), and tRNA-miRNA (69, 93).
To determine transcript abundance, we first verified that there is a linear response in spike-in control (Agilent) intensities as a function of their concentration. We calculated a scaling factor from the linear fit of the spike-ins versus their concentration and scaled all the probe intensities with this scaling factor for each array. To estimate the percentage of probes expressed, 1 standard deviation (SD) of all viral probes at 0 h NIH 3T3 fibroblast cell infection or at 0 h HE2 cell infection was used as the cutoff. Nonspecific background signals were removed by subtracting the log2 probe expression values in the control samples (uninfected fibroblasts and untreated HE2 cells).
For hierarchical clustering, the expression value of each ORF was calculated as the median of the background-subtracted log2 signal of all tiling probes enclosed inside the ORF and then clustered by Cluster (version 3.0) software (University of Tokyo, Human Genome Center) using uncentered correlation for the similarity metric calculation and complete linkage as the clustering method. Cluster data were visualized using Java TreeView software (version 1.1.4r3). To address concerns of 3′ bias due to poly(A)-dependent labeling, array data sets were also calculated using the log2 median probe value of the 3′ 300 nt of each ORF. In a hierarchical analysis, the array data at each time point that were calculated using the median log2-normalized value of the 3′ 300 nt of the ORF clustered with the array data set that was calculated by the median values of the full-length ORF. The log2 median signal of the full-length ORFs was used for all downstream analysis.
Silhouette analysis provides a graphical representation of the robustness of the clusters (71). A higher Silhouette score (Si) indicates a tighter grouping of the genes within each cluster and their separation from the other clusters. The average cluster Silhouette scores of three to six clusters for the de novo infection were 0.58 for 3, 0.56 for 4, 0.53 for 5, and 0.39 for 6 clusters, and for the TPA-stimulated B cell line, reactivation the scores were 0.69 for 3, 0.65 for 4, 0.64 for 5, and 0.61 for 6 clusters. In addition, the individual cluster scores and gene contents were examined. For the de novo infection, five clusters provided maximal resolution by isolating ORF75A as a single-gene cluster that we identify as an outlier, leading to four major temporal clusters, clusters I to IV. For the TPA-stimulated B cell line reactivation data set, five clusters provided maximal resolution while maintaining strong Silhouette scores for each individual cluster. The ORF7 single-gene cluster was identified as an outlier, leading to four major temporal clusters, clusters I to IV.
To elucidate the transcript boundaries, data sets were quantile normalized to remove the systematic differences, and then we utilized the segmentation algorithm implemented in the tilingArray package (34). The segment function in the tilingArray package fits a piecewise constant curve to estimate the change points in the array data. The segmented data were plotted along with the ORF annotation to visualize the transcript boundaries. The change point estimate often corresponded to transcript boundaries. A heat map was also generated for the nonsegmented data using the plotAlnogChrom function in the tilingArray package.
Northern blot and probe preparation.
Ten micrograms of total RNA was loaded per lane on a 1% agarose formaldehyde gel, and RNA Millenium Markers (Ambion, Austin, TX) were used for size estimation. After electrophoresis, RNA was transferred to a Hybond-XL membrane (GE Healthcare Life Sciences, Piscataway, NJ) through downward capillary transfer, and a UV source was used to cross-link the RNA to the membrane. Prehybridization and hybridization were performed at 68°C in ULTRAhyb hybridization buffer (Ambion). Single-stranded 32P-labeled RNA probes were generated from linearized plasmid complementary to bp 86,312 to 86,569 using Ambion's Maxiscript SP6/T7 kit. Unincorporated nucleotides were removed using Amicon centrifugal spin columns (Millipore, Bedford, MA). Membranes were exposed to a phosphor storage plate and scanned using a Storm 840 PhosphorImager (Molecular Dynamics, GE Healthcare).
RACE analysis.
Rapid amplification of cDNA ends (RACE)-ready cDNA was prepared using RNA from WT MHV68-infected NIH 3T3 cells at 4, 12, and 18 hpi for ORF73, 10 hpi for ORF63, and 6 hpi for ORF6 analysis using a GeneRacer kit (Invitrogen, Carlsbad, CA). Briefly, RNA adaptors were ligated to the 5′ end of decapped mRNA and then reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) and the GeneRacer oligo(dT) primer sequence 5′-GCTGTCAACGATACGCTACGTAACGGCATGACAGTG(T)24-3′. Next, for 5′ RACE of cDNA, PCR was performed using the following outer and inner GeneRacer primers and gene-specific primers for 5′ RACE: (i) ORF73 (5′-GATCGTCTGTCTCTCCTACATCTAAACC-3′), (ii) ORF73nested1 (5′-AGGAGGCATGGCTGCTGGTTTGTTGAAG-3′), (iii) ORF73nested2 (5′-CATCTGTACTTGCCAGCAGCTCATC-3′), (iv) ORF63 (5′-ACTTCTGTACCCGCGACTGTTGCTGAGA-3′), (v) ORF63nested (5′-TTCGTAGATTAGTGGTGGAAGGCGCTCC-3′), (vi) ORF6 (5′-AAGTACCTGCACCGGACCTTGTCGAGAC-3′), and (vii) ORF6nested (5′-TGGTTGAAGTGAAGTCGGTTTCGGACAG-3′). For 3′ RACE, PCR was performed using outer and inner GeneRacer primers and ORF63-specific PCR primer ORF63 (5′-ATGGCTCCACCCACAGACACGTCAATGA-3′). PCR products were electrophoresed on 1.0 to 1.5% agarose gels, and specific bands were excised and prepared for ligation using a Qiagen gel extraction kit (Qiagen, Valencia, CA). Products were cloned into pCR-Blunt II-TOPO plasmid (Invitrogen), and 9 to 12 clones were analyzed by DNA sequencing.
Reporter assay.
The putative regulatory region of ORF6 (genomic coordinates 9,894 to 11,218 bp) was amplified with primers (i) ORF6p-F (5′-GATCGAGCTCCTGCGCAGTAATTGGTGGGGA-3′) and (ii) ORF6p-R (5′-GATCCTCGAGCATGATGAGTGTCCAAAAGCAGAGAGG-3′) and cloned into a firefly luciferase reporter vector (Hsp70-Luc, courtesy of David Lukac) (55).
For transfection followed by infection assays, NIH 3T12 cells were seeded at 8.0 × 105 cells per 100-mm plate and were transfected with 5 to 10 μg reporter vectors ORF57p (courtesy of Samuel Speck) (53) and ORF6p or empty vector Hsp70-Luc 24 h later using lipid-based transfection (Superfect, Qiagen). pMaxGFP (Lonza, Rockland, ME) was used to monitor transfection efficiency by immunofluorescence microscopy (Olympus, Melville, NY). At 24 h posttransfection, the cells were seeded at 1.0 × 105 cells per well of 12-well plates. The next day, the cells were infected in triplicate at an MOI of 5. The cells were lysed in 1× Promega passive lysis buffer (Promega, Madison, WI) at 24 hpi, and luciferase activity was measured per the manufacturer's recommendations (Lumat LB9507; EG&G Berthold, Bad Wildbad, Germany).
For cotransfection experiments, HEK 293T cells were seeded at 1.25 × 105 cells per well into 24-well plates. Twenty-four hours later, the cells were transfected via calcium phosphate with luciferase reporter vectors ORF57p and ORF6p or empty vector Hsp70-Luc with filler pCMV-Tag2B. The lytic transactivator RTA expression plasmid pSG50 (66) was cotransfected into the cells at 5-fold excess of the reporters. pRLnull (Promega) was transfected for normalization of the data. At 48 h posttransfection, the cells were lysed in 1× Promega passive lysis buffer. Luciferase activity was measured using Promega Dual-Luciferase reagents.
Microarray data accession numbers.
Microarray transcript data have been deposited in the Gene Expression Omnibus (GEO) database under the series entry GSE35866. To view the transcript data, users need to install Integrated Genome Browser (IGB) software and then configure IGB to add Data Sources and set type QuickLoad and URL http://www.osa.sunysb.edu/bioinformatics/igb/SBU-MHV68/. Under data access, choose M_herpesvirus_May_2009 in pulldown menus.
RESULTS
Genome-wide analysis of gene expression during productive infection examined by overlapping tiled array.
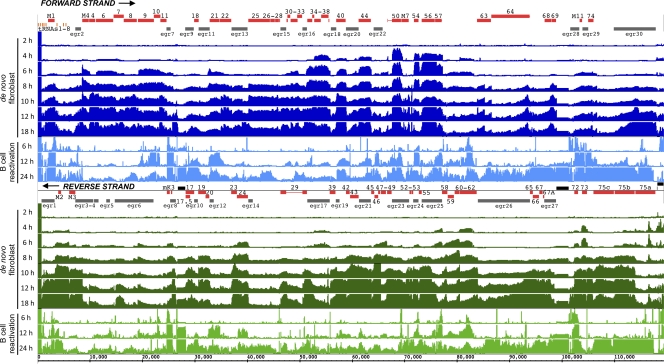
Understanding how virus gene expression is regulated during de novo fibroblast infection and upon reactivation from B cell latency is a critical aspect of herpesvirus pathogenesis. To examine the kinetics of gene expression and better define transcript structures, we designed a tiled viral microarray that has 11,940 overlapping 60-mer probes offset by 20-base intervals to provide strand-specific, triple coverage of the viral genome. RNA was prepared during a time course of productive infection in NIH 3T3 fibroblast cells at an MOI of 10.0 with WT MHV68 (WUMS strain) and during a reactivation time course of TPA-stimulated A20-HE2 B cells latently infected with a BAC-excised recombinant MHV68 encoding hygromycin-enhanced green fluorescent protein (22). RNA samples were Cy3 labeled in parallel with a Cy5-labeled reference RNA sample using a poly(A)-dependent linear amplification kit and hybridized to the custom Agilent array to enable the detection of strand-specific transcript signals. For de novo infection, 2-h intervals were chosen on the basis of previous gene expression analyses and single-step growth curves (2, 18, 44, 59). The last time point examined was 18 hpi since infectious particle production peaks at between 18 and 24 h after infection at a high MOI (44). After signal normalization using spike-in controls and background subtraction, the linear ratio of the sample signal to a reference RNA signal from infected cells was determined for each oligonucleotide on the array. The signal ratio for every 20 nucleotides is the mean ratio of the three staggered 60-mer oligonucleotides that encompass those 20 nucleotides. These normalized data for all 11,940 probes across the MHV68 genome were visualized in the Integrated Genome Browser software (version 6.4) (Fig. 1).
Fig 1.
Tiled array analysis reveals a distinct MHV68 transcript profile during productive fibroblast infection and upon TPA-stimulated viral reactivation from a latently infected B cell line. RNA was isolated from newly infected NIH 3T3 fibroblast cells at a multiplicity of infection of 10 PFU per cell (dark bars; de novo fibroblast) or from TPA-treated A20-HE2 B cells to induce reactivation of MHV68 (light bars; B cell reactivation) at the indicated time points and processed for hybridization to the tiled array containing 11,940 60-mer oligonucleotides (∼6,000 overlapping probes per strand with a 20-nt interval spacing). The expression value of each probe was represented by the linear ratio of normalized sample signal versus normalized reference signal. For Integrated Genome Browser (version 6.4) data visualization, each strand (forward strand or reverse strand), annotated ORFs (red bars), EGRs (gray bars), viral tRNAs (orange hatch marks), and repeats (black bars) were created on the basis of the sequence with NCBI accession number NC_001826 and reference 40. The exons of ORF29 and ORF50 are connected by thin horizontal lines. Underneath these annotations are bars that represent the mean signal ratio of the three probes that covered each 20-nt interval. De novo time course signal ratios are shown on a scale of from 0 to 3.0, and TPA-stimulated B cell reactivation signal ratios are on a scale of from 0 to 0.5.
Upon de novo infection of fibroblasts, transcription in ORFs that were previously classified as immediate-early genes, ORF50/mRTA, ORF57, and ORF73/mLANA, was readily apparent by 4 hpi (Fig. 1). As the infection progressed, signals increased in areas associated with annotated ORFs and in non-ORF-annotated regions defined by Johnson et al. (40) as EGRs. By 18 hpi, greater than 98% of the genome had a transcript signal intensity greater than 1 SD above the mean expression at 0 hpi.
The linear ratio of the transcript signals during the TPA-stimulated B cell reactivation time course compared to the reference RNA signal revealed a distinct transcription profile (Fig. 1). At the earliest time point examined during the TPA-stimulated B cell reactivation time course, transcription in ORF54/dUTPase and ORF72/v-cyclin exceeded that in ORF50/RTA, ORF57/vMAP, and ORF73/mLANA. We also examined the median of the log2-normalized probe signals for each ORF and EGR. By 24 h after TPA stimulation, greater than 97% of the median ORF signals exceeded 1 standard deviation of the untreated control A20-HE2 sample signal. The average of all the median ORF signals at 24 h after TPA stimulation in the A20-HE2 cells was ∼8-fold lower than the average of all the median ORF signals at 18 hpi in fibroblasts. Hence, in Fig. 1, a smaller scale is utilized for visualization of the HE2 TPA-stimulated reactivation data than for visualization of the de novo infection time course data. The average of all the median ORF signals exceeded the average of all the median EGR signals by approximately 5-fold between 6 and 18 hpi in the de novo fibroblast time course and 3.5-fold at 24 h in the TPA-stimulated B cell reactivation time course.
The expression kinetics for each genomic feature were also determined using the median log2-normalized signal and are illustrated as a heat map in genomic order in Fig. S1 in the supplemental material. Quantitative RT-PCR of select MHV68 regions representing IE, E, and L genes confirmed the trend in transcript accumulation based on the tiled array for both de novo fibroblast infection and TPA-stimulated B cell reactivation time courses (see Fig. S2 in the supplemental material). The fold increase in expression is also presented as bar graphs for select de novo fibroblast infection time points (see Fig. S3 in the supplemental material) and the TPA-stimulated B cell reactivation time course (see Fig. S4 in the supplemental material). Expression data were also calculated as the percentage of peak gene expression in Table 1 for the ORFs and in Table S1 in the supplemental material for the EGRs.
Table 1.
Kinetic classifications and boundary analysis of ORFs during productive infection
| ORF |
De novo fibroblasts |
TPA-stimulated reactivation A20-HE2 B cells |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % peak expression at the following times (h) postinfectiona: |
Kinetic classification |
Predicted transcript structured |
% peak expression at the following times (h) postinductiona: |
Kinetic arraye | ||||||||||||
| 2 | 4 | 6 | 8 | 10 | 12 | 18 | Arrayb | Previousc | Strand | 5′ boundary | 3′ boundary | 6 | 12 | 24 | ||
| M1-M1, secreted superantigen-like | 3.5 | 4.8 | 11.5 | 29.3 | 39.8 | 46.1 | 100.0 | IV | E-L | + | 1,960–1,980 | 3,340–3,360 | 10.2 | 71.5 | 100.0 | II |
| M2-M2, adaptor protein | 1.2 | 5.6 | 13.9 | 19.7 | 34.6 | 48.5 | 100.0 | II | E-L | − | 5,931–5,911 | 3,391–3,371 | 22.3 | 43.4 | 100.0 | III |
| M3-M3, chemokine-binding protein | 1.0 | 9.0 | 36.0 | 64.3 | 76.8 | 91.0 | 100.0 | II | E-L | − | 7,311–7,291 | 6,031–6,011 | 7.6 | 61.0 | 100.0 | II |
| M4-M4, secreted protein | 1.2 | 11.4 | 54.2 | 84.6 | 78.0 | 76.0 | 100.0 | II | IE | + | 8,520–8,540 | 9,840–9,860 | 21.2 | 73.4 | 100.0 | II |
| ORF4, complement control protein | 1.5 | 2.0 | 7.4 | 32.9 | 67.2 | 70.0 | 100.0 | III | E-L | + | 9,880–9,900 | NDf | 56.0 | 65.9 | 100.0 | IV |
| ORF6, single-stranded DNA-binding protein | 1.9 | 12.1 | 38.7 | 53.6 | 71.5 | 75.3 | 100.0 | II | E-L | + | 11,180–11,200 | 14,900–14,920 | 35.2 | 72.8 | 100.0 | III |
| ORF7, DNA-packaging terminase subunit 2 | 9.3 | 12.8 | 23.5 | 36.1 | 45.3 | 49.8 | 100.0 | IV | E-L | + | ND | ND | 81.5 | 84.1 | 100.0 | ND |
| ORF8, envelope glycoprotein B | 1.9 | 2.3 | 13.4 | 49.2 | 78.0 | 83.0 | 100.0 | III | L | + | 16,460–16,480 | 19,120–19,140 | 11.9 | 37.1 | 100.0 | III |
| ORF9, DNA polymerase catalytic subunit | 5.4 | 13.7 | 54.3 | 61.3 | 59.2 | 59.0 | 100.0 | II | E, E-L | + | 19,220–19,240 | ND | 53.2 | 100.0 | 88.0 | I |
| ORF10, protein G10 | 2.3 | 8.5 | 42.1 | 61.5 | 62.9 | 65.5 | 100.0 | II | E-L | + | ND | ND | 24.2 | 100.0 | 62.7 | I |
| ORF11, virion protein G11 | 4.0 | 7.3 | 28.6 | 54.9 | 66.5 | 67.4 | 100.0 | II | L | + | 23,420–23,440 | 24,680–24,700 | 25.1 | 68.4 | 100.0 | II |
| ORF12, mK3, E3 ubiquitin ligase MIR1 | 2.4 | 10.4 | 39.8 | 62.2 | 56.5 | 58.4 | 100.0 | II | IE, L | − | 25,831–25,811 | 24,711–24,691 | 6.4 | 95.3 | 100.0 | II |
| ORF17, capsid maturation protease | 1.8 | 2.1 | 10.6 | 44.7 | 75.1 | 76.2 | 100.0 | III | L | − | 29,991–29,971 | 28,271–28,251 | 11.8 | 40.5 | 100.0 | III |
| ORF17.5, capsid scaffold protein | 2.2 | 2.4 | 17.8 | 71.9 | 91.8 | 99.8 | 100.0 | III | ND | − | ND | 28,271–28,251 | 8.9 | 33.4 | 100.0 | III |
| ORF18, protein UL79 | 1.0 | 1.9 | 23.3 | 43.4 | 49.3 | 52.1 | 100.0 | II | L | + | ND | 30,760–30,780 | 2.3 | 24.2 | 100.0 | III |
| ORF19, DNA-packaging tegument protein UL25 | 3.2 | 3.4 | 16.3 | 69.8 | 99.8 | 95.7 | 100.0 | III | L | − | ND | 30,711–30,691 | 13.4 | 47.1 | 100.0 | III |
| ORF20, nuclear protein UL24 | 2.4 | 2.9 | 14.3 | 62.9 | 93.2 | 93.9 | 100.0 | III | E-L | − | 32,971–32,951 | ND | 41.1 | 96.3 | 100.0 | II |
| ORF21, thymidine kinase | 1.8 | 5.6 | 45.8 | 81.2 | 92.3 | 86.1 | 100.0 | II | E-L | + | 32,860–32,880 | ND | 7.2 | 100.0 | 81.7 | II |
| ORF22, envelope glycoprotein H | 0.9 | 3.6 | 25.3 | 70.6 | 88.1 | 94.2 | 100.0 | II | L | + | ND | 37,000–37,020 | 5.8 | 52.2 | 100.0 | II |
| ORF23, protein UL88 | 0.9 | 1.3 | 10.7 | 35.7 | 58.2 | 68.2 | 100.0 | III | L | − | ND | 37,011–36,991 | 9.3 | 35.5 | 100.0 | III |
| ORF24, virion protein UL87 | 2.7 | 4.1 | 37.5 | 54.5 | 49.3 | 53.7 | 100.0 | II | E | − | ND | ND | 33.1 | 100.0 | 99.1 | II |
| ORF25, major capsid protein | 1.1 | 1.4 | 16.3 | 61.6 | 91.3 | 76.7 | 100.0 | III | E-L | + | 40,240–40,260 | ND | 19.9 | 36.2 | 100.0 | IV |
| ORF26, capsid triplex subunit 2 | 0.9 | 1.2 | 15.9 | 65.4 | 89.8 | 89.7 | 100.0 | III | L | + | ND | ND | 5.5 | 23.6 | 100.0 | III |
| ORF27, envelope glycoprotein 48 | 0.9 | 1.2 | 20.3 | 73.5 | 94.2 | 97.7 | 100.0 | III | L | + | ND | ND | 7.2 | 31.5 | 100.0 | III |
| ORF28, envelope glycoprotein 150 | 0.2 | 0.4 | 20.9 | 81.0 | 97.4 | 100.0 | 99.8 | III | L | + | ND | 46,380–46,400 | 4.6 | 17.9 | 100.0 | III |
| ORF29, DNA-packaging terminase subunit 1 | 1.0 | 1.7 | 8.7 | 28.3 | 38.0 | 51.7 | 100.0 | III | L | − | ND (exon 1)/ 47,531–47,511 (exon 2) | 50,571–50,551 (exon 1)/-46,411–46,391 (exon 2) | 39.5 | 55.3 | 100.0 | IV |
| ORF30, protein UL91 | 1.4 | 3.5 | 22.1 | 52.8 | 75.9 | 80.6 | 100.0 | II | E-L | + | ND | ND | 19.2 | 100.0 | 61.8 | II |
| ORF31, protein UL92 | 1.0 | 3.5 | 28.1 | 66.4 | 80.8 | 89.0 | 100.0 | II | E-L | + | ND | 48,300–48,320 | 15.8 | 100.0 | 54.0 | I |
| ORF32, DNA-packaging tegument protein UL17 | 1.8 | 5.0 | 27.2 | 43.8 | 56.2 | 59.5 | 100.0 | II | E-L | + | 48,300–48,320 | ND | 26.9 | 64.3 | 100.0 | III |
| ORF33, tegument protein UL16 | 1.4 | 2.2 | 17.7 | 53.2 | 74.8 | 75.3 | 100.0 | III | L | + | ND | 50,880–50,900 | 8.6 | 22.8 | 100.0 | IV |
| ORF34, protein UL95 | 3.3 | 10.4 | 59.0 | 64.1 | 58.2 | 61.3 | 100.0 | II | E-L | + | 51,460–51,480 | ND | 58.1 | 95.8 | 100.0 | II |
| ORF35, tegument protein UL14 | 1.2 | 6.2 | 44.8 | 64.8 | 66.8 | 66.7 | 100.0 | II | E-L | + | ND | ND | 57.8 | 77.6 | 100.0 | III |
| ORF36, tegument serine/threonine protein kinase | 1.1 | 40.1 | 75.5 | 78.0 | 77.9 | 81.8 | 100.0 | I | E | + | 52,820–52,840 | ND | 27.4 | 66.8 | 100.0 | III |
| ORF37, SOX, shutoff, and exonuclease | 0.6 | 44.8 | 73.1 | 76.8 | 72.0 | 82.3 | 100.0 | I | E-L | + | ND | ND | 6.4 | 77.1 | 100.0 | II |
| ORF38, myristylated tegument protein | 0.6 | 37.5 | 58.8 | 79.2 | 82.3 | 95.3 | 100.0 | I | IE | + | ND | 55,780–55,800 | 5.6 | 35.5 | 100.0 | II |
| ORF39, envelope glycoprotein M | 1.1 | 1.5 | 10.0 | 55.6 | 79.3 | 91.2 | 100.0 | III | L | − | 57,051–57,031 | 55,791–55,771 | 3.6 | 15.5 | 100.0 | III |
| ORF40, helicase/primase subunit | 0.7 | 9.9 | 24.8 | 32.2 | 38.2 | 50.6 | 100.0 | II | E-L | + | 56,960–56,980 | 58,840–58,860 | 12.0 | 71.7 | 100.0 | II |
| ORF42, tegument protein UL7 | 0.9 | 3.9 | 18.8 | 51.0 | 68.0 | 74.7 | 100.0 | II | L | − | ND | 58,871–58,851 | 8.5 | 61.8 | 100.0 | II |
| ORF43, capsid portal protein | 0.4 | 0.7 | 6.4 | 29.2 | 49.4 | 58.0 | 100.0 | III | E-L | − | ND | ND | 13.4 | 35.0 | 100.0 | III |
| ORF44, helicase/primase helicase subunit | 1.5 | 28.3 | 66.4 | 65.7 | 55.2 | 66.6 | 100.0 | I | E-L | + | 61,260–61,280 | 63,620–63,640 | 21.0 | 96.4 | 100.0 | II |
| ORF45, tegument protein G45 | 0.9 | 2.7 | 13.6 | 50.8 | 71.2 | 86.3 | 100.0 | II | L | − | ND | 63,651–63,631 | 4.3 | 22.6 | 100.0 | III |
| ORF46, uracil-DNA glycosylase | 0.4 | 2.1 | 10.7 | 26.9 | 53.3 | 65.6 | 100.0 | II | L | − | ND | ND | 7.8 | 39.0 | 100.0 | III |
| ORF47, envelope glycoprotein L | 0.5 | 2.0 | 9.0 | 18.3 | 35.2 | 48.8 | 100.0 | II | E-L | − | ND | ND | 13.5 | 30.3 | 100.0 | IV |
| ORF48, tegument protein G48 | 1.0 | 14.1 | 67.5 | 84.8 | 74.7 | 78.4 | 100.0 | II | L | − | 66,591–66,571 | ND | 9.3 | 100.0 | 98.6 | II |
| ORF49, protein G49 | 0.4 | 5.1 | 10.7 | 28.2 | 40.8 | 54.6 | 100.0 | II | E-L | − | 67,731–67,711 | ND | 21.2 | 58.3 | 100.0 | III |
| ORF50, RTA, viral transactivator | 3.4 | 70.6 | 72.2 | 68.7 | 60.8 | 79.8 | 100.0 | I | IE | + | 66,720–66,740 (exon 1)/67,640–67,660 (exon 2) | 66,800–66,820 (exon 1)/69,440–69,460 (exon 2) | 28.0 | 75.8 | 100.0 | II |
| ORF51, M7, envelope glycoprotein gp150 | 9.3 | 10.8 | 12.2 | 32.0 | 48.0 | 55.0 | 100.0 | IV | L | + | ND | 70,920–70,940 | 36.8 | 50.8 | 100.0 | IV |
| ORF52, virion protein G52 | 1.0 | 3.2 | 10.9 | 52.1 | 79.8 | 94.5 | 100.0 | II | L | − | ND | 70,951–70,931 | 2.4 | 10.1 | 100.0 | III |
| ORF53, envelope glycoprotein N | 0.6 | 0.7 | 6.5 | 50.3 | 81.4 | 91.5 | 100.0 | III | L | − | ND | ND | 0.9 | 9.6 | 100.0 | III |
| ORF54, deoxyuridine triphosphatase | 1.1 | 51.0 | 100.0 | 80.4 | 43.7 | 47.4 | 35.4 | I | E | + | 71,780–71,800 | 72,740–72,760 | 14.8 | 100.0 | 60.3 | I |
| ORF55, tegument protein UL51 | 1.1 | 1.4 | 8.5 | 39.9 | 60.3 | 64.7 | 100.0 | III | E-L | − | 73,351–73,331 | ND | 6.5 | 26.4 | 100.0 | III |
| ORF56, helicase/primase primase subunit | 3.4 | 29.8 | 64.1 | 41.5 | 35.2 | 47.2 | 100.0 | I | E-L | + | 73,220–73,240 | ND | 61.6 | 82.9 | 100.0 | III |
| ORF57, multifunctional expression regulator and vMAP | 1.4 | 45.5 | 75.0 | 66.2 | 46.7 | 69.9 | 100.0 | I | IE | + | 75, 980–76,000 (exon 2) | 75,880–75,900 (exon 1)/77,160–77, 180 (exon 2) | 6.3 | 100.0 | 87.7 | II |
| ORF58, envelope protein UL43 | 0.8 | 10.7 | 48.2 | 78.4 | 79.7 | 90.6 | 100.0 | II | L | − | ND | 77,191–77,171 | 5.5 | 52.8 | 100.0 | II |
| ORF59, DNA polymerase processivity subunit | 1.8 | 16.7 | 81.8 | 96.1 | 82.5 | 88.6 | 100.0 | II | L | − | ND | ND | 6.1 | 85.5 | 100.0 | II |
| ORF60, ribonucleotide reductase subunit 2 | 0.7 | 6.4 | 69.1 | 91.8 | 73.6 | 68.9 | 100.0 | II | E-L | − | 80,471–80,451 | ND | 19.9 | 80.6 | 100.0 | II |
| ORF61, ribonucleotide reductase subunit 1 | 1.4 | 44.2 | 65.9 | 73.1 | 71.1 | 91.5 | 100.0 | I | L | − | 82,911–82,891 | 80,491–80,471 | 18.5 | 100.0 | 81.7 | I |
| ORF62, capsid triplex subunit 1 | 1.0 | 1.5 | 5.7 | 38.3 | 67.5 | 80.9 | 100.0 | III | L | − | 83,971–83,951 | ND | 48.7 | 69.4 | 100.0 | III |
| ORF63, tegument protein UL37 | 15.9 | 17.8 | 30.0 | 65.2 | 83.8 | 83.6 | 100.0 | III | E | + | 83,760–83,780 | 86,540–86,560 | 42.1 | 58.0 | 100.0 | IV |
| ORF64, large tegument protein | 14.3 | 16.5 | 22.5 | 46.2 | 63.5 | 69.6 | 100.0 | IV | E | + | 86,580–86,600 | 93,840–93,860 | 36.8 | 54.9 | 100.0 | IV |
| ORF65, M9, small capsid protein | 1.2 | 1.7 | 7.9 | 46.6 | 72.6 | 90.8 | 100.0 | III | L | − | ND | 93,851–93,831 | 2.1 | 9.0 | 100.0 | IV |
| ORF66, protein UL49 | 1.2 | 1.6 | 7.7 | 42.0 | 68.4 | 85.9 | 100.0 | III | L | − | ND | ND | 3.2 | 11.7 | 100.0 | III |
| ORF67, nuclear egress type 2 membrane protein | 1.0 | 1.2 | 5.2 | 35.2 | 62.9 | 79.7 | 100.0 | III | L | − | ND | ND | 3.6 | 18.3 | 100.0 | III |
| ORF67A, DNA-packaging protein UL33 | 0.3 | 0.4 | 3.4 | 33.3 | 63.3 | 79.5 | 100.0 | III | ND | − | ND | ND | 1.9 | 6.2 | 100.0 | IV |
| ORF68, DNA-packaging protein UL32 | 6.8 | 8.6 | 24.3 | 90.7 | 96.3 | 100.0 | 64.7 | III | E | + | ND | ND | 8.2 | 19.3 | 100.0 | IV |
| ORF69, nuclear egress lamina protein | 1.0 | 1.7 | 13.4 | 54.1 | 79.6 | 80.2 | 100.0 | III | L | + | ND | 98,940–98,960 | 3.8 | 30.1 | 100.0 | III |
| ORF72, v-cyclin | 0.6 | 7.2 | 16.3 | 20.4 | 25.4 | 41.4 | 100.0 | II | E-L | − | 103,271–103,251 (ORF exon) | 102,431–102,411 | 13.4 | 100.0 | 50.6 | I |
| M11, v-bcl-2 | 7.6 | 13.7 | 65.9 | 100.0 | 88.7 | 93.2 | 85.4 | II | E-L | + | ND | ND | 11.1 | 100.0 | 68.7 | I |
| ORF73, mLANA | 2.5 | 34.1 | 44.5 | 33.4 | 39.9 | 51.8 | 100.0 | I | IE | − | 104,891–104,871 (ORF exon), 105,011–104,911 | 103,771–103,751 | 29.8 | 51.8 | 100.0 | III |
| ORF74, v-GPCR | 1.2 | 3.9 | 29.5 | 49.8 | 53.5 | 59.9 | 100.0 | II | E-L | + | 104,720–104,740 | 105,800–105,820, 106,620–106,640 | 9.9 | 69.6 | 100.0 | II |
| ORF75C, tegument protein G75C | 1.5 | 2.5 | 6.3 | 38.8 | 74.9 | 69.5 | 100.0 | III | L | − | 110,031–110,011 | 105,831–105,811 | 4.9 | 15.2 | 100.0 | IV |
| ORF75B, protein G75B | 3.2 | 9.7 | 17.4 | 40.3 | 64.5 | 58.4 | 100.0 | II | E | − | ND | ND | 8.3 | 13.0 | 100.0 | IV |
| ORF75A protein G75A | 35.3 | 58.1 | 66.1 | 61.1 | 59.0 | 67.5 | 100.0 | ND | IE, E-L | − | ND | ND | 18.5 | 23.3 | 100.0 | IV |
Values represent the percentage of peak expression based on the median normalized, background-subtracted log2 probe value of each ORF.
Classifications based on heat map clusters determined in Fig. 2.
Classification based on previous studies that are used for color codes of ORF names in Fig. 2 and 3 (2, 18, 40, 59).
Consistent with transcripts previously reported: M2 (16); M3 (2, 84); ORF6, ORF8, ORF11, ORF12, ORF51, ORF54, and ORF57 (35); ORF50 (29, 53); ORF57 (56); ORF72 (3, 4); ORF73 (4, 12); ORF74 (86); and ORF52 and ORF67 (56).
Classifications based on heat map clusters determined in Fig. 3.
ND, not determined.
Hierarchical analysis of gene expression during de novo infection of fibroblasts.
Herpesvirus gene expression during a productive infection typically follows a temporal cascade whereby genes can be classified as IE, E, and L. A formal designation as an IE gene requires transcript insensitivity to the inhibition of protein synthesis. E genes are dependent on IE gene expression but insensitive to the inhibition of viral DNA replication. L genes are dependent on viral DNA replication for robust expression. This classical analysis of MHV68 gene expression has been applied in previous array studies of productive infections (2, 18, 40, 59). However, a kinetic clustering of viral genes in a protracted and naturally progressing time course experiment using a tiled array has not been described.
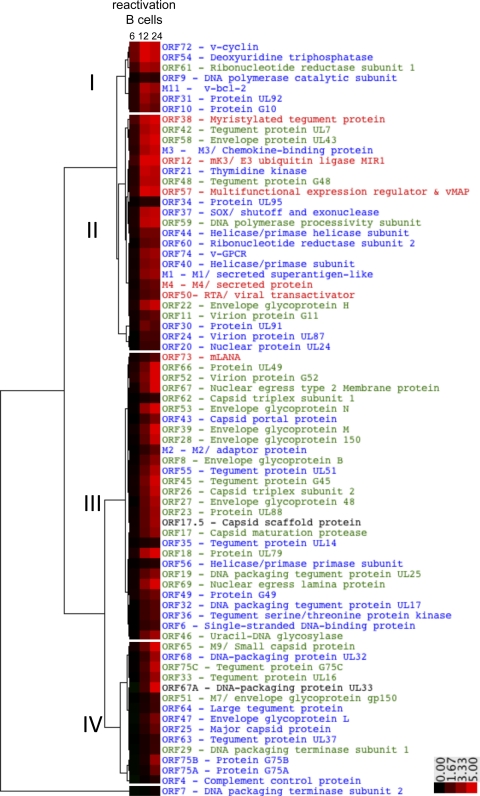
To this end, we performed a hierarchical analysis of the median log2-normalized signals of the probes that encompass an annotated ORF at each time point using uncentered correlation and complete linkage. The dendrogram output in Fig. 2 illustrates major branch points. The cluster designations were based on an analysis of optimal Silhouette scores within and across the clusters (data not shown) (71). While the TPA-stimulated B cell reactivation analysis is described in more detail below, we included the heat map for each ORF at 6, 12, and 24 h after TPA stimulation to aid comparison between de novo fibroblast and B cell reactivation transcription profiles (Fig. 2).
Fig 2.
Temporal cascade of lytic gene expression upon de novo infection of NIH 3T3 fibroblast cells differs from that from TPA-stimulated reactivation from latency in B cells. The median log2-normalized probe signal within an ORF at each time point (2, 4, 6, 8, 10, 12, and 18 hpi) was determined, and hierarchical cluster analysis was performed using the uncentered correlation for the similarity metric calculation and complete linkage for the clustering method in the Cluster program (version 3.0). The clustering was visualized in TreeView (version 1.1.4r3) and revealed four major clusters of gene expression during de novo infection. The average Silhouette score for the five clusters was 0.53, and individual cluster scores were 0.75 (I), 0.33 (II), 0.72 (III), 0.59 (IV), and 1.0 (single-gene outlier ORF75A). ORFs 30, 40, 45, 48, 52, 59, and 72 had the lowest Silhouette scores of cluster II genes. The transcript abundance at each time point for each ORF is represented as a box in the heat map. The black-to-red scale indicates an increase in transcript abundance. The color-coded text labels reflect classifications of genes as IE (red), E/E-L (blue), or L (green), based on prior de novo productive infection arrays described elsewhere (2, 18, 40, 59). As a point of comparison, the unclustered heat map in the right panel represents the kinetics of gene expression at 6, 12, and 24 h after TPA stimulation in the B cell reactivation time course for each ORF in the cluster analysis of the de novo infection time course.
As seen in the left panel of the heat map of Fig. 2 and as summarized in Table 1, MHV68 genes clustered into four major groups (de novo clusters I, II, III, and IV), based on their pattern of expression over the de novo infection time course. The expression kinetics of representative genes from each cluster are provided in Fig. S5 in the supplemental material. Transcripts in de novo cluster I had rapid expression kinetics and were detected by 4 hpi. Cluster I comprised the previously characterized IE genes encoding the lytic gene transactivators ORF50/RTA (66, 91) and ORF73/mLANA (21, 65) and the ORF57 gene, which encodes both a multifunctional expression regulator, ORF57, and a mitochondrial antiapoptotic protein, vMAP (19). Cluster I also included genes encoding factors for nucleotide metabolism and DNA replication (ORF64/ribonucleotide reductase subunit 1 and ORF44 and ORF56/helicase-primase subunits). Several de novo cluster I genes that were transcribed by 4 hpi encode host modulatory molecules and are classified as E or E-L genes (ORF36/protein kinase, ORF37/vSOX, and ORF54/deoxyuridine triphosphatase [dUTPase]). ORF75A was identified as an outlier from cluster I on the basis of the small change in expression over the time course and the grouping of ORF75A as a single-gene cluster in the Silhouette analysis of the hierarchical cluster (Fig. 2). Cluster II genes had intermediate expression kinetics, rapidly increasing between 4 and 6 hpi. This cluster encodes proteins involved in DNA replication (ORF60/RNR subunit, ORF21/thymidine kinase, ORF9/DNA polymerase, and ORF6/ssDBP), modulation of cellular processes (M11/v-bcl-2 and ORF74/vGPCR), secreted proteins (M3 and M4), and tegument (ORF42 and ORF75B). Within cluster II, ORFs 30, 40, 45, 48, 52, 59, and 72 were the most dissimilar on the basis of the Silhouette analysis (data not shown). Cluster III genes were delayed, transcribed by 6 to 8 hpi, and were largely comprised of capsid, tegument, and glycoprotein genes. Cluster IV genes were expressed with slow kinetics at low levels during infection and included those for M1/superantigen-like protein, ORF7/DNA-packaging terminase subunit, ORF51/gp150, and ORF64/large tegument protein.
The consensus classifications of MHV68 genes based on prior expression studies using chemical inhibitors of protein synthesis and viral DNA replication are indicated by the color-coded text labels in Fig. 2. We next examined for general trends and specific differences between these previous classifications and the clustering based on the hierarchical analysis. IE genes were found in both rapid cluster I genes and intermediate cluster II genes. Two genes classified as IE genes on the basis of their transcription in the presence of cycloheximide (ORF12/13 M and M4) did not cluster as class I genes since their expression was not detected until 6 hpi, with a peak in expression occurring between 6 and 12 hpi. E genes were found in all cluster groups yet tended to be rapid cluster I or intermediate cluster II genes. Genes designated E-L or L were identified as intermediate cluster II genes and comprised the majority of delayed cluster III. Taken together, transcript classification based on the kinetics of gene expression in a naturally progressing infection by tiled array is similar to yet distinct from previous classifications based on the sensitivity to chemical treatments that block replication processes dependent on protein synthesis and DNA replication (2, 18, 40, 59).
Hierarchical analysis of TPA-stimulated reactivation from a latent B cell line.
The phorbol ester TPA is a widely used stimulus for reactivation of gammaherpesviruses from latency and has been demonstrated to lead to AP-1-dependent upregulation of KSHV RTA via the protein kinase C (PKC) pathway (9). We examined transcript changes in the A20-HE2 cell line, a TPA-inducible, latently infected B cell line that has both a low incidence of spontaneous reactivation and a rapid induction response, based on <1% ORF59-positive cells and <50 PFU/ml of virus production in untreated cells compared to greater than 50% ORF59-positive cells and 1,000 PFU/ml of virus production upon TPA stimulation (22). Intervals of 6, 12, and 24 h after TPA stimulation were examined for the changes in gene expression in the course of reactivation, based on analysis of transcript, protein, and infectious particle production (22).
In the untreated A20-HE2 cells, signal reaching +2 SDs of the uninfected A20 B cell signal was detected only in the repeats, near the termini, and in the EGR7 region opposite ORF12 (data not shown). Signals detected at 6 to 24 h were determined as log2-normalized signals, calculated by subtracting the uninduced A20-HE2 transcript signals, and represented newly generated transcripts. All median ORF signals except those for ORF4 and ORF7 exceeded +2 SDs of the unstimulated latent A20-HE2 0-h signal by 24 h. Quantitative RT-PCR confirmed the general trend in transcript abundance across several regions of the genome, including ORF50/mRTA, ORF73/mLANA, M11/v-bcl-2, and ORF26, at 24 h after TPA stimulation (see Fig. S2B in the supplemental material). The average of all the 24-h median ORF signals following TPA stimulation of reactivation was ∼8-fold lower than the average of all the 18-h median ORF signals in the de novo infection time course. A deceased viral transcript signal is consistent with a lower percentage of the A20-HE2 B cells than fibroblast cells supporting productive infection (∼50%), based on viral antigen detection and also the several-log-magnitude decreased output of infectious particles in reactivating B cell cultures compared to fibroblast infections (22).
The kinetics of gene expression were analyzed for each ORF at 6, 12, and 24 h after TPA stimulation of A20-HE2 cells. Hierarchical cluster analysis of the transcripts detected upon TPA-stimulated B cell reactivation revealed four major clusters of genes (Fig. 3), with representative genes shown in Fig. S5B in the supplemental material. In general, transcripts in B cell reactivation cluster I had rapid expression kinetics, peaking at 12 h, while those in cluster II were intermediate in kinetics and expressed at similar levels at both 12 and 24 h postreactivation. Transcription in cluster III genes was delayed, detected at lower levels than cluster II genes12 h after TPA stimulation, and increased in expression between 12 and 24 h. Cluster IV genes had the slowest kinetic profile, consistent for genes that encode the structural components of the virion. ORF7 was an outlier; the signal in ORF7 was not detectable above the uninfected B cell background. ORF27 and ORF65 had the lowest Silhouette scores of cluster II and IV genes, respectively, in the Silhouette analysis (data not shown).
Fig 3.
Temporal cascade of lytic gene expression upon TPA-stimulated reactivation from latent B cells. The median log2-normalized probe signal within an ORF at each time point (6, 12, and 24 h after TPA stimulation) was determined, and hierarchical cluster analysis was performed using the uncentered correlation for the similarity metric calculation and complete linkage for the clustering method in the Cluster program (version 3.0). The clustering was visualized in TreeView (version 1.1.4r3) and revealed four major clusters of gene expression during reactivation induced by TPA stimulation of the latent HE2 B cell line. The average Silhouette score for the five clusters was 0.64, and individual cluster scores were 0.63 (I), 0.60 (II), 0.73 (III), 0.58 (IV), and 1.0 (single-gene outlier ORF7). ORF27 and ORF65 had the lowest Silhouette scores of cluster II and IV genes, respectively. The transcript abundance at each time point for each ORF is represented as a box in the heat map. The black-to-red scale indicates increasing transcript abundance. The color-coded text labels reflect classifications of genes as IE (red), E/E-L (blue), or L (green), based on prior de novo productive infection arrays described elsewhere (2, 18, 40, 59).
Differences in ORF heat map patterns between the de novo fibroblast infection and TPA-stimulated B cell reactivation time courses that were apparent in Fig. 2 were confirmed by comparing the hierarchical clusters of the two productive infections. B cell reactivation cluster I genes in Fig. 3 included genes from multiple de novo clusters identified in Fig. 2. These include genes involved in DNA synthesis (ORF9/DNA polymerase, ORF61/ribonucleotide reductase, and ORF54/dUTPase), in addition to viral modulators of cell survival (M11/v-bcl-2) and cell growth (ORF72/v-cyclin). ORF54 and ORF72 were especially notable for being the most highly induced transcripts 6 h after TPA stimulation (see Fig. S4 in the supplemental material). Other B cell reactivation cluster I genes included ORF31 and ORF10, which are expressed as intermediate cluster II genes upon de novo infection of fibroblasts. Genes that comprised cluster I during de novo infection (Fig. 2) had distinct kinetic profiles upon TPA-stimulated reactivation from B cell latency (Fig. 3). Unlike de novo infection, ORF57/vMAP, ORF50/RTA, and ORF37/vSOX transcripts had intermediate class II kinetics, while ORF36/protein kinase and ORF73/mLANA transcript levels were lower and delayed, leading to a B cell reactivation cluster III designation.
The majority of genes (16 of 23) comprising TPA-induced B cell reactivation class II also clustered together as class II genes during de novo infection. The TPA-induced B cell reactivation class III and class IV genes demonstrated a gradient in expression that spiked dramatically between 12 and 24 h and comprised genes that predominantly overlapped with de novo clusters II and III. ORF38, ORF67A, and ORF53 were among the highest expressed during both TPA-induced B cell reactivation and de novo infection, yet ORF75A was expressed at higher levels at 24 h upon TPA stimulated-reactivation compared to any de novo infection time point (see Fig. S3 and S4 in the supplemental material). We observed a steady increase in transcript signal within most of the EGR boundaries over the de novo and TPA-induced B cell reactivation time courses, with the exception of the signals for EGR5, EGR15, and EGR22, which peaked at 8 to 10 hpi during de novo infection, and EGR7 and EGR12, which peaked 12 h after TPA-induced B cell reactivation (see Table S1 in the supplemental material).
We also analyzed whether the expression profiles might be more comparable at particular time points. Quantile normalized log2 median ORF data sets from the de novo and TPA-stimulated B cell reactivation time course experiments did not cluster together by arrays at any time point in a hierarchical analysis (data not shown). Next, all possible time point comparisons were analyzed by linear regression. While the correlation coefficient did not exceed 0.60 in pairwise comparisons of time points between the two time course experiments, linear regression analysis indicates that the A20-HE2 cell 6-h time point weakly correlates with an early de novo time point (for NIH 3T3, 4 hpi; r2 = 0.55) and the A20-HE2 cell 24-h time point weakly correlates with late de novo time points (NIH 3T3, 10 to 18 hpi; r2 = 0.56). This suggests that the profiles of gene expression between de novo infection of fibroblasts and TPA-stimulated reactivation from the A20-HE2 B cell line are related and have some commonalities, as might be expected in a comparison of two types of productive infections.
Novel transcript boundaries identified by tiled array.
The tiled array design provides triple probe coverage of nearly every nucleotide in the genome to ensure redundant and robust coverage for kinetic analysis of ORF expression. Since, the 60-mer oligonucleotides overlap by 20 nt, we examined if transcript boundaries could be mapped to within the 20-nt resolution of this design. To elucidate the transcript boundaries, we utilized the segmentation algorithm implemented in the tilingArray package (34). The segment function in the tilingArray package fits a piecewise constant curve to estimate the change points in the array data. We combined this bioinformatic transcript boundary analysis with visualization of the absolute transcript signal and signal ratios in the Integrated Genome Browser to predict start and stop sites for each ORF. This approach enabled the confirmation of exons previously reported for spliced ORF50/mRTA (29, 53) and ORF57 (56) transcripts during both de novo fibroblast infection and TPA-stimulated B cell reactivation (see Fig. S6 in the supplemental material). The coordinates of 55 ORFs are provided within the 20-nt window resolution of the array in Table 1. As noted in Table 1, the tiled array boundaries are in good agreement with those for ORFs that have previously been mapped using RNase protection assays, Northern blot hybridization, cDNA cloning, and RACE analysis (2–4, 11, 12, 29, 35, 56, 84, 86).
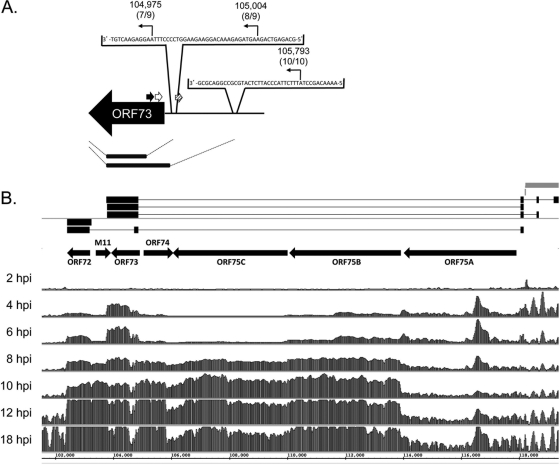
ORF72/v-cyclin has been mapped as a spliced transcript that initiates in the right terminal repeat with an exon that includes a portion of ORF73 (4). In addition, ORF72/v-cyclin is also detected as an unspliced transcript during lytic infection (3). In the tiled array, transcription of the major ORF72 exon initiates between bp 103,271 and 103,251 and terminates between bp 102,431 and 102,411 (Fig. 4 and Table 1). This is within 20 bp of the previously mapped 5′ boundary of bp 103,258 to 103,238 based on 5′ RACE and primer extension analysis and 3′ termination at bp 102,412 by 3′RACE (3).
Fig 4.
Tiled array identifies a novel ORF73 transcript and distinct transcript profiles of genes at the right end of the genome during de novo infection. (A) Boundary analysis of ORF73. Arrows indicate positions of 5′ RACE gene-specific primers; black, outer primer; white, inner primer 1; hatched, inner primer 2. Sequencing results mapped the 5′ transcriptional start sites to bp 104,975 for 7/9 clones at 18 hpi of NIH 3T3 fibroblasts, bp 105,004 for 8/9 clones at 12 hpi, and bp 105,793 for 10/10 clones at 4 hpi. (B) Tiled array bar graphs. For each time point indicated, bars represent the mean linear signal ratio (sample/reference RNA) of the three probes that covered each 20-nt interval on the reverse strand of the ORF72-ORF75A genomic regions at the indicated time points for de novo-infected fibroblasts. Gray bars indicate terminal repeats at the right end of the genome. Connected horizontal black boxes are previously reported spliced structures for ORF73 and ORF72 (3, 4, 12). Arrows indicate the orientation of the ORFs.
The tiled array signal in the ORF73/mLANA locus was strongest in the region directly overlapping the annotated open reading frame (Fig. 4B). This is especially evident at early time points in productively infected fibroblasts. However, at later times during infection, we detected a minus-strand signal upstream of ORF73/mLANA predicted by the array to initiate at bp 105,771 to 105,791. These data suggested (i) the presence of a novel minus-strand transcript contiguous with ORF73/mLANA and overlapping with ORF74/v-GPCR encoded on the positive strand or (ii) the presence of a large 5′ noncoding region that extends the ORF73/mLANA transcript during productive MHV68 infection and that has not been previously described. To define transcripts in the ORF73/mLANA locus and validate the array data, we performed nested 5′ RACE using an oligonucleotide anchored in the ORF73/mLANA coding sequence for round 1 amplification, followed by oligonucleotides directed against the ORF73/mLANA coding sequence or sequence directly upstream of ORF73 for round 2. With this approach we detected three separate transcript initiation points: bp 104,975, 105,004, and 105,793 (Fig. 4A). These data support the array-predicted ORF73 transcript and are consistent with a previous study that detected, but did not map, an ORF73 unspliced transcript initiating well upstream of the ORF using RNase protection assays and RT-PCR (12). Overall, these data indicate that ORF72 and ORF73 genes have multiple regulatory regions that lead to distinct transcript structures and expression.
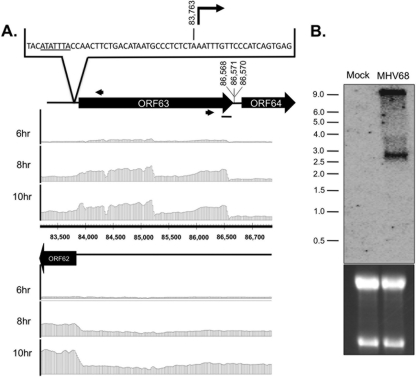
KSHV ORF63 was recently identified to be an inhibitor of inflammasome activation (31). MHV68 ORF63 has 21.6% amino acid identity to KSHV ORF63/vNLR1, but its role in inflammasome activation has not been investigated. Discontinuity of the array signal across the ORF suggested that there might be two smaller transcripts within ORF63 of MHV68 (Fig. 5A). While one shorter transcript ending at bp 85,643 (7/9 clones) was detected by 3′ RACE using a primer anchored at bp 85,097 (data not shown), we were unable to isolate a RACE clone ending at the lower signal boundary. RACE analysis identified 5′ transcription starts at bp 83,763 (8/8 clones), approximately 33 bp downstream from a TATA box element, and a major 3′ transcript boundary at bp 86,568 (8/10) (Fig. 5A). In agreement with RACE analysis, an approximate 2.8-kb transcript was detected by strand-specific Northern analysis in infected RNA at 10 hpi (Fig. 5B). Thus, a full-length MHV68 ORF63 protein is likely expressed during infection.
Fig 5.
Characterization of ORF63 transcript structure. (A) Boundary analysis of ORF63. Tiled array bar graphs representing the transcript signal ratio for each strand of the ORF62-ORF64 genomic regions (bp 83,200 to 86,950) are provided at the indicated time points. Black arrows indicate positions of 5′ and 3′ RACE gene-specific primers. Sequencing results mapped the 5′ transcriptional start sites to bp 83,763 (n = 8) at 10 hpi of fibroblasts. Underlined nucleotides represent a TATA box element located (33 bp) upstream of the transcriptional start. Sequencing results for the 3′ transcript end mapped to bp 86,568 (n = 8), 86,570 (n = 1), and 86,571 (n = 1) at 10 hpi of fibroblasts. (B) Northern blot analysis of ORF63 region with strand-specific RNA probe (black bar in panel A) identifies RNA of ∼2.8 kb at 10 hpi of fibroblasts. ORF63 transcript in MHV68-infected cells, consistent with the full-length transcript predicted by tiled array and RACE analysis. Numbers to the left indicate the kilobase sizes of the RNA ladder. 28S and 18S rRNA bands visualized in an ethidium bromide-stained polyacrylamide gel demonstrate equal loading.
Prediction and validation of the regulatory region of a lytic gene.
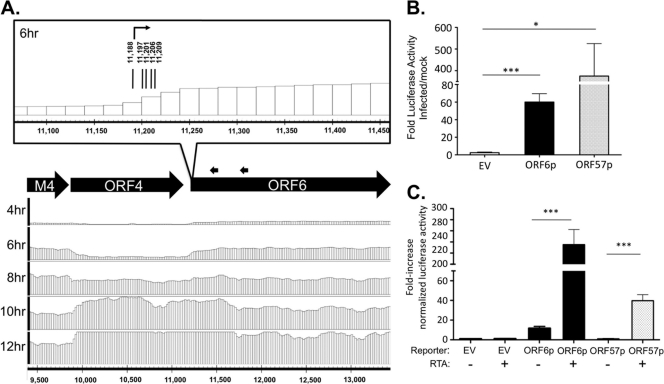
We next sought to determine the utility of the tiled array in predicting transcription initiation sites to guide promoter analysis. The conserved gammaherpesvirus ORF6/ssDBP is essential for MHV68 replication and the establishment of splenic latency (81, 90). In addition, ORF6 is an antigenic target for CD8+ T cells in the early stages of virus infection (23, 78). ORF6 expression depends on protein synthesis but not DNA replication, designating it an early gene (18, 40, 59). However, the regulation of ORF6 gene expression has not been well-defined. In the tiled array time course analysis, at 4 and 6 hpi the transcriptional start site of ORF6 was apparent by the increase in signal with probes that cover the genomic regions bp 11,180 to 11,200 (Fig. 6A). We utilized RACE to confirm the 5′ terminus of the ORF6 transcript at 6 hpi. Five transcription start sites between bp 11,188 and 11,209 were identified, with the majority of clones (6/10) initiating at bp 11,188 (Fig. 6A). An approximate 1.3-kb region upstream of the 5′ end of ORF6 cloned into the minimal promoter reporter vector Hsp70-Luc was activated 60-fold during a fibroblast infection (Fig. 6B), similar to ORF57p, the previously characterized ORF57 promoter region activated by RTA (53). The ORF6 promoter region was also directly activated 20-fold by the MHV68 transactivator RTA in HEK 293T cells (Fig. 6C). These data are consistent with previous reports of increased ORF6 expression upon infection with a recombinant MHV68 that overexpresses RTA (33, 59). In addition, these data demonstrate the utility of the tiled array in mapping transcript boundaries to enable the identification of viral promoters.
Fig 6.
ORF6 transcription start site and upstream regulatory region predicted by tiled array. (A) Analysis of the 5′ boundary of ORF6. Tiled array bar graphs for transcript signal ratio in the genomic region upstream of and including ORF6 (forward strand, bp 9,500 to 13,400) are provided at the indicated time points. Black arrows indicate positions of 5′ RACE gene-specific primers. As illustrated in the inset of the genomic region, sequencing results mapped the 5′ transcriptional start sites to bp 11,188 (n = 6), 11,197 (n = 1), 11,201 (n = 1), 11,206 (n = 1), and 11,209 (n = 1) at 6 hpi of fibroblasts. (B) Identification of ORF6 promoter activity. The genomic region (9,894 to 11,218 bp) upstream of ORF6 transcription initiation was cloned into the firefly luciferase vector Hsp70-Luc and transfected into NIH 3T12 fibroblast cells prior to infection at an MOI of 5 in triplicate. Bars indicate the fold increase in luciferase activity of infected cells compared to uninfected cells. (C) ORF6 promoter is RTA responsive. HEK 293T cells were cotransfected with the indicated reporter constructs, a Renilla luciferase reporter for normalization, and the lytic transactivator MHV68 RTA. Bar graphs represent luciferase activity relative to that of an empty vector (EV). ORF57p is a previously characterized RTA-responsive promoter (53). Error bars indicate the mean and standard deviation. Significance was determined by Student's t test (*, P < 0.0126; ***, P < 0.0005).
DISCUSSION
Here, we have applied a tiled array to better analyze and compare MHV68 transcript structures, transcript expression levels, and transcript kinetics during a time course of de novo fibroblast infection and upon TPA-stimulated reactivation from a latent B cell line. On the basis of their kinetic profiles during de novo infection and upon TPA-stimulated B cell reactivation in the absence of chemical inhibitors, the annotated genes clustered into four major classes that were distinct from the previously defined IE, E, and L gene classes. Except for ORF54 and ORF61, genes with rapid expression kinetics that comprised de novo fibroblast infection cluster I and TPA-stimulated B cell reactivation cluster I genes shared little overlap. Boundaries of previously undefined transcripts for ORF6, ORF63, and ORF73 that were observed by microarray at early times after de novo infection were confirmed by RACE. This led to the initial characterization of the ∼1.3-kb region upstream of ORF6 transcription initiation as an RTA-responsive regulatory region. Lastly, we found regions of transcription consistent with the previously defined expressed genomic regions that were differentially expressed both during de novo infection and upon phorbol ester-stimulated reactivation from latency in the A20-HE2 B cell line. Our results indicate that the gammaherpesvirus transcriptome is dynamic and distinct during both types of productive infections, providing a foundation for further global analyses of the diverse gene expression programs that likely exist in the infected host.
Comparison with previous array studies.
The genome-wide profile in the annotated ORFs obtained using 11,940 60-mers with 40-nt overlap described here was in strong agreement with the profiles obtained at 8 and 18 hpi using 42,545 tiled 60-mers with a much closer 2- to 5-nt overlap (40). That study applied tiled arrays to define the transcriptome using a classical approach, wherein the authors compared the profiles at an early 8-hpi time point in the absence and presence of cycloheximide to identify IE genes and they compared the profiles at an 18-hpi time point in the absence and presence of cidofovir to identify E genes (40). Both array time course experiments identified transcript signals extending from previously annotated ORF regions, in addition to transcripts between ORFs that have been named EGRs (40). Some EGRs comprised a 5′ extension of a gene, such as those for ORF67A or ORF12/13 M, as verified by RACE and strand-specific Northern analysis (11, 40). Other EGRs are completely within the boundaries of the ORF on the opposite strand, suggesting that these EGRs might have antisense regulatory properties. The cidofovir sensitivity of most EGRs previously identified by Johnson et al. (40) led to their classification as late genes, consistent with our analysis of increased accumulation for most EGRs over the time course experiments. Extensive transcripts in non-ORF annotated regions have also been identified during KSHV infection and have been proposed to impact antisense viral gene expression (8). The regulation and functional consequence of these novel RNAs will be interesting to determine; non-ORF annotated KSHV transcripts have been reported to encode small peptides with unknown function (92).
Hierarchical analysis identifies a class of genes rapidly expressed upon de novo infection.
Viral gene expression is dynamic and complex and is regulated by viral transcription factors and changes in the host transcription factor milieu based on virus-driven impacts on cellular signaling. Thus, while the requirement for protein synthesis differentiates an immediate-early from an early gene and provides insight into its regulation, it does not preclude the possibility that an early gene is expressed with rapid kinetics in an unhindered natural infection. For instance, ORF36 and ORF37 transcript accumulation is impaired in the absence of early infection events (2, 18, 40, 59), yet transcription across both ORFs was robust and rapidly detected when protein synthesis was not blocked (Fig. 1 and 2). ORF36 is a protein kinase that induces a DNA damage response and interferes with interferon regulatory factor 3 (IRF3) activation of beta interferon production (36, 79). In the absence of ORF36, lytic replication in macrophages and fibroblasts is impaired and splenic latency is reduced in infected mice (36, 80). While the mSOX protein, encoded by ORF37, is necessary for virus replication (14), a mutant with a point mutation revealed that the mSOX host-shutoff function was dispensable for replication but important for splenic latency in vivo (70). Consistent with the tiled array data presented here, mSOX protein was detected by 8 hpi and its expression occurred in the absence of virus DNA replication (14). Taken together, even though ORF36 and ORF37 were classified as E/E-L genes in experiments using chemical inhibitors, their rapid expression kinetics in the de novo fibroblast infection time course support their roles in modulating host processes to favor early events in virus infection. Hierarchical classification may complement studies that seek to define gene function in the context of progressive, productive infection.
TPA-stimulated reactivation from latency in B cells.
We also extended the findings of previous array approaches by examining the kinetics and boundaries of viral transcripts during TPA-stimulated reactivation from latency in B cells. The MHV68-positive S11 tumor cell line was isolated from an infected mouse that was immunosuppressed and has been used in previous studies of latency-associated gene expression (59, 83). However, the S11 cells lack a negative-control cell line and undergo spontaneous reactivation. This led us to examine viral gene expression in A20-HE2 cells, A20 B cells selected to maintain MHV68 that encodes a hygromycin-eGFP resistance cassette. The A20-HE2 cell line is both tightly latent and inducible for reactivation, producing infectious virus by 24 h following stimulation with TPA (22). Previous nonquantitative RT-PCR analysis indicated a global induction of lytic transcripts upon TPA treatment (22), but the kinetics were not characterized in detail. Transcripts in the M2, M11/v-bcl-2, ORF73/mLANA, and ORF74/vGPCR genomic regions have been detected in the latently infected S11E cell line (59) and in the splenocytes of B cell-deficient mice (84), yet the signal in these regions of the tiled array in the untreated 0-h A20-HE2 RNA was not higher than that for the uninfected parental A20 B cells, which indicates either a decreased sensitivity in the tiled array or differences in the latency programs.
In the transcriptome analysis of TPA-induced reactivation of MHV68 from the A20-HE2 cells, viral genes were expressed in a temporal cascade of gene expression that grouped into four major clusters. There was substantial agreement in the genes that comprise B cell reactivation cluster III and IV genes with de novo cluster III genes (Fig. 2 and 3). These late genes encode proteins involved in virion structure and assembly. However, there were clear distinctions in the genes that peaked earliest in de novo fibroblast infection and upon TPA stimulation of the latent A20-HE2 B cell line to undergo reactivation. It is unclear whether this is due to direct effects of the TPA stimulus used to trigger B cell reactivation or a different intracellular milieu of host signaling and transcription factors in B cells compared to fibroblasts. In addition, variables such as genome copy number and incoming tegument proteins may impact the gene expression programs of these two biologically distinct types of productive infections.
TPA stimulation likely drives reactivation via multiple signaling pathways such as the PKC pathway (9) that in turn directly activates viral promoters such as RTA or indirectly impacts viral promoters via histone acetylation and the activation of other signaling pathways. ORF50 is both necessary for de novo infection and sufficient to trigger viral reactivation in latently infected B cells (66, 91). However, ORF50/RTA does not function in isolation, as both host factors, such as NF-κB (7, 38), HMGB1 (76), and PI3K-AKT (67), and viral proteins, including LANA, ORF49, and ORF57, modulate ORF50 transactivation of viral genes (46, 48, 54, 77). While transcript levels determined by microarray analysis do not indicate protein levels, clustering of genes with rapid expression kinetics at 6 h after TPA stimulation that are distinct from RTA expression kinetics further suggests that viral gene expression is driven by factors in addition to or in combination with RTA. For example, the rapid expression of ORF72 upon TPA stimulation prior to peak RTA expression was unexpected since ORF72 is RTA responsive in cell culture studies (33, 59). However, an RTA-responsive region in the ORF72 promoter is dispensable for reactivation in vivo (3) and might be indicative of RTA-independent regulation of ORF72. Therefore, examination of viral gene expression in the absence of a functional RTA or RTA-regulatory region is needed to determine whether the ORFs that were expressed in advance or concomitantly with peak RTA transcription under TPA-stimulated conditions are RTA dependent. In addition, transcriptional profiles of newly infected B cells in cell culture and, ideally, in the context of the host would also provide significant insight regarding cell type-dependent impacts on viral gene expression. These studies would likely require deep sequencing technology since B cells have low permissivity for de novo infection (39).
Transcripts of distinction.
ORF54/dUTPase and ORF61/ribonucleotide reductase subunit 1 were expressed with rapid kinetics during both types of productive infection. Recently, the ORF54/dUTPase has been reported to promote the degradation of the type I interferon receptor protein IFNAR1 in a dUTPase-dependent manner to play a critical role in establishing latency in lymphocytes (47). ORF61 is required for acute replication and dissemination to lymphoid tissues in infected mice (26). ORF12/mK3 was not formally designated a cluster I gene in the hierarchical analyses of productive infection here, but it was expressed at relatively high levels at the earliest time points, consistent with earlier classifications as an immediate-early gene (18). Overall, the expression profiles of these genes suggest a mode of regulation that is independent of cell type that might serve as a novel target for intervention.
In MHV68, there are three ORF75 genes encoding tegument proteins with a formyl-glycineamide-phosphoribosyl-amidotransferase (FGARAT) motif that likely arose from a gene duplication event (15, 24, 52). ORF75A, ORF75B, and ORF75C share only approximately 28 to 31% amino acid identity (24, 52). ORF75C but not ORF75A and ORF75B mediates PML body disruption (52) and is essential for virus replication (24). This seeming lack of redundancy in function is supported by the unique expression profiles during de novo infection and upon TPA-stimulated reactivation from latent B cells as described here. ORF75A transcription was at a low level throughout NIH 3T3 infection, while ORF75B transcription was detected by 4 hpi and increased over the time course. ORF75C expression was delayed and peaked at ∼10 hpi, consistent with ORF75C designation as a late gene (18). In reactivating B cells, ORF75A was expressed at higher levels than during fibroblast infection, at levels comparable to those for ORF75B. Interestingly, the discontinuous signal across ORF75A observed in the tiled array (Fig. 4B) was also evident in a DNA-based probe array of MHV68 gene expression in the S11 latent cell line, as reported by Martinez-Guzman et al. (59). In that report, the probe in the 5′ region of ORF75A had a higher signal than a 3′ probe in ORF75A. The 5′ probe used corresponds to the area demonstrating the highest transcript signal in ORF75A detected by tiled array. This might be indicative of a novel spliced transcript structure that should be pursued in future studies.
Novel transcript structures identified by tiled array.
The 60-mer tiled oligonucleotides overlapped by 40 nt, affording a 20-nt resolution for transcript boundary predictions. Given these constraints, the tiled array signal boundaries were consistent with numerous transcriptional mapping studies (2–4, 12, 16, 29, 35, 53, 56, 84, 86) and accurately predicted boundaries for three previously undefined transcripts of ORF73/mLANA, ORF63, and ORF6. The extension of the 5′ untranslated region (UTR) for ORF73/mLANA identified by tiled array and confirmed by 5′ RACE suggests that ORF73 is expressed as an unspliced transcript and is consistent with observations from RNase protection assays by Coleman et al. (12). The tiled array predicted the 5′ and 3′ boundaries of ORF63 that were verified by RACE and Northern analysis. The inflammasome is a proinflammatory host cell response to infection that is activated during KSHV infection via the nuclear sensor IFI16 (41). Interestingly, the KSHV ORF63 gene product inhibits NLRP1-dependent inflammasome activation (31). The full-length transcript structure for the MHV68 ORF63 homolog will facilitate studies to examine whether the impairment of inflammasome function reported by KSHV ORF63/vNLRP1 is a conserved property of rhadinoviruses (31).
The ssDBP encoded by MHV68 ORF6 is a conserved herpesvirus protein that is essential for virus replication (49, 81). ORF6 is critical for the establishment of latency in splenocytes but not peritoneal cells in MHV68-infected mice (49). Understanding how ORF6 is regulated should provide significant insight into viral gene expression programs that precede and govern virus latency in specific cell types. ORF6 was a candidate RTA-responsive gene based on overexpression studies (33). Here, we identified an approximately 1.3-kb genomic region upstream of ORF6 transcription initiation that promotes transcription during fibroblast infection and is responsive to RTA in 293T cells (Fig. 6). This information will allow the fine mapping of ORF6 promoter elements that are responsive to RTA and other factors.
The presence of overlapping transcripts in MHV68 confounded boundary determinations for some regions of the genome, as indicated in Table 1, especially in regions that have previously been predicted to utilize a common poly(A) site. A constant transcript signal spanning ORF65 to ORF67A precluded the identification of boundaries for the overlapping ORF65, ORF66, and ORF67 genes, consistent with overlapping coterminal transcripts that utilize the poly(A) site at bp 93,855 to 93,860. However, an extension of the 5′ UTR of the ORF67A gene is suggested by transcript signal initiation more than 600 bp further upstream (Fig. 1). In KSHV, the ORF58-ORF62 locus is characterized by nested transcripts that share a poly(A) signal sequence (57). A similar pattern of transcript signal was observed by tiled array for the MHV68 ORF58-ORF62 region at later time points. However, a monocistronic ORF61 transcript was observed at 4 hpi during de novo infection and 12 h after TPA-stimulated B cell reactivation, prior to the upregulation of neighboring genes. ORF62 transcripts were detected at 8 hpi in the de novo time course. Transcripts in the ORF30-ORF33 locus are predicted to share a single poly(A) signal (bp 50,887 to 50,892). The ORF33 transcript signal terminated at this poly(A) site, but distinct ORF transcript structures and expression patterns in this region suggest that the ORF transcripts are not coterminal. While ORF30 and ORF31 were nearly indistinguishable, the normalized ORF32 transcript signal was more abundant than the signals of ORF30-ORF31 and ORF33 early during de novo infection (Fig. 1). ORF32 clustered with ORF31 as class II genes during de novo fibroblast infection (Fig. 2), but the genes that comprise this locus had distinct kinetic profiles during B cell reactivation induced by TPA stimulation (Fig. 3 and Table 1). Thus, the incremental analysis during the infection time course provided windows of opportunity to identify transcript structures within the overlapping transcripts predicted from consensus poly(A) signals.
There were limitations in the tiled array in mapping transcripts during latency and in analyzing transcripts, especially in the termini and in the tRNA-miRNA region of the left end of the genome. Several spliced structures, including the exon 1- and exon 2-containing ORF73 transcripts (4, 12) and exon 1 of M2 (16, 35), were not clearly evident by tiled array. At the left end, the tRNA-miRNA transcripts have a great deal of secondary structure that might lead to a higher level of self-priming that also contributed to a higher transcript signal.
Segmentation analysis of the tiled array data accurately predicted 3′ boundaries yet seemed less accurate with 5′ boundaries. There were also areas of obvious, albeit weaker, transcript signal that confound boundary and splicing predictions, as visualized in the 5′ exons of ORF50 and ORF57 in Fig. S6 in the supplemental material. In addition, since ORF64 is the largest ORF at 7,374 bp, its detection may have been impacted by the poly(A)-dependent labeling method employed in our analysis. Alternative detection methods, including the use of random primers for reverse priming or antibodies that detect RNA-DNA hybrids, might overcome these technical limitations.
Utility of the tiled microarray in pathogenesis studies.
One powerful tool used in the MHV68 model of gammaherpesvirus pathogenesis is the ability to generate recombinant viruses with the insertion of transgenes, including cre, to mediate recombinatorial deletion of loxP-flanked host genes (63), fluorescent proteins (13, 27), luciferase reporters (37, 61), or a β-lactamase tag for marking infected cells (64) or with the expression of a transdominant negative mutant factor that targets a specific signaling pathway (44). However, care must be taken to avoid disruption of neighboring genes. Current loci used include the genomic region left of M1 (37), between ORF11 and the gene for mK3 (17), ORF27-ORF29 (disrupting the nonessential gene ORF28) (43, 44, 60, 63), and ORF57-ORF58 (26, 61). Additional nontranscribed regions between convergent annotated ORF transcripts that might prove suitable for transgene insertion were located in the ORF11-ORF12 and ORF51-ORF52 intergenic regions (Fig. 1). In addition, while the convergent ORF pairs ORF28-ORF29B, ORF38-ORF39, and ORF41-ORF42 overlap slightly, the 3′ ends of these open reading frames could be added back to the flanking arms of an inserted element.
MHV68 is a genetically tractable system that has increased our understanding of the role of viral proteins in replication and latency in vivo, the function of innate and adaptive immune responses during acute and chronic stages of gammaherpesvirus infection, and virus-host interactions that transpire in particular tissues. This system has the potential to dissect the molecular basis of latency and reactivation in the context of specific cell types in the host. Thus, fundamental knowledge regarding gene structure and regulation and gene expression programs will better enable the full potential of the murine gammaherpesvirus model system to be realized. This ∼12,000-tiled oligonucleotide microarray is an economical approach to rapidly define the viral transcriptome during infections of distinct cell types and in the context of altered virus-host interactions. We expect this comparative analysis of productive infection to serve as a foundation for future transcriptome studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hong Wang, Robert Stockton, Sumar Hayan, Huei Mei, and Nina Rehage Chen for technical support and the laboratories of Erich Mackow, Nancy Reich, and Martha Furie for sharing critical equipment. We thank Bruce Futcher, Robert D. Allen III, and all members of the Krug lab for helpful discussions and advice.
L.T.K. was supported by an American Cancer Society research scholar grant, RSG-11-160-01-MPC. A.S. was supported by a National Science Foundation Graduate Bridge to the Doctorate fellowship (HRD1832598), and S.J. was supported by Howard Hughes Medical Institute grant 52005887.
B.Y.H.C., A.S., J.C.F., Y.Z., J.L., and L.T.K. designed the experiments. B.Y.H.C., A.S., E.S., Y.Z., S.J., and L.T.K. executed the experiments. J.Z., A.S., S.K., J.C.F., J.L., and L.T.K. analyzed the data. J.Z., A.S., S.K., J.C.F., and L.T.K. prepared the manuscript.
Footnotes
Published ahead of print 8 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Adler H, Messerle M, Wagner M, Koszinowski UH. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 74:6964–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn JW, Powell KL, Kellam P, Alber DG. 2002. Gammaherpesvirus lytic gene expression as characterized by DNA array. J. Virol. 76:6244–6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen RD, III, Dezalia MN, Speck SH. 2007. Identification of an Rta responsive promoter involved in driving gammaHV68 v-cyclin expression during virus replication. Virology 365:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen RD, III, Dickerson S, Speck SH. 2006. Identification of spliced gammaherpesvirus 68 LANA and v-cyclin transcripts and analysis of their expression in vivo during latent infection. J. Virol. 80:2055–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barton E, Mandal P, Speck SH. 2011. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu. Rev. Immunol. 29:351–397 [DOI] [PubMed] [Google Scholar]
- 6. Barton ES, Lutzke ML, Rochford R, Virgin HW., IV 2005. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J. Virol. 79:14149–14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown HJ, et al. 2003. NF-kappaB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandriani S, Xu Y, Ganem D. 2010. The lytic transcriptome of Kaposi's sarcoma-associated herpesvirus reveals extensive transcription of noncoding regions, including regions antisense to important genes. J. Virol. 84:7934–7942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen A, Brodie C, Sarid R. 2006. An essential role of ERK signalling in TPA-induced reactivation of Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 87:795–802 [DOI] [PubMed] [Google Scholar]
- 10. Coleman CB, Nealy MS, Tibbetts SA. 2010. Immature and transitional B cells are latency reservoirs for a gammaherpesvirus. J. Virol. 84:13045–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coleman HM, Brierley I, Stevenson PG. 2003. An internal ribosome entry site directs translation of the murine gammaherpesvirus 68 MK3 open reading frame. J. Virol. 77:13093–13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coleman HM, Efstathiou S, Stevenson PG. 2005. Transcription of the murine gammaherpesvirus 68 ORF73 from promoters in the viral terminal repeats. J. Gen. Virol. 86:561–574 [DOI] [PubMed] [Google Scholar]
- 13. Collins CM, Boss JM, Speck SH. 2009. Identification of infected B-cell populations by using a recombinant murine gammaherpesvirus 68 expressing a fluorescent protein. J. Virol. 83:6484–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Covarrubias S, Richner JM, Clyde K, Lee YJ, Glaunsinger BA. 2009. Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J. Virol. 83:9554–9566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davison AJ. 2002. Evolution of the herpesviruses. Vet. Microbiol. 86:69–88 [DOI] [PubMed] [Google Scholar]
- 16. DeZalia M, Speck SH. 2008. Identification of closely spaced but distinct transcription initiation sites for the murine gammaherpesvirus 68 latency-associated M2 gene. J. Virol. 82:7411–7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dutia BM, et al. 2009. A novel Cre recombinase imaging system for tracking lymphotropic virus infection in vivo. PLoS One 4:e6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ebrahimi B, et al. 2003. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 84:99–109 [DOI] [PubMed] [Google Scholar]
- 19. Feng P, et al. 2007. A novel inhibitory mechanism of mitochondrion-dependent apoptosis by a herpesviral protein. PLoS Pathog. 3:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forrest JC, Krug LT, Speck SH. 2008. Murine gammaherpesvirus 68 infection of mice: a small animal model for characterizing basic aspects of gamma-herpesvirus pathogenesis. In Damania B, Pipas J. (ed), DNA tumor viruses. Springer, New York, NY [Google Scholar]
- 21. Forrest JC, Paden CR, Allen RD, Collins J, Speck SH. 2007. ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J. Virol. 81:11957–11971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forrest JC, Speck SH. 2008. Establishment of B-cell lines latently infected with reactivation-competent murine gammaherpesvirus 68 provides evidence for viral alteration of a DNA damage-signaling cascade. J. Virol. 82:7688–7699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freeman ML, et al. 2010. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J. Virol. 84:2881–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaspar M, Gill MB, Lösing J-B, May JS, Stevenson PG. 2008. Multiple functions for ORF75c in murid herpesvirus-4 infection. PLoS One 3:e2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaspar M, et al. 2011. Murid herpesvirus-4 exploits dendritic cells to infect B cells. PLoS Pathog. 7:e1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gill MB, May JS, Colaco S, Stevenson PG. 2010. Important role for the murid herpesvirus 4 ribonucleotide reductase large subunit in host colonization via the respiratory tract. J. Virol. 84:10937–10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gillet L, Gill MB, Colaco S, Smith CM, Stevenson PG. 2006. Murine gammaherpesvirus-68 glycoprotein B presents a difficult neutralization target to monoclonal antibodies derived from infected mice. J. Gen. Virol. 87:3515–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodwin MM, Canny S, Steed A, Virgin HW. 2010. Murine gammaherpesvirus 68 has evolved gamma interferon and Stat1-repressible promoters for the lytic switch gene 50. J. Virol. 84:3711–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gray KS, Allen RD, Farrell ML, Forrest JC, Speck SH. 2009. Alternatively initiated gene 50/RTA transcripts expressed during murine and human gammaherpesvirus reactivation from latency. J. Virol. 83:314–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gray KS, Forrest JC, Speck SH. 2010. The de novo methyltransferases DNMT3a and DNMT3b target the murine gammaherpesvirus immediate-early gene 50 promoter during establishment of latency. J. Virol. 84:4946–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gregory SM, et al. 2011. Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331:330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. 2009. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog. 5:e1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hair JR, Lyons PA, Smith KGC, Efstathiou S. 2007. Control of Rta expression critically determines transcription of viral and cellular genes following gammaherpesvirus infection. J. Gen. Virol. 88:1689–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huber W, Toedling J, Steinmetz LM. 2006. Transcript mapping with high-density oligonucleotide tiling arrays. Bioinformatics 22:1963–1970 [DOI] [PubMed] [Google Scholar]
- 35. Husain SM, et al. 1999. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8(+) T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 96:7508–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hwang S, et al. 2009. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe 5:166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hwang S, et al. 2008. Persistent gammaherpesvirus replication and dynamic interaction with the host in vivo. J. Virol. 82:12498–12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Izumiya Y, et al. 2009. NF-kappaB serves as a cellular sensor of Kaposi's sarcoma-associated herpesvirus latency and negatively regulates K-Rta by antagonizing the RBP-Jkappa coactivator. J. Virol. 83:4435–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jarousse N, Chandran B, Coscoy L. 2008. Lack of heparan sulfate expression on B cell lines: implications for Kaposi's sarcoma-associated virus and murine gammaherpesvirus 68 infections. J. Virol. 82:12591–12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson LS, Willert EK, Virgin HW. 2010. Redefining the genetics of murine gammaherpesvirus 68 via transcriptome-based annotation. Cell Host Microbe 7:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kerur N, et al. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim IJ, et al. 2003. Maintenance of long term gamma-herpesvirus B cell latency is dependent on CD40-mediated development of memory B cells. J. Immunol. 171:886–892 [DOI] [PubMed] [Google Scholar]
- 43. Krug LT, Collins CM, Gargano LM, Speck SH. 2009. NF-kappaB p50 plays distinct roles in the establishment and control of murine gammaherpesvirus 68 latency. J. Virol. 83:4732–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krug LT, Moser JM, Dickerson SM, Speck SH. 2007. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog. 3:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krug LT, et al. 2010. Inhibition of NF-kappaB signaling reduces virus load and gammaherpesvirus-induced pulmonary fibrosis. Am. J. Pathol. 177:608–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lan K, Kuppers DA, Verma SC, Robertson ES. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 78:6585–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leang RS, et al. 2011. The anti-interferon activity of conserved viral dUTPase ORF54 is essential for an effective MHV-68 infection. PLoS Pathog. 7:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee S, et al. 2007. The ORF49 protein of murine gammaherpesvirus 68 cooperates with RTA in regulating virus replication. J. Virol. 81:9870–9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li H, Ikuta K, Sixbey JW, Tibbetts SA. 2008. A replication-defective gammaherpesvirus efficiently establishes long-term latency in macrophages but not in B cells in vivo. J. Virol. 82:8500–8508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li X, et al. 2010. Tpl2/AP-1 enhances murine gammaherpesvirus 68 lytic replication. J. Virol. 84:1881–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. 2009. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog. 5:e1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ling PD, Tan J, Sewatanon J, Peng R. 2008. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. J. Virol. 82:8000–8012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu S, Pavlova IV, Virgin HW, IV, Speck SH. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu F, Day L, Gao S-J, Lieberman PM. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J. Virol. 80:5273–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lukac DM, Garibyan L, Kirshner JR, Palmeri D, Ganem D. 2001. DNA binding by Kaposi's sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J. Virol. 75:6786–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mackett M, et al. 1997. Genetic content and preliminary transcriptional analysis of a representative region of murine gammaherpesvirus 68. J. Gen. Virol. 78(Pt 6):1425–1433 [DOI] [PubMed] [Google Scholar]
- 57. Majerciak V, Yamanegi K, Zheng Z-M. 2006. Gene structure and expression of Kaposi's sarcoma-associated herpesvirus ORF56, ORF57, ORF58, and ORF59. J. Virol. 80:11968–11981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marques S, Efstathiou S, Smith KG, Haury M, Simas JP. 2003. Selective gene expression of latent murine gammaherpesvirus 68 in B lymphocytes. J. Virol. 77:7308–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martinez-Guzman D, et al. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488–10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. May JS, Coleman HM, Boname JM, Stevenson PG. 2005. Murine gammaherpesvirus-68 ORF28 encodes a non-essential virion glycoprotein. J. Gen. Virol. 86:919–928 [DOI] [PubMed] [Google Scholar]
- 61. Milho R, et al. 2009. In vivo imaging of murid herpesvirus-4 infection. J. Gen. Virol. 90:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moser JM, Farrell ML, Krug LT, Upton JW, Speck SH. 2006. A gammaherpesvirus 68 gene 50 null mutant establishes long-term latency in the lung but fails to vaccinate against a wild-type virus challenge. J. Virol. 80:1592–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moser JM, Upton JW, Allen RD, III, Wilson CB, Speck SH. 2005. Role of B-cell proliferation in the establishment of gammaherpesvirus latency. J. Virol. 79:9480–9491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nealy MS, Coleman CB, Li H, Tibbetts SA. 2010. Use of a virus-encoded enzymatic marker reveals that a stable fraction of memory B cells expresses latency-associated nuclear antigen throughout chronic gammaherpesvirus infection. J. Virol. 84:7523–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paden CR, Forrest JC, Moorman NJ, Speck SH. 2010. Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J. Virol. 84:7214–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pavlova IV, Virgin HW, IV, Speck SH. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peng L, et al. 2010. Inhibition of the phosphatidylinositol 3-kinase-Akt pathway enhances gamma-2 herpesvirus lytic replication and facilitates reactivation from latency. J. Gen. Virol. 91:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Polcicova K, et al. 2008. Up-regulation of murid herpesvirus 4 ORF50 by hypoxia: possible implication for virus reactivation from latency. Virus Res. 132:257–262 [DOI] [PubMed] [Google Scholar]
- 69. Reese TA, et al. 2010. Identification of novel microRNA-like molecules generated from herpesvirus and host tRNA transcripts. J. Virol. 84:10344–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Richner JM, et al. 2011. Global mRNA degradation during lytic gammaherpesvirus infection contributes to establishment of viral latency. PLoS Pathog. 7:e1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rousseeuw P. 1987. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20:53–65 [Google Scholar]
- 72. Sarawar SR, et al. 2002. Chemokine induction and leukocyte trafficking to the lungs during murine gammaherpesvirus 68 (MHV-68) infection. Virology 293:54–62 [DOI] [PubMed] [Google Scholar]
- 73. Sarawar SR, Lee BJ, Giannoni F. 2004. Cytokines and costimulatory molecules in the immune response to murine gammaherpesvirus-68. Viral Immunol. 17:3–11 [DOI] [PubMed] [Google Scholar]
- 74. Shannon-Lowe C, Rowe M. 2011. Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PLoS Pathog. 7:e1001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Siegel AM, Rangaswamy US, Napier RJ, Speck SH. 2010. Blimp-1-dependent plasma cell differentiation is required for efficient maintenance of murine gammaherpesvirus latency and antiviral antibody responses. J. Virol. 84:674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Song MJ, et al. 2004. The DNA architectural protein HMGB1 facilitates RTA-mediated viral gene expression in gamma-2 herpesviruses. J. Virol. 78:12940–12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Speck SH, Ganem D. 2010. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8:100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stevenson PG, Belz GT, Castrucci MR, Altman JD, Doherty PC. 1999. A gamma-herpesvirus sneaks through a CD8(+) T cell response primed to a lytic-phase epitope. Proc. Natl. Acad. Sci. U. S. A. 96:9281–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tarakanova VL, et al. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, Gauld SB. 2010. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology 405:50–61 [DOI] [PubMed] [Google Scholar]
- 81. Tibbetts SA, Suarez F, Steed AL, Simmons JA, Virgin HW., IV 2006. A gamma-herpesvirus deficient in replication establishes chronic infection in vivo and is impervious to restriction by adaptive immune cells. Virology 353:210–219 [DOI] [PubMed] [Google Scholar]
- 82. Uldrick TS, Whitby D. 2011. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma. Cancer Lett. 305:150–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Usherwood EJ, Stewart JP, Nash AA. 1996. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. J. Virol. 70:6516–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Virgin HW, IV, Presti RM, Li XY, Liu C, Speck SH. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 73:2321–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Virgin HW, IV, et al. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wakeling MN, Roy DJ, Nash AA, Stewart JP. 2001. Characterization of the murine gammaherpesvirus 68 ORF74 product: a novel oncogenic G protein-coupled receptor. J. Gen. Virol. 82:1187–1197 [DOI] [PubMed] [Google Scholar]
- 87. Weck KE, Barkon ML, Yoo LI, Speck SH, Virgin HI. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Willer DO, Speck SH. 2005. Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40. J. Virol. 79:2891–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Willer DO, Speck SH. 2003. Long-term latent murine gammaherpesvirus 68 infection is preferentially found within the surface immunoglobulin D-negative subset of splenic B cells in vivo. J. Virol. 77:8310–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Woisetschlaeger M, Strominger JL, Speck SH. 1989. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 86:6498–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu TT, Usherwood EJ, Stewart JP, Nash AA, Sun R. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xu Y, Ganem D. 2010. Making sense of antisense: seemingly noncoding RNAs antisense to the master regulator of Kaposi's sarcoma-associated herpesvirus lytic replication do not regulate that transcript but serve as mRNAs encoding small peptides. J. Virol. 84:5465–5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhu JY, et al. 2010. Identification and analysis of expression of novel microRNAs of murine gammaherpesvirus 68. J. Virol. 84:10266–10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.