Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is a complex retrovirus associated with the lymphoproliferative disease adult T-cell leukemia/lymphoma (ATL) and the neurodegenerative disorder tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM). Replication of HTLV-1 is under the control of two major trans-acting proteins, Tax and Rex. Previous studies suggested that Tax activates transcription from the viral long terminal repeat (LTR) through recruitment of cellular CREB and transcriptional coactivators. Other studies reported that Rex acts posttranscriptionally and allows the cytoplasmic export of unspliced or incompletely spliced viral mRNAs carrying gag/pol and env only. As opposed to HIV's Rev-responsive element (RRE), the Rex-responsive element (RxRE) is present in all viral mRNAs in HTLV-1. However, based on indirect observations, it is believed that nuclear export and expression of the doubly spliced tax/rex RNA are Rex independent. In this study, we demonstrate that Rex does stimulate Tax expression, through nuclear-cytoplasmic export of the tax/rex RNA, even though a Rex-independent basal export mechanism exists. This effect was dependent upon the RxRE element and the RNA-binding activity of Rex. In addition, Rex-mediated export of tax/rex RNA was CRM1 dependent and inhibited by leptomycin B treatment. RNA immunoprecipitation (RNA-IP) experiments confirmed Rex binding to the tax/rex RNA in both transfected cells with HTLV-1 molecular clones and HTLV-1-infected T cells. Since both Rex and p30 interact with the tax/rex RNA and with one another, this may offer a temporal and dynamic regulation of HTLV-1 replication. Our results shed light on HTLV-1 replication and reveal a more complex regulatory network than previously anticipated.
INTRODUCTION
Like all other retroviruses, human T-cell leukemia virus type 1 (HTLV-1) has limited genetic information restricted by the small size of its genome. To overcome its small genome while reducing its dependency on the host cell machinery, HTLV-1 has evolved many strategies, including ribosome frameshifts, overlapping reading frames, complex alternative splicing patterns, reverse-sense RNA transcripts, and posttranslational processing/modifications of viral proteins which ascribe distinct functions to them. Early observations of the existence of three major viral RNAs expressed in HTLV-1-infected cells, i.e., gag/pol, env, and tax/rex RNAs, led to the hypothesis that Rex positively regulates gag/pol and env RNAs while concomitantly reducing the abundance of the tax/rex RNA (25). Hence, HTLV-1 replication has been thought to be regulated by Tax and Rex proteins (14, 27), though evidence suggests that additional viral proteins may also play an important role during the virus replication cycle (5, 32).
Tax interaction with CREB family members and coactivators (CBP, P300, and PCAF) results in a high-molecular-weight complex with high affinity for binding to and transactivating the viral long terminal repeat (LTR) (15, 23, 26, 30). Rex has at least three domains that are important for its functions: an arginine-rich region important for binding to the Rex-responsive element (RxRE) (8); a region required for multimerization of Rex, a step required for assembly onto the RxRE (6, 9); and a leucine-rich nuclear export signal that interacts with the nuclear export receptor, CRM1 (17, 19). The ability of Rex to regulate expression of the gag and env structural genes posttranscriptionally is rigorously dependent on the presence of the RxRE in the 3′-LTR sequence. In HTLV-1, the mRNA 3′ cleavage site is not positioned 10 to 30 nucleotides downstream of the highly conserved polyadenylation AAUAAA hexamer motif but is located more than 250 nucleotides upstream of the actual pre-mRNA cleavage site. This is surprising, as prior studies have shown the rigid requirement for close spacing of these elements for efficient use of the poly(A) site. Binding of Rex to its RxRE stabilizes a secondary structure that spatially juxtaposes the separated AAUAAA hexamer and GU-rich elements and promotes the stable binding of PF2 and CF1 that commits this poly(A) site to 3′ processing. Rex is thought not only to regulate mRNA transport from the nucleus to the cytoplasm (3, 22) but also to inhibit splicing, increase the stability of mRNAs, and/or enhance translation of incompletely spliced mRNAs (16); these functions of Rex appear to be regulated by phosphorylation (1, 2).
In contrast to the positive regulation exerted by Tax and Rex, we previously found that HTLV-1 p30 negatively regulates the relative amount of tax/rex mRNA in the cytoplasm (31). Thus, Rex and p30 viral proteins have opposite effects on viral production. Rex increases genomic and structural (Gag and Env) as well as enzymatic (reverse transcriptase, protease, and integrase) RNA transport, thus increasing virus production. p30, in contrast, decreases virus production by decreasing Rex and Tax function through a posttranscriptional mechanism. Additional studies also found that binding of p30 to Rex prevents Rex-RxRE interactions, impeding its nuclear export function (35). In addition, p30 interacts efficiently with Rex only when p30 is bound to RNA. Since p30 can interact with the tax/rex but not gag/pol or env mRNA, these data explain why p30 is able to prevent tax/rex nuclear export and why p30 has little or no effect on other viral mRNAs (35).
The data presented here challenge the current dogma regarding Rex dependency of tax/rex RNA for expression. In this study, we found that Rex does bind to the RxRE to stimulate tax/rex mRNA export to the cytoplasm, in a CRM1-dependent manner, and to increase Tax expression.
MATERIALS AND METHODS
Plasmids.
The envelope-LTR sequence, amplified by PCR from CMV-env-LTR (kindly provided by C. Pique), was cloned into the pcDNA3.1(−) vector (Invitrogen) between the XbaI and EcoRI sites. After replacing the XbaI-TthIIII fragment in the pcDNA3.1(−) env-LTR vector with the XbaI-TthIIII fragment from RLTK-tax/rex (31), the CMV-tax/rex-LTR vector was generated. Site-directed mutagenesis of the CMV-tax/rex-LTR vector was used to generate a Rex-deficient CMV-tax/rex-LTR vector [CMV-tax/rex-LTR (Rex−)]. The ATG for Rex was mutated to AGG. The pc-Tax plasmid and all pc-Tax-derived mutants (M22, M47, and K88) have been reported previously (23, 36). A Rex-deficient pc-Tax plasmid [pc-Tax (Rex−)] was constructed using site-directed mutagenesis. The mutagenic primers were rexmF (5′-GACTCCTCAAGCGAGTAGCCCAAGACCCGTCGGAG-3′) and rexmR (5′-CTCCGACGGGTCTTGGGCTACTCGCTTGAGGAGTC-3′). The CTG in pc-Tax was mutated to TGA, a stop codon. The primers for Rex were RexF (5′-CCCAAGCTTCCATGCCCAAGACCCGTCGG-3′) and RexR (5′-TTTGAATTCGAGGGGCAGGAGGGG-3′). Rex and Rexlys (a Rex mutant with lysine substitutions) were amplified from pc-Rex and pc-Rexlys by using PCR (21) and were cloned into the pMH vector (Roche), which provides a hemagglutinin (HA) tag at the C terminus. HTLV-1 LTR-Luc, pCMV-XRE-CAT, pHTLV-Rex1L, and pHTLV-XMT have been described previously (10, 13, 20). The HTLV-LTR-Luciferase vector was constructed by inserting the HTLV-1 LTR into the pGL3-basic vector (Invitrogen).
Cell culture, transfections, and luciferase assays.
The 293T cell line was maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (FCS) (Atlas Biologicals, Fort Collins, CO), 100 units of penicillin/ml, and 100 μg of streptomycin/ml (Invitrogen). MT-2 cell lines were maintained in RPMI 1640 medium supplemented with 10% FCS (Atlas Biologicals), 100 units of penicillin/ml, and 100 μg of streptomycin/ml (Invitrogen). All transient-transfection assays were performed using PolyFect (Qiagen) according to the manufacturer's instructions. For dual transfection, 50 ng of RLTK vector was added to each transfection mixture to control for transfection efficiency and toxicity. Luciferase assays were performed with a dual-luciferase assay system (Promega). Transfected cells were harvested and lysed in 1× passive lysis buffer. The relative luciferase units were measured using luciferase assay reagent II (Promega) according to the manufacturer's instructions. The Student t test was used to calculate P values for all experiments.
Western blots.
293T cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mm Tris-Cl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS) containing Complete protease inhibitor (Roche Diagnostics). Cell lysates were prepared by centrifugation at 12,000 × g for 10 min at 4°C. Lysates were subjected to SDS-PAGE and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, MA). Blots were incubated with mouse anti-Tax monoclonal antibody, rabbit anti-Rex polyclonal antibody, and goat anti-β-actin followed by appropriate horseradish peroxidase-conjugated secondary antibodies. For detection of proteins containing the HA tag, the blots were incubated with horseradish peroxidase-conjugated mouse anti-HA 3F10 monoclonal antibody (Roche). All blots were developed using chemiluminescent reagents (Supersignal; Pierce).
RNA preparation and quantitative real-time RT-PCR.
Total RNA was prepared from 293T cells by use of TRIzol reagent (Invitrogen). Cytoplasmic RNA was prepared as previously described (29). After DNase I treatment, the RNA was reverse transcribed and the cDNA was used for PCR and real-time PCR. The PCR primers RPX3 (5′-ATCCCGTGGAGACTCCTCAA-3′) and RPX4 (5′-AACACGTAGACTGGGTATCC-3′) were used for tax/rex RNA, and primers LTR2 (5′-CCTACCTGAGGCCGCCATCCACGCGGTTG-3′) and USDR1 (5′-CTATAGAATGGGCTGTCGCTGGC-3′) were used for gag/pol RNA. Real-time reverse transcription-PCR (RT-PCR) was performed using RT2 SYBR green qPCR master mix (SA Bioscience) with the following primers: LTR2, tax/rexR (5′-GGAAGTGGGCCATGGTG-3′), GADPH F (5′-GAAGGTGAAGGTCGGAGTC-3′), and GADPH R (5′-GAAGATGGTGATGGGATTTC-3′). The expression of tax/rex was normalized to that of GAPDH.
RNA immunoprecipitation.
293T cells were transfected with the p-HTLV-1XMT molecular clone, its counterpart ablated for Rex expression (pHTLV-Rex1L), or pcDNA. Transfected and MT-2 control cells were lysed in NP-40 lysis buffer containing protease and phosphatase inhibitors as well as RNase inhibitors (RNAseOut) and ribosyl complexes. Equal amounts of proteins were immunoprecipitated at 4°C overnight, using a Rex rabbit antiserum. Protein A/G agarose was added for 2 h, and protein-RNA complexes were pulled down by centrifugation, washed with NP-40 buffer, and extracted with TRIzol. Equal volumes were then used for one-step RT-PCR using primers specific to gag/pol or tax/rex RNA.
RESULTS
In HTLV-1, the Rex-responsive element (RxRE) is present at the 3′ end of all viral mRNAs. To date, it remains unclear how the interaction of Rex with RxRE is regulated to result in selective Rex-dependent export of some but not other viral mRNAs. Since increased Rex expression has been found to result in increased gag/pol RNA and decreased tax/rex RNA, it was proposed that Rex does not regulate tax/rex RNA cytoplasmic export. However, these observations cannot exclude effects on RNA splicing and stability in addition to the nuclear-cytoplasmic RNA export function of Rex. In fact, there are some reports that Rex can control the expression of HTLV-1-spliced mRNAs as well as the cDNA corresponding to the doubly spliced mRNA encoding the x-II open reading frame (ORF) product Tof, which was shown to be strictly Rex dependent (12).
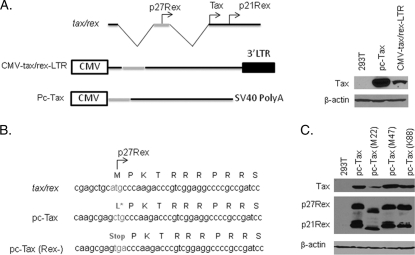
To determine whether the presence of the RxRE sequence is sufficient to confer Rex responsiveness to tax/rex mRNA expression, we constructed the CMV-tax/rex-LTR vector (Fig. 1A). This vector allows constitutive, Tax-independent expression of the viral tax/rex mRNA. The RNA produced truly mimics the natural tax/rex spliced mRNA product, with a flanking 3′ U3R LTR sequence. In addition, this vector has the advantage of allowing the study of Rex-mediated export uncoupled from Rex's effects on splicing. To characterize our vector, we compared the plasmids CMV-Tax (also known as pc-Tax and referred to as such from this point forward) and CMV-tax/rex-LTR, which differ by the presence of the viral LTR (Fig. 1A). The same amount of each vector was analyzed in transient-transfection assays. We found that the presence of a 3′-LTR sequence significantly reduced the amount of Tax protein expressed (Fig. 1A). Unexpectedly, pc-Tax, described as a Tax-only expression vector, was found to express both p27Rex and p21Rex proteins (Fig. 1C).
Fig 1.
CMV-tax/rex-LTR and pc-Tax express both Tax and Rex. (A) Schematic representation of tax/rex RNA, pc-Tax, and the CMV-tax/rex-LTR construct and detection of Tax expression from CMV-tax/rex-LTR and pc-Tax. 293T cells were transfected with 4 μg of pc-Tax or CMV-tax/rex-LTR plasmid. Forty-eight hours after transfection, cell lysates were prepared and subjected to Western blotting. (B) Sequence comparison between wild-type tax/rex, pc-Tax, and pc-Tax (Rex−). (C) 293T cells were transfected with 4 μg of pc-Tax, pc-Tax (M22), pc-Tax (M47), or pc-Tax (K88). Tax and Rex were detected by Western blotting.
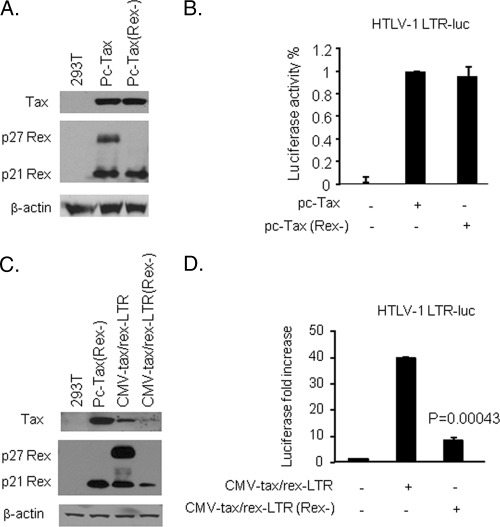
After careful examination, we discovered that the SphI site used to delete the Rex ATG codon resulted in the creation of an in-frame leucine codon (Fig. 1B), which was used for initiation of translation under the strong cytomegalovirus (CMV) promoter. In support of this observation, all previous Tax mutants (M22, M47, and K88) generated using pc-Tax as a backbone were found to express significant amounts of p27Rex and p21Rex proteins (Fig. 1C). Although p21Rex shares the carboxy terminus of p27Rex, it is deprived of the nuclear/nucleolar targeting signal and the RNA-binding motif. Since the aim of this study was to study the effect of p27Rex on the tax/rex mRNA, we used site-directed mutagenesis to eliminate the p27Rex initiation codon from pc-Tax and from CMV-tax/rex-LTR. Tax expression was then demonstrated by Western blot analyses after transfection of 293T cells (Fig. 2A and C) or by transactivation assays using an HTLV-1-LTR-Luciferase reporter vector (Fig. 2B and D). The absence of Rex expression from the mutated CMV-tax/rex-LTR vector was confirmed by Western blotting (Fig. 2A and C). While removing p27Rex from pc-Tax had no effect on Tax expression as measured by Western blotting or luciferase values (Fig. 2A and B), Tax expression and activity from the CMV-tax/rex-LTR vector decreased when Rex was mutated (Fig. 2C and D).
Fig 2.
In the presence of the 3′-LTR, Tax expression was decreased and partly dependent on Rex. (A) 293T cells were transfected with 4 μg of pc-Tax or pc-Tax (Rex−) plasmid. Forty-eight hours after transfection, Tax and Rex were detected by Western blotting. (B) 293T cells were cotransfected with pc-Tax or pc-Tax (Rex−) (0.1 μg) and an HTLV-1 LTR-luc reporter (0.5 μg). The results shown are representative of at least 3 experiments. (C) 293T cells were transfected with 4 μg of pc-Tax (Rex−), CMV-tax/rex-LTR, or CMV-tax/rex-LTR (Rex−) plasmid. Forty-eight hours after transfection, Tax and Rex were detected by Western blotting. (D) 293T cells were cotransfected with CMV-tax/rex-LTR or CMV-tax/rex-LTR (Rex−) (0.1 μg) and an HTLV-1 LTR-luc reporter (0.5 μg).
The results of these studies suggest that Rex does not affect Tax transactivation or protein stability (Fig. 2A and B) and, as previously suggested (28), that the presence of the 3′-LTR sequence generates a posttranscriptional block that can be alleviated by Rex expression. Our results also suggest that a small fraction of tax/rex RNA can be expressed in the complete absence of Rex, since CMV-tax/rex-3′LTR (Rex−) still expressed a little of the Tax protein (Fig. 2C). This may have important implications, since both Tax and Rex are expressed from the same mRNA and because some Tax is needed to initiate viral replication after infection.
We then reasoned that if tax/rex mRNA expressed from CMV-tax/rex-LTR is Rex dependent, then adding exogenous Rex should increase Tax expression. For these experiments, we transfected the CMV-tax/rex-LTR (Rex−) vector along with increasing amounts of Rex (Fig. 3A). Our data indicated a dose-dependent increase in Tax as measured by transactivation of the HTLV-1-LTR-Luciferase plasmid (Fig. 3A). This effect was specific to the CMV-tax/rex-LTR (Rex−) vector, since Rex alone had no effect and increasing amounts of Rex did not affect luciferase values obtained using pc-Tax (data not shown). Rescue of Tax expression by Rex was also demonstrated by Western blotting after transient transfection of 293T cells. Our results showed that the Tax protein level expressed from the CMV-tax/rex-LTR (Rex−) vector was rescued by exogenous Rex to levels similar to those with the CMV-tax/rex-LTR vector (Fig. 3B). Rex posttranscriptional functions rely upon its RNA-binding activity on the RxRE present in the 3′-LTR. The arginine-rich basic domain comprising amino acids 1 to 19 of the Rex protein has been shown to be required for efficient binding to the RxRE (4). To gain some insight into the mechanisms involved in Rex-mediated rescue of Tax expression from CMV-tax/rex-LTR, we used a Rex mutant (Rexlys) in which the RNA-binding motif (Arg stretch) was replaced with lysines to conserve the charge, since this region is also a nuclear/nucleolar localization signal. This mutant has previously been shown to be localized like wild-type Rex but is unable to interact with the RxRE and unable to support RxRE-mediated export of HTLV-1 RNAs. Our results demonstrated that Rexlys did not increase Tax expression from CMV-tax/rex-LTR, as shown by luciferase and Western blot assays (Fig. 3C and D). Together, these data demonstrate that the effect of Rex on Tax expression from the CMV-tax/rex-LTR vector requires Rex RNA-binding activity, and they suggest a posttranscriptional effect.
Fig 3.
Rex RNA-binding activity is required for nuclear export of tax/rex RNA. (A) 293T cells were cotransfected with an HTLV-1 LTR-luc reporter construct (1 μg), CMV-tax/rex-LTR (Rex−) (1 μg), and increasing amounts of pc-Rex (0.25, 0.5, and 1 μg) or pMH-Rexlys (0.25, 0.5, and 1 μg). The experiments were performed four times. The values are presented as means and standard deviations (SD). (B) 293T cells were transfected with 4 μg of CMV-tax/rex-LTR, CMV-tax/rex-LTR (Rex−), or CMV-tax/rex-LTR (Rex−) plus pc-Rex (2 μg). Tax and Rex were detected by Western blotting 48 h after transfection. (C) Tax expression was monitored indirectly by measuring luciferase activity as a surrogate for Tax-mediated trans-activation of the HTLV-1 LTR. (D) 293T cells were transfected with 4 μg of CMV-tax/rex-LTR, CMV-tax/rex-LTR (Rex−), CMV-tax/rex-LTR (Rex−) plus pMH-Rex (2 μg), or pMH-Rexlys (2 μg). Tax, Rex, and Rexlys were detected by Western blotting 48 h after transfection.
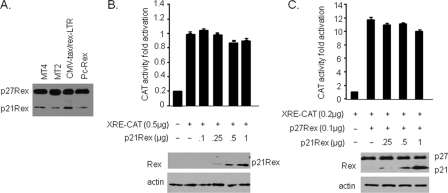
Since the pcRex vector was found to express both p27Rex and p21Rex, we next investigated if p21Rex affected p27Rex functions. Since both p27Rex and p21Rex are recognized by the same Rex antiserum used in this study, we performed Western blot analyses to estimate the relative levels of these proteins expressed in MT-2 and MT-4 HTLV-1-transformed cells as well as from pcRex or CMV-tax/rex-LTR following transient transfection of 293T cells. Results from these experiments indicated that p27Rex was expressed in great excess relative to p21Rex (Fig. 4A). MT-4 cells have a mutated Rex protein coding sequence with an early termination codon after amino acid 138, which deletes part of the multimerization domain.
Fig 4.
p21Rex has no effect on RNA export and does not interfere with p27Rex RNA export functions. (A) Western blot analyses of p27Rex and p21Rex expression in HTLV-1-transformed MT-4, MT-2, and 293T cells transfected with pcRex or CMV-tax/rex-LTR. (B) Effects of p21Rex on nuclear RNA export were tested using the CAT-RxRE vector in transiently transfected 293T cells. Values are expressed as means with top ranges of values for two independent experiments. (C) The potential effects of p21Rex on p27Rex RNA export functions were tested by transient transfection of CAT-RxRE in the absence or presence of p27Rex and increasing amounts of p21Rex expression vector. Values are expressed as means with top ranges of values for two independent experiments.
We next investigated whether p21Rex can export RxRE-bearing RNA or interfere with p27Rex-mediated export. Our results clearly demonstrate that p21Rex cannot export RNA from the nucleus to the cytoplasm (Fig. 4B) and does not affect p27Rex export function even when overexpressed at suboptimal physiological levels (Fig. 4C). Our results contradict a previous paper suggesting that p21Rex may inhibit p27Rex (24). In that study, the authors used a 4- to 10-fold excess of a p21RexGFP expression vector in order to see an effect on p27Rex-mediated export. Clearly, such conditions are not physiological given the results presented in Fig. 4A showing that HTLV-1-transformed cells express p27Rex at a large excess relative to p21Rex. Under our experimental conditions, even with an excess of p21Rex, there was no effect on p27Rex. It is possible that a large excess of p21Rex fused to green fluorescent protein (GFP) acts differently from our p21Rex vector.
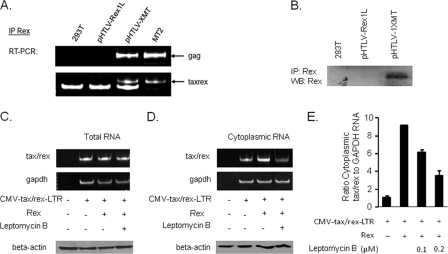
To further investigate the effect of Rex on the tax/rex RNA, we transfected 293T cells with pCDNA, the wild-type HTLV-1 molecular clone pHTLV-1XMT, or pHTLV-1XMT mutated to abolish the expression of Rex (pHTLV-Rex1L). The absence of Rex expression from pHTLV-Rex1L was confirmed by transient-transfection, immunoprecipitation, and Western blot analyses (Fig. 5B). Our data confirmed the absence of Rex expression from this molecular clone and suggested that mutation of the Rex Met initiation codon to Leu has a different outcome that is context and promoter dependent (compare expression of Rex from pc-Tax and pHTLV-Rex1L) (Fig. 1 and 5B). We next investigated the ability of Rex to interact with the tax/rex mRNA by using RNA immunoprecipitation (RNA-IP) assays. For these experiments, specific primers were used to amplify tax/rex or gag, used as a control because numerous reports have established that gag RNA is posttranscriptionally regulated by Rex. Following formaldehyde cross-linking, Rex was immunoprecipitated with a Rex rabbit antiserum and bound RNA detected by RT-PCR. While gag RNA bound to Rex was readily detected, we also found a fraction of the tax/rex RNA bound to Rex. Importantly, no amplification was seen when we used an HTLV-1 molecular clone not expressing Rex, and similar results were also obtained with MT-2, an HTLV-1-replicating transformed cell line (Fig. 5A). These data demonstrate that Rex does indeed interact with the tax/rex mRNA.
Fig 5.
Rex interacts with tax/rex RNA and mediates CRM1-dependent export. (A) Chromatin immunoprecipitation assays. 293T cells were transfected with the HTLV-1 molecular clone pHTLV-1XMT and its Rex counterpart by the calcium phosphate precipitation method. Mock-transfected and HTLV-1-transformed MT-2 cells were used as negative and positive controls, respectively. Cells were lysed in NP-40 lysis buffer containing a phosphatase inhibitor (sodium orthovanadate), a protease inhibitor (phenylmethylsulfonyl fluoride [PMSF]), and RNase inhibitors (ribosyl complexes and RNAseOut). Rex was then immunoprecipitated using a Rex rabbit antiserum (a gift from M. Duc Dodon), and bound RNAs were extracted with TRIzol. RT-PCR was performed with a Qiagen one-step RT-PCR kit, using primers specific for gag (LTR2 and USDR1) or tax/rex (RPX3 and RPX4) RNA. (B) The absence of Rex expression from pHTLV-Rex1L was confirmed by transient transfection of 293T cells and IP and Western blot (WB) analyses of Rex expression. (C and D) Effects of Rex on tax/rex expression in the total RNA fraction (C) or cytoplasmic RNA fraction (D) in the absence or presence of LMB (a CRM1 inhibitor). General toxicity was monitored by Western blot analysis of beta-actin, using equal amounts of proteins as determined by Bradford assay. (E) One microgram of CMV-tax/rex-LTR and 0.5 μg pMH-Rex were used for transfection of 293T cells. After 16 h, LMB was added to the cells, and 8 h later, cytoplasmic RNA was prepared from the cells. Quantitative RT-PCR was used to measure the effects of Rex on tax/rex expression in the cytoplasmic RNA fraction in the absence or presence of an increasing dose of LMB (0.1 and 0.2 μM), used as a CRM1 inhibitor. Results are derived from two independent experiments, each performed in duplicate.
Several studies have established that Rex-mediated nucleocytoplasmic export of viral RNA requires binding to human chromosomal region maintenance 1 (hCRM1; also known as exportin 1 [XPO1]) (18), a member of the importin family, in collaboration with a GTP-bound form of the small G protein Ran (Ran-GTP). The complex formed by Rex, hCRM1, Ran-GTP, and the viral mRNA containing the RxRE is then transported to the cytoplasm. Leptomycin B (LMB), an antifungal antibiotic, has been reported to act as a specific inhibitor of CRM1-mediated RNA export (29, 33). As expected, either Rex or leptomycin B had a limited effect on the amount of total tax/rex RNA expressed from the CMV-tax/rex-LTR plasmid (Fig. 5C). However, exogenous Rex expression significantly increased the cytoplasmic amount of tax/rex RNA expressed from CMV-tax/rex-LTR (Fig. 5D). Western blots for beta-actin were performed to control for general toxicity of LMB treatment (Fig. 5C and D). Our results were specific to Rex posttranscriptional functions, as the use of leptomycin B efficiently decreased the amount of cytoplasmic tax/rex RNA in a dose-dependent manner, by more than 50% (Fig. 5E). Altogether, our results demonstrate that Rex interacts with the RxRE-containing tax/rex RNA to facilitate its CRM1-dependent cytoplasmic export.
DISCUSSION
Based upon indirect evidence, it has long been accepted that the tax/rex mRNA is Rex independent for its nuclear-cytoplasmic export and expression. In this study, we demonstrate that this concept is imprecise, showing that the tax/rex mRNA is largely dependent upon Rex expression for efficient RNA export and Tax expression and that p21Rex has no significant effects on p27Rex RNA export functions. We constructed a vector to express tax/rex cDNA under the control of a CMV promoter and a 3′-LTR. Our design allows study of tax/rex mRNA expression and export in a Tax-independent manner. In addition, this construct contained the RxRE and allowed us to uncouple splicing and export to study the posttranscriptional effect of Rex on nuclear-cytoplasmic export of viral RNA.
Our results clearly demonstrated that in the absence of Rex, tax/rex RNA was exported to the cytoplasm less efficiently, resulting in smaller amounts of Tax protein expression. These effects were dependent on the presence of the RxRE, inasmuch as Rex had no effect on the same cDNA when the 3′-LTR sequence was replaced by the simian virus 40 (SV40) polyadenylation signal. We also demonstrated that the RNA-binding activity of Rex was required, since a Rex mutant (Rexlys) unable to interact with the RxRE had no significant effect on tax/rex export and could not increase Tax expression. These results were confirmed and extended to tax/rex RNA expressed in a physiological context by use of HTLV-1 infectious molecular clones or HTLV-1-transformed MT-2 cells. In both situations, RNA-IP assays demonstrated binding of Rex to the tax/rex RNA with natural expression of viral components. Moreover, a molecular clone deleted for Rex expression was used as a control and displayed no interactions, confirming the specificity of our observations.
Finally, consistent with previous studies that showed a requirement of CRM1 for HTLV-1 Rex export functions, the use of leptomycin B, an inhibitor of CRM1, abolished Rex-mediated export of tax/rex RNA. Altogether, these compelling data demonstrate that HTLV-1 tax/rex mRNA is in fact Rex dependent for export and expression of both the Tax and Rex proteins. Tightly controlled regulation of tax/rex RNA expression is essential for HTLV-1, since high expression of these proteins appears to be toxic to newly infected cells and stimulates the expression of viral antigens. Along these lines, the posttranscriptional repressor HTLV p30 prevents export of the tax/rex RNA and may play an essential role in maintaining a balanced expression of Tax and Rex. However, the function of p30 in repressing viral gene expression presents an apparent conundrum. Although it is essential for HTLV-1 to reduce its expression to evade immune clearance, “true” latency would not benefit the virus, which in its early stages needs some viral gene expression to transmit virus to neighboring cells, alter cell cycle checkpoints, alter DNA repair, and extend the life span of infected cells, thus facilitating transformation.
Interestingly, our data obtained using the CMV-tax/rex-LTR (Rex−) vector reconcile this paradox. We found that a small fraction of tax/rex RNA is exported to the cytoplasm and expressed independently of viral proteins. This suggests either a leaky export of viral RNA or that cellular factors can stimulate export, albeit inefficiently, of incompletely spliced RNA to the cytoplasm. This step is critically important, since initiation of virus replication requires some Tax and Rex proteins, and in the absence of Rex-independent export, infection would be abortive and lead to latently infected cells unable to replicate the virus. Our studies suggest that expression of Tax and Rex is largely Rex dependent. This is in sharp contrast to the case for HIV. In the HIV genome, the Rev-responsive element (RRE) is not placed in the 3′-LTR and is spliced out from some viral mRNAs, including the RNA encoding Tat and Rev (34). We think that this stratagem may have important implications for the establishment of latency and virus reexpression. Along these lines, it may be easier for HTLV-1 to hide but more difficult for it to reinitiate the virus cycle.
It has long been known that simple retroviruses, such as Mason-Pfizer monkey virus (MPMV) and avian leukemia viruses (7, 11), use a constitutive transport element (CTE) to allow nuclear export of incompletely spliced viral RNA. If a cellular protein/pathway is involved in Rex-independent export of tax/rex RNA, additional studies are warranted, since it may offer a target for manipulating HTLV-1 latency and either preventing de novo infection and virus spread or uncovering virally infected cells for presentation to the host immune system.
ACKNOWLEDGMENTS
This work was supported by National Cancer Institute grant AI 058944 to C.N.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published ahead of print 8 February 2012
REFERENCES
- 1. Adachi Y, et al. 1992. Phosphorylation of the Rex protein of human T-cell leukemia virus type I. J. Biol. Chem. 267:21977–21981 [PubMed] [Google Scholar]
- 2. Adachi Y, Nosaka T, Hatanaka M. 1990. Protein kinase inhibitor H-7 blocks accumulation of unspliced mRNA of human T-cell leukemia virus type I (HTLV-I). Biochem. Biophys. Res. Commun. 169:469–475 doi:0006-291X(90)90355-Q [DOI] [PubMed] [Google Scholar]
- 3. Ahmed YF, Gilmartin GM, Hanly SM, Nevins JR, Greene WC. 1991. The HTLV-I Rex response element mediates a novel form of mRNA polyadenylation. Cell 64:727–737 doi:0092-8674(91)90502-P [DOI] [PubMed] [Google Scholar]
- 4. Ballaun C, et al. 1991. Functional analysis of human T-cell leukemia virus type I Rex-response element: direct RNA binding of Rex protein correlates with in vivo activity. J. Virol. 65:4408–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bindhu M, Nair A, Lairmore MD. 2004. Role of accessory proteins of HTLV-1 in viral replication, T cell activation, and cellular gene expression. Front. Biosci. 9:2556–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogerd H, Greene WC. 1993. Dominant negative mutants of human T-cell leukemia virus type I Rex and human immunodeficiency virus type 1 Rev fail to multimerize in vivo. J. Virol. 67:2496–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bogerd HP, Echarri A, Ross TM, Cullen BR. 1998. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 72:8627–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bogerd HP, Huckaby GL, Ahmed YF, Hanly SM, Greene WC. 1991. The type I human T-cell leukemia virus (HTLV-I) Rex trans-activator binds directly to the HTLV-I Rex and the type 1 human immunodeficiency virus Rev RNA response elements. Proc. Natl. Acad. Sci. U. S. A. 88:5704–5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogerd HP, Tiley LS, Cullen BR. 1992. Specific binding of the human T-cell leukemia virus type I Rex protein to a short RNA sequence located within the Rex-response element. J. Virol. 66:7572–7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Copeland KF, Haaksma AG, Derse D, Heeney JL. 1994. Detection of human T-cell leukaemia virus 1 permissive cells using cell lines producing selectable recombinant virions. J. Virol. Methods 50:219–225 [DOI] [PubMed] [Google Scholar]
- 11. Cullen BR. 2003. Nuclear mRNA export: insights from virology. Trends Biochem. Sci. 28:419–424 doi:S0968000403001427 [DOI] [PubMed] [Google Scholar]
- 12. D'Agostino DM, Ciminale V, Zotti L, Rosato A, Chieco-Bianchi L. 1997. The human T-cell lymphotropic virus type 1 Tof protein contains a bipartite nuclear localization signal that is able to functionally replace the amino-terminal domain of Rex. J. Virol. 71:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delamarre L, Rosenberg AR, Pique C, Pham D, Dokhelar MC. 1997. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J. Virol. 71:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franchini G, Nicot C, Johnson JM. 2003. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv. Cancer Res. 89:69–132 [DOI] [PubMed] [Google Scholar]
- 15. Giebler HA, et al. 1997. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol. Cell. Biol. 17:5156–5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grone M, Koch C, Grassmann R. 1996. The HTLV-1 Rex protein induces nuclear accumulation of unspliced viral RNA by avoiding intron excision and degradation. Virology 218:316–325 [DOI] [PubMed] [Google Scholar]
- 17. Hakata Y, Umemoto T, Matsushita S, Shida H. 1998. Involvement of human CRM1 (exportin 1) in the export and multimerization of the Rex protein of human T-cell leukemia virus type 1. J. Virol. 72:6602–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hakata Y, Yamada M, Shida H. 2001. Rat CRM1 is responsible for the poor activity of human T-cell leukemia virus type 1 Rex protein in rat cells. J. Virol. 75:11515–11525 doi:10.1128/JVI.75.23.11515-11525.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hakata Y, Yamada M, Shida H. 2003. A multifunctional domain in human CRM1 (exportin 1) mediates RanBP3 binding and multimerization of human T-cell leukemia virus type 1 Rex protein. Mol. Cell. Biol. 23:8751–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamaia S, Casse H, Gazzolo L, Duc DM. 1997. The human T-cell leukemia virus type 1 Rex regulatory protein exhibits an impaired functionality in human lymphoblastoid Jurkat T cells. J. Virol. 71:8514–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammes SR, Greene WC. 1993. Multiple arginine residues within the basic domain of HTLV-I Rex are required for specific RNA binding and function. Virology 193:41–49 doi:S0042-6822(83)71101-3 [DOI] [PubMed] [Google Scholar]
- 22. Hanly SM, et al. 1989. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 3:1534–1544 [DOI] [PubMed] [Google Scholar]
- 23. Harrod R, et al. 1998. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 18:5052–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heger P, Rosorius O, Hauber J, Stauber RH. 1999. Titration of cellular export factors, but not heteromultimerization, is the molecular mechanism of trans-dominant HTLV-1 Rex mutants. Oncogene 18:4080–4090 doi:10.1038/sj.onc.1202762 [DOI] [PubMed] [Google Scholar]
- 25. Hidaka M, Inoue J, Yoshida M, Seiki M. 1988. Post-transcriptional regulator (Rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 7:519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang H, et al. 1999. PCAF interacts with Tax and stimulates Tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136–8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kashanchi F, Brady JN. 2005. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene 24:5938–5951 [DOI] [PubMed] [Google Scholar]
- 28. King JA, et al. 1998. Nucleocytoplasmic transport of HTLV-1 RNA is regulated by two independent LTR encoded nuclear retention elements. Oncogene 16:3309–3316 doi:10.1038/sj.onc.1201884 [DOI] [PubMed] [Google Scholar]
- 29. Kudo N, et al. 1998. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242:540–547 doi:S0014-4827(98)94136-2 [DOI] [PubMed] [Google Scholar]
- 30. Lemasson I, Polakowski NJ, Laybourn PJ, Nyborg JK. 2004. Transcription regulatory complexes bind the human T-cell leukemia virus 5′ and 3′ long terminal repeats to control gene expression. Mol. Cell. Biol. 24:6117–6126 doi:10.1128/MCB.24.14.6117-6126.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicot C, et al. 2004. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 10:197–201 doi:10.1038/nm984 [DOI] [PubMed] [Google Scholar]
- 32. Nicot C, Harrod RL, Ciminale V, Franchini G. 2005. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene 24:6026–6034 [DOI] [PubMed] [Google Scholar]
- 33. Otero GC, Harris ME, Donello JE, Hope TJ. 1998. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus Rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J. Virol. 72:7593–7597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz S, Felber BK, Pavlakis GN. 1991. Expression of human immunodeficiency virus type 1 Vif and Vpr mRNAs is Rev-dependent and regulated by splicing. Virology 183:677–686 doi:0042-6822(91)90996-O [DOI] [PubMed] [Google Scholar]
- 35. Sinha-Datta U, Datta A, Ghorbel S, Dodon MD, Nicot C. 2007. Human T-cell lymphotrophic virus type I Rex and p30 interactions govern the switch between virus latency and replication. J. Biol. Chem. 282:14608–14615 doi:10.1074/jbc.M611219200 [DOI] [PubMed] [Google Scholar]
- 36. Smith MR, Greene WC. 1990. Identification of HTLV-I Tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875–1885 [DOI] [PubMed] [Google Scholar]