Abstract
Vaccination is the primary form of protection from influenza virus infection. We recently developed a replication-incompetent PB2-knockout (PB2-KO) influenza virus that possesses a reporter gene (the green fluorescent protein gene) in the coding region of the PB2 segment. This virus replicated to high titers in PB2-expressing, but not unmodified, cells, suggesting its potential safety and feasibility as a vaccine. Here, we tested its efficacy in a murine model. The levels of IgG and IgA antibodies against influenza virus in sera, nasal washes, and bronchoalveolar lavage fluids of mice immunized with the PB2-KO virus were higher than those induced by a conventional inactivated vaccine. All PB2-KO virus-immunized mice survived challenges with lethal doses of influenza virus. Moreover, importantly, mice immunized with the PB2-KO virus produced antibodies against the reporter protein, suggesting that the PB2-KO virus has potential as a multivalent vaccine to combat infection with not only influenza virus but also other pathogens.
INTRODUCTION
Vaccine and therapeutic antiviral research on the prevention and control of influenza viruses is essential to limit the strain placed on health care systems by influenza epidemics and global pandemics. Intensive research has led to the discovery of therapeutic interventions to combat influenza infections; however, due to the virus's error-prone polymerase, the hemagglutinin (HA) and neuraminidase (NA) influenza viral proteins are subject to point mutations, known as antigenic or genetic drift (12), that allow the virus to escape host immune responses or result in some types of drug resistance (13). Vaccination is one of the most effective means of preventing influenza-associated morbidity and mortality.
Currently, inactivated vaccines are widely used for the prevention of influenza; however, inactivated influenza vaccines provide short protection periods and limited efficacy, especially in young children and the elderly (3, 17). In addition, due to the inability to effectively elicit cell-mediated immunity, inactivated vaccines are generally less immunogenic, and hence less potent, than live attenuated vaccines, which are approved for use in a limited number of countries such as the Unites States. Intranasally administered live attenuated viruses are considered superior to inactivated vaccines for children because they elicit robust mucosal immunity and humoral and cellular immune responses coupled with long-lasting protective efficacy (1). However, live attenuated vaccines are currently licensed only for individuals aged 2 through 49 who lack chronic medical conditions and who are not pregnant or immunocompromised, even though licensed live attenuated influenza viruses are considered safe and stable with respect to the underlying risk of the emergence of revertant viruses. Hence, the development of improved influenza vaccines is critical to control future outbreaks.
Recently, our group genetically engineered PB2-knockout (PB2-KO) influenza viruses that are able to harbor a reporter gene, such as the green fluorescent protein (GFP) gene, between the packaging signals (the noncoding and coding 120 bases at both the 5′ and 3′ ends) of the PB2 gene (16). Growth of such PB2-KO viruses is restricted to a cell line stably expressing the PB2 protein and yields high virus titers (>108 PFU/ml). The HA and NA genes of a heterologous influenza virus could be accommodated in the PB2-KO virus (16). Furthermore, the recombinant PB2/reporter gene was stably incorporated into progeny PB2-KO virions and was retained through sequential passages. Therefore, the PB2-KO virus can be tailored to encode not only desirable combinations of the main influenza virus antigens, namely, HA and NA, but also non-influenza virus antigens, suggesting that the PB2-KO virus could be used as a multivalent vaccine. Here, we tested the vaccine potential of the PB2-KO virus by immunizing mice and examining antibody responses and protective efficacy.
MATERIALS AND METHODS
Cells.
293 and 293T (a derivative of the 293 line into which the gene for simian virus 40 T antigen was inserted [2]) human embryonic kidney cells were maintained in Dulbecco's modified Eagle medium (Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA). Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium (MEM) (Invitrogen) supplemented with 5% newborn calf serum (NCS) (Sigma, St. Louis, MO). AX4 cells, which are an MDCK-derived cell line with enhanced expression of human-type receptors for influenza virus and were produced by stable transfection of a plasmid expressing the human α-2,6-sialyltransferase (8), were maintained in 5% NCS–MEM supplemented with puromycin (2 μg/ml). AX4/PB2 cells (AX4 cells stably expressing the PB2 protein derived from A/Puerto Rico/8/34 [H1N1, PR8], established by transduction with a retroviral vector [16]) were maintained in 5% NCS–MEM supplemented with puromycin (2 μg/ml) and blasticidin (10 μg/ml). All cells were maintained in a humidified incubator at 37°C in 5% CO2.
Plasmid-driven reverse genetics.
The wild-type PR8 and PB2-KO viruses used in this study were engineered by using reverse genetics, as previously described (15). For expression of viral RNA (vRNA), plasmids contained the cloned cDNAs of PR8 genes between the human RNA polymerase I promoter and the mouse RNA polymerase I terminator (referred to as PolI plasmids). A plasmid [pPolIPB2(120)GFP(120)] was constructed to replace the PolI plasmid encoding the PB2 segment and contained the A/WSN/33(H1N1)-derived 3′ PB2 noncoding region, 120 nucleotides that correspond to the PB2-coding sequence at the 3′ end of the vRNA followed by the GFP-coding sequence, 120 nucleotides that correspond to the PB2-coding sequence at the 5′ end of the vRNA, and finally the 5′ PB2 noncoding region (14). To generate the PB2-KO virus, pPolIPB2(120)GFP(120) and the remaining 7 PolI plasmids were cotransfected into 293T cells along with eukaryotic protein expression plasmids for PB2, PB1, PA, and NP derived from A/WSN/33 by use of the TransIT 293 transfection reagent (Mirus, Madison, WI), following the manufacturer's instructions. At 48 h posttransfection, the supernatants containing the PR8 or PB2-KO virus were harvested and inoculated into 10-day-old embryonated chicken eggs or AX4/PB2 cells, respectively. Both viruses were titrated by use of plaque assay with AX4/PB2 cells.
Preparation of formalin-inactivated virus.
Egg-propagated PR8 viruses were concentrated and purified by ultracentrifugation of the infected allantoic fluid through a 10% to 50% sucrose density gradient and resuspended in phosphate-buffered saline (PBS). Formalin (final concentration, 0.1%) was added to inactivate the purified PR8 virus at 4°C for 1 week. Inactivation of the virus was confirmed by passaging viruses twice in MDCK cells and examining the cytopathic effect or lack thereof.
Experimental infection of mice with PB2-KO virus.
To test the safety of the PB2-KO virus in mice, six 4-week-old female BALB/c mice were intranasally inoculated with 106 PFU/mouse of the virus. The body weight and survival of the infected mice were monitored daily for 14 days postinoculation. Also, on days 1, 3, and 6 postinoculation, lungs and nasal turbinates from the inoculated mice were harvested, homogenized, and subjected to plaque assays to detect the presence of virus.
Immunization and protection test.
Eight-, six-, or four-week-old female BALB/c mice (3 mice per group) were anesthetized with isoflurane and intranasally inoculated with 50 μl of medium (MEM containing 0.3% bovine serum albumin fraction V), formalin-inactivated PR8 (64 hemagglutination units, which is equivalent to 106 PFU of the PB2-KO virus), or PB2-KO virus (106 PFU) once, twice, or three times at 2-week intervals, respectively. Three weeks after the final inoculation, mice were intranasally challenged with 0.5 or 5 50% mouse lethal doses (MLD50) of PR8 virus. On days 3 and 6 postinfection, lungs and nasal turbinates of mice (3 mice per group) were collected, homogenized in 1 ml of PBS by using TissueLyser II (Qiagen, Valencia, CA), and clarified by low-speed centrifugation (5,000 rpm for 10 min at 4°C). Virus titers in homogenates were determined by using plaque assays with AX4/PB2 cells. The body weight and survival of the remaining challenged mice (3 mice per group) were monitored daily for 14 days.
Detection of virus-specific antibodies.
Sera from mice (3 mice per group) were obtained via mandibular vein bleeding prior to each immunization and via the femoral artery 1 day before challenge. Nasal wash and bronchoalveolar lavage (BAL) fluid samples (3 mice per group) were also obtained 1 day before challenge from mice sacrificed by cervical dislocation. Incisions were made to insert a cannula into the trachea. The lungs were then perfused with 1 ml of PBS by using a syringe. The lavage fluid was recovered and stored in microtubes on ice. Nasal wash was collected by passing 400 μl of PBS through the nasal cavity. IgG and IgA antibodies in the sera, nasal washes, and BAL fluid samples were detected by using an enzyme-linked immunosorbent assay (ELISA) as previously described (11). Each well was coated with purified PR8 disrupted with 0.05 M Tris-HCl (pH 7.8) containing 0.5% Triton X-100 and 0.6 M KCl. After incubation of the virus-coated plates with the test samples, IgA and IgG antibodies in the samples were detected by use of goat anti-mouse IgA or IgG antibodies conjugated to horseradish peroxidase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Neutralizing antibody titers in sera of immunized mice were also evaluated as previously described (9). Briefly, virus (100 50% tissue culture infectious doses [TCID50]) was incubated with 2-fold serial dilutions of receptor-destroying enzyme-treated sera for 30 min at 33°C, and the mixtures were added to confluent MDCK cells on 96-well microplates to determine the neutralizing activity.
IFA for detection of antibodies against GFP.
293 cells grown in 35-mm glass-bottom dishes (Asahi Techno Glass) were transfected with a plasmid expressing GFP and incubated for 48 h prior to the immunofluorescence assay (IFA). Cells were fixed in PBS containing 4% paraformaldehyde (Wako Pure Chemical Industries Ltd.) for 15 min and permeabilized with 0.1% Triton X-100 for 5 min. They were incubated for 1 h with 20-fold-diluted serum collected from mice mock immunized with medium or immunized with formalin-inactivated PR8 or with the PB2-KO virus. Anti-GFP antibody (clone GFP-20; Sigma-Aldrich)-treated cells served as a positive control. All cells were then further incubated for 1 h with an Alexa Fluor 594-labeled goat anti-mouse secondary antibody (Invitrogen) and Hoechst 33342 (Invitrogen) for the detection of GFP antibody and nuclear staining, respectively. Samples were observed under a confocal laser microscope (LSM510META; Carl Zeiss, Jena, Germany).
RESULTS
Characterization of the PB2-KO virus in mice.
We demonstrated that the PB2-KO virus was replication incompetent in AX4 cells but yielded high titers similar to those of PR8 in AX4/PB2 cells (16). To determine whether the PB2-KO virus could serve as an influenza vaccine, we assessed its safety profile in mice by intranasally inoculating each mouse with the PB2-KO virus (106 PFU in 50 μl) and monitoring body weight for 2 weeks. Mice steadily achieved stable growth increments and appeared unperturbed by PB2-KO virus infection (data not shown). Lungs and nasal turbinates obtained on days 1, 3, and 6 postinoculation were homogenized and subjected to plaque assays in AX4/PB2 cells to assess the growth of the PB2-KO virus in mice. No plaques were detected from organs of mice infected with the PB2-KO virus, whereas a high virus titer (108 PFU/g) was found in lung tissue of mice infected with 106 PFU of PR8 virus. These results indicate that the PB2-KO virus did not grow in mice and that reversion to a replication-competent virus did not occur.
Virus-specific antibody responses in mice inoculated with the PB2-KO virus.
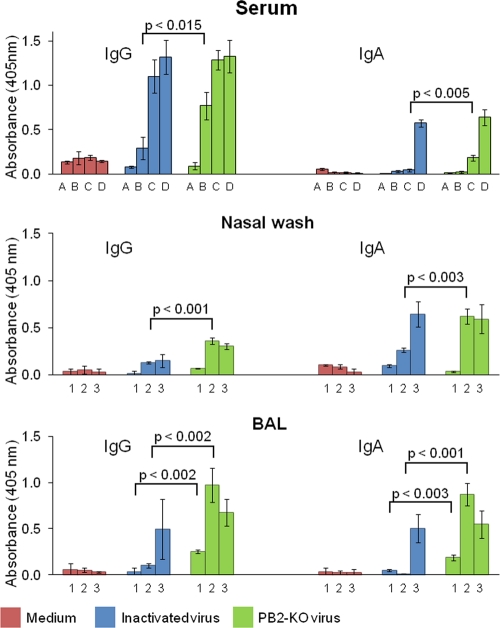
The level of antibody responses elicited by the PB2-KO virus was examined in mice that were intranasally inoculated with the PB2-KO virus once, twice, or three times at 2-week intervals. For comparison, mice were also mock immunized with medium or immunized with formalin-inactivated PR8 virus at a dose equivalent to 106 PFU of the PB2-KO virus. Sera were collected at various time points to determine the presence of different levels of antibodies over time. Also, at 3 weeks after the final inoculation, the levels of IgG and IgA antibodies against PR8 in the sera, nasal washes, and BAL fluid samples were examined by using an ELISA (Fig. 1). Neither the IgG nor the IgA response in any sample was appreciable in mice inoculated with medium. In contrast, mice immunized with the formalin-inactivated PR8 and PB2-KO viruses exhibited a time-dependent increase in serum IgG and IgA levels. After three immunizations, similar antibody levels were detected in both inactivated virus- and PB2-KO virus-immunized mice. Interestingly, when mice were immunized once or twice, significantly higher serum IgG or IgA titers, respectively, were observed in PB2-KO virus-immunized mice than in mice immunized with the formalin-inactivated virus (Fig. 1, top panel). In nasal washes of mice inoculated with PB2-KO virus twice and in BAL fluids of mice inoculated once and twice, IgG and IgA levels were significantly higher than those in mice inoculated with the formalin-inactivated virus (Fig. 1, middle and bottom panels, respectively). Thus, PB2-KO virus efficiently induced IgG and IgA antibody responses in this murine model. Sera obtained from mice mock immunized with medium had no neutralizing antibodies, whereas those from PB2-KO-treated mice had neutralizing antibody titers of 1:16, which was approximately 2-fold higher than that in mice treated with inactivated virus (data not shown). We did not detect neutralizing activities in any nasal wash sample or BAL fluid (data not shown).
Fig 1.
Virus-specific antibody responses in immunized mice. Purified PR8 virus was used as an antigen to analyze IgG and IgA antibody titers in the sera, nasal washes, and BAL fluids (top, middle, and bottom, respectively) of mice mock immunized with medium or immunized with the formalin-inactivated virus or with the PB2-KO virus. Sera (top panels) were obtained at different time points, i.e., prevaccination (bars A), before the second vaccination (bars B), before the third vaccination (bars C), and before challenge (bars D). Nasal washes and BAL fluids (middle and bottom panels, respectively) were obtained 1 day before challenge from mice given 1 vaccination (bars 1), 2 vaccinations (bars 2), or 3 vaccinations (bars 3). Values are expressed as the mean absorbance ± standard deviation (SD) (n = 3). Statistical significance between samples obtained from mice immunized with inactivated virus and PB2-KO virus is indicated.
Vaccine efficacy of the PB2-KO virus.
To assess the vaccine efficacy of the PB2-KO virus, we challenged mice with 0.5 or 5 MLD50 of PR8 virus. The former challenge dose was tested to mimic natural infections, in which individuals are usually infected with a relatively low virus dose (certainly not a lethal dose).
(i) Body weight changes and survival of immunized mice after challenge.
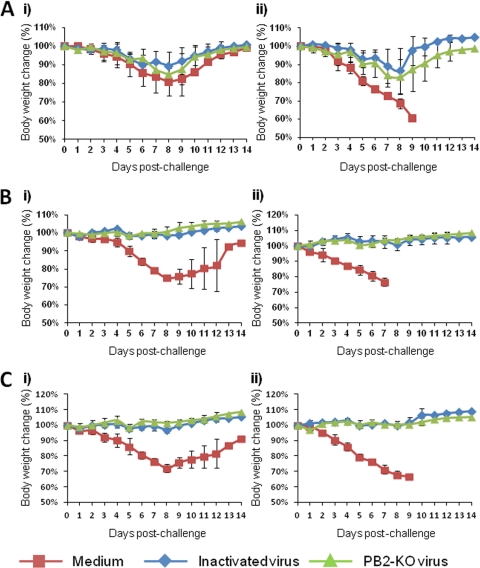
To assess the vaccine efficacy of the PB2-KO virus, we examined body weight changes and survival of mice immunized with the PB2-KO virus after they were challenged with the PR8 virus. Mice mock immunized with medium and challenged with 0.5 MLD50 of PR8 experienced substantial body weight loss, which they subsequently recovered (Fig. 2, left panels). On the other hand, all mice mock immunized with medium and challenged with 5 MLD50 of PR8 showed substantial body weight loss and died at approximately 1 week postinfection (Fig. 2, right panels). Mice immunized once with the formalin-inactivated or PB2-KO virus experienced weight loss (15%) after each challenge dose (Fig. 2A). It is noteworthy that 100% of mice immunized once with the PB2-KO virus survived, whereas one out of three mice immunized once with the formalin-inactivated virus died on day 8 postinfection after being challenged with 5 MLD50 of the PR8 virus (data not shown). All mice immunized twice and three times with the inactivated and PB2-KO viruses survived without any appreciable body weight loss (Fig. 2B and C).
Fig 2.
Body weight change after challenge in mice. Mice immunized with the indicated agents once (A), twice (B), or three times (C) were challenged with 0.5 (i) or 5 (ii) MLD50 of PR8 virus. Values are expressed as mean changes in body weight ± SD (n = 3).
(ii) Virus replication in lungs and nasal turbinates.
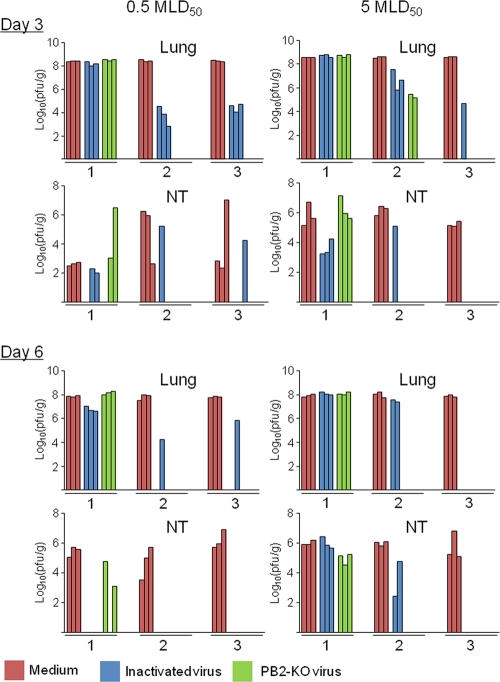
To evaluate virus replication in the lungs and nasal turbinates of mice immunized with the PB2-KO virus, both organs were collected on days 3 and 6 postchallenge with the PR8 virus. Figure 3 shows the extent of virus replication in these organs. The PR8 virus replicated to a high titer in the lungs and nasal turbinates of all mock-immunized mice. Although the potency of the PB2-KO vaccine was similar to that of the formalin-inactivated vaccine in mice immunized once, in mice that received two or three vaccinations, the PB2-KO vaccine was more efficacious than the formalin-inactivated vaccine, with virus titers in both organs being considerably lower in mice immunized with the former than in those immunized with the latter. Taken together, these results indicate that the PB2-KO virus has better potency as an influenza vaccine than the formalin-inactivated virus.
Fig 3.
Virus titers in the lungs and nasal turbinates (NT) of immunized mice after challenge. The numbers on the x axis indicate the number of vaccinations. Three BALB/c mice per group were intranasally infected with the indicated doses of PR8 virus (50 μl per mouse) and sacrificed on days 3 and 6 postinfection for virus titration. Bars indicate the virus titer in each organ of each mouse. The absence of bars indicates that virus titers were below the detection limit of 5 PFU/ml/organ.
Detection of antibodies against GFP in mice inoculated with the PB2-KO virus.
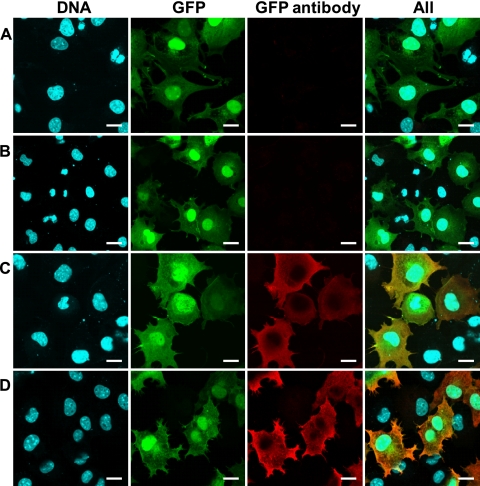
Finally, we examined whether the PB2-KO virus could induce antibodies against GFP, because the PB2-KO virus used here possesses the GFP gene in its PB2-coding region and GFP was expressed in PB2-KO virus-infected culture cells (data not shown). The detection of an anti-GFP antibody in the sera of mice inoculated with the PB2-KO virus would suggest the potential for this system as a platform for the development of an influenza virus-based multivalent vaccine. We therefore collected sera from mice on day 3 postchallenge and tested them in an IFA. We did not detect GFP with sera from mock-immunized mice or from those immunized with the inactivated vaccine (Fig. 4); however, sera from mice immunized with the PB2-KO virus, as well as a commercial anti-GFP antibody (which served as a positive control), detected GFP expression. These results indicate that an antibody against GFP was induced in mice immunized with the PB2-KO virus, suggesting the potential application of the PB2-KO virus as a multivalent vaccine.
Fig 4.
Detection of antibodies against GFP in the sera of mice immunized with the PB2-KO virus. Confluent 293 cells that transiently express GFP were treated with sera (1/20 dilution) obtained from mice inoculated with medium (A), the formalin-inactivated virus (B), or the PB2-KO virus (C) or were treated with a commercial anti-GFP antibody (D). DNA (first column) was stained with Hoechst 33342. GFP (second column) represents cells transfected with a plasmid for the expression of GFP. GFP antibody (third column) represents the presence of the GFP antibody in the samples. These three images were merged (fourth column). Scale bars, 20 μm.
DISCUSSION
Here, we demonstrated that a replication-incompetent PB2-KO virus elicits virus-specific protective antibody responses and that this virus also induces antibodies against the reporter protein encoded in the coding region of its PB2 segment. These results suggest that this replication-incompetent PB2-KO virus can serve as a platform for an influenza vaccine as well as for a multivalent vaccine if the PB2-coding region is replaced with the antigenic portion of another pathogen.
Both live attenuated and most inactivated influenza vaccines are currently propagated in embryonated chicken eggs, although cell-based vaccines have been licensed in Europe. Since a prerequisite for successful egg-based vaccine propagation is the selection of variants adapted to embryonated chicken eggs at the time of implementation, the virus in the vaccine may be slightly different from the circulating viruses in terms of antigenicity (4, 7, 18). Because of the propensity of egg proteins in these vaccines to induce allergies, parenterally administered inactivated vaccines produced in eggs are associated with adverse or anaphylactic reactions in some individuals (5). An added complication is the possible depletion of chicken stocks in the event of an outbreak of a highly pathogenic avian influenza pandemic, which could compromise mass vaccine production (6). In contrast, cell-based alternatives offer several advantages over conventional egg-based vaccine propagation. Manufacturing capacity can be readily scaled up in proportion to demand. In addition, unlike for viruses grown in eggs, the antigenicity of viruses grown in cells matches that in animals and humans (10, 19). Thus, the cell-based PB2-KO vaccine proposed in this study eliminates various obstacles to vaccine preparation and delivery.
We previously demonstrated that replication-incompetent virus-like particles (VLPs) efficiently elicit mucosal and systemic immune responses in a murine model. VLPs that lack NS2 protect mice against various lethal doses of influenza viruses (20). However, the absence of a cell line that constitutively expresses NS2 precludes the efficient production of sufficient VLPs to elicit protective efficacy. We established a cell line that stably expresses PB2 (AX4/PB2) and demonstrated that the PB2-KO virus could efficiently replicate in this cell line (i.e., at a level comparable to that for wild-type virus) (16). We also showed that our viral system stably incorporated and maintained the GFP gene during virus replication (16). These data clearly establish the feasibility of using this system for efficient vaccine production.
The formalin-inactivated vaccine efficiently protected mice from challenges with lethal doses of the PR8 virus by eliciting immune responses (Fig. 1, 2, and 3). However, even though the outcomes in terms of survival and body weight loss were similar for mice immunized with the formalin-inactivated vaccine and those immunized with the PB2-KO vaccine (Fig. 2), the virus titers in the lungs and nasal turbinates of the mice immunized with the former vaccine were higher than in those in mice immunized with the latter (Fig. 3). This finding likely reflects differences in the levels of immune responses (Fig. 1). It is also plausible that cytotoxic T lymphocyte (CTL) responses were activated by the PB2-KO virus but not by the formalin-inactivated virus, since inactivated antigens are thought not to induce CTL responses, although we did not examine CTL responses in this study.
Streptococcus pneumoniae is a respiratory pathogen that causes secondary bacterial infection following influenza virus infection, which is associated with elevated mortality in the elderly. Parainfluenza viruses, such as respiratory syncytial virus and human parainfluenza virus type 1, are respiratory pathogens that cause severe manifestations in infants. No vaccines are currently available for parainfluenza virus. We demonstrated here that the PB2-KO virus could be used as a multivalent vaccine because an antibody against the reporter gene product (GFP in this case) encoded in the coding region of the PB2 segment was induced in place of authentic PB2 (Fig. 4). If major antigens of the above-described pathogens are similarly encoded in the coding region of the PB2 segment, the PB2-KO virus could induce immune responses against those antigens as well as against influenza viral proteins, thereby protecting infants and the elderly from these serious respiratory diseases.
In conclusion, given that the PB2-KO virus elicited effective immune responses, induced antibodies against the product of a reporter gene encoded in its PB2 segment, is easily propagated, and can be safely administered as a vaccine, the PB2-KO virus represents a credible, safe, and efficacious vaccine candidate.
ACKNOWLEDGMENTS
We thank Susan Watson for editing the manuscript.
This work was supported by a grant-in-aid for Specially Promoted Research and by the Japan Initiative for Global Research Network on Infectious Diseases from the Ministries of Education, Culture, Sports, Science, and Technology, by grants-in-aid from Health, Labor, and Welfare of Japan, by ERATO (Japan Science and Technology Agency), and by National Institute of Allergy and Infectious Diseases Public Health Service research grants. S.T.V. is the recipient of scholarships from the Otsuka Toshimi and Honjo International Scholarship Foundations.
Footnotes
Published ahead of print 1 February 2012
REFERENCES
- 1. Cox RJ, Brokstad KA, Ogra P. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 59:1–15 [DOI] [PubMed] [Google Scholar]
- 2. DuBridge RB, et al. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiore AE, et al. 2010. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recommend. Rep. 59:1–62 [PubMed] [Google Scholar]
- 4. Fulvini AA, et al. 2011. Gene constellation of influenza A virus reassortants with high growth phenotype prepared as seed candidates for vaccine production. PLoS One 6:e20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halperin SA, et al. 2002. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 20:1240–1247 [DOI] [PubMed] [Google Scholar]
- 6. Hampson AW. 2008. Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat. Ann. Acad. Med. Singapore 37:510–517 [PubMed] [Google Scholar]
- 7. Hardy CT, Young SA, Webster RG, Naeve CW, Owens RJ. 1995. Egg fluids and cells of the chorioallantoic membrane of embryonated chicken eggs can select different variants of influenza A (H3N2) viruses. Virology 211:302–306 [DOI] [PubMed] [Google Scholar]
- 8. Hatakeyama S, et al. 2005. Enhanced expression of an α2,6-linked sialic acid on MDCK cells improves isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J. Clin. Microbiol. 43:4139–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwatsuki-Horimoto K, et al. 2011. Seroprevalence of pandemic 2009 (H1N1) influenza A virus among schoolchildren and their parents in Tokyo, Japan. Clin. Vaccine Immunol. 18:860–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz JM, Wang M, Webster RG. 1990. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J. Virol. 64:1808–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kida H, Brown LE, Webster RG. 1982. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology 122:38–47 [DOI] [PubMed] [Google Scholar]
- 12. Lin YP, Gregory V, Bennett M, Hay A. 2004. Recent changes among human influenza viruses. Virus Res. 103:47–52 [DOI] [PubMed] [Google Scholar]
- 13. Moss RB, Davey RT, Steigbigel RT, Fang F. 2010. Targeting pandemic influenza: a primer on influenza antivirals and drug resistance. J. Antimicrob. Chemother. 65:1086–1093 [DOI] [PubMed] [Google Scholar]
- 14. Muramoto Y, et al. 2006. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J. Virol. 80:2318–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neumann G, et al. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. U. S. A. 96:9345–9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ozawa M, et al. 2011. Replication-incompetent influenza A viruses that stably express a foreign gene. J. Gen. Virol. 92:2879–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palese P, Garcia-Sastre A. 2002. Influenza vaccines: present and future. J. Clin. Invest. 110:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson JS. 1993. Clinical influenza virus and the embryonated hen's egg. Rev. Med. Virol. 3:97–106 [Google Scholar]
- 19. Robertson JS, et al. 1991. Sequence analysis of the haemagglutinin (HA) of influenza A (H1N1) viruses present in clinical material and comparison with the HA of laboratory-derived virus. J. Gen. Virol. 72:2671–2677 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe T, Watanabe S, Neumann G, Kida H, Kawaoka Y. 2002. Immunogenicity and protective efficacy of replication-incompetent influenza virus-like particles. J. Virol. 76:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]