Abstract
The human immunodeficiency virus type 1 (HIV-1) evades the immune responses of natural killer (NK) cells through mechanisms that have been partially deciphered. Here we show that in HIV-1-infected T lymphocytes, the early viral Nef protein downmodulates PVR (CD155, Necl-5), a ligand for the activating receptor DNAM-1 (CD226) expressed by all NK cells, CD8+ T cells, and other cell types. This novel Nef activity is conserved by Nef proteins of laboratory HIV-1 strains (NL4-3, SF2) and of a patient-derived virus, but it is not maintained by HIV-2. Nef uses the same motifs to downregulate PVR and HLA-I molecules, likely by the same mechanisms. Indeed, as previously demonstrated for HLA-I, Nef reduces the total amounts of cell-associated PVR. Optimal downregulation of cell surface PVR by Nef also requires the presence of the late viral factor Vpu. In line with PVR reduction, the NK cell-mediated lysis of T cells infected by a wild-type but not Nef-deficient virus is virtually abrogated upon blocking of both DNAM-1 and another activating receptor, NKG2D, previously shown to mediate killing of HIV-infected cells. Together, these data demonstrate that the PVR downmodulation by Nef and Vpu is a strategy evolved by HIV-1 to prevent NK cell-mediated lysis of infected cells. The PVR downregulation reported here has the potential to affect the immune responses of other DNAM-1-positive cells besides NK cells and to alter multiple PVR-mediated cellular processes, such as adhesion and migration, and may thus greatly influence HIV-1 pathogenesis.
INTRODUCTION
Several studies have shown that the human immunodeficiency virus type 1 (HIV-1) has evolved the capacity to evade both innate and adaptive immunity and that one of the key viral factors involved in these processes is the Nef protein. Nef is a membrane-associated protein abundantly expressed early in infection and essential for efficient viral replication in vivo and disease progression (19, 34, 35). By means of a remarkable number of activities, including alteration of signal transduction pathways and downregulation of cell surface CD4, human leukocyte antigen class I (HLA-I), CD28, and CXCR4, Nef protects infected cells against the immune system and facilitates viral spread at different levels (3, 36). First of all, by triggering accelerated endocytosis and intracellular retention of HLA-I molecules, Nef reduces their expression on the surface of infected cells and prevents recognition and killing by HIV-specific CD8+ T cells (18). Importantly, Nef downregulates HLA-A and -B but spares HLA-C and -E, which can be recognized by specific inhibitory receptors of natural killer (NK) cells (17). NK cells are regulated by a balance between signals delivered by activating and inhibitory receptors, with the inhibitory signals received from autologous HLA-I molecules usually being predominant to maintain NK cells in a resting state (37). Therefore, the selective activity of Nef on cell surface HLA-I expression simultaneously shields HIV-infected cells from the immune response of both T and NK cells, at least of those NK cells expressing inhibitory receptors specific for HLA-C or -E. Moreover, Nef has the capacity to inhibit the cell surface expression of activating molecules that can trigger the cytotoxic function of NK cells (12, 26). In fact, infection with HIV-1 can induce the expression of ligands for NKG2D (12, 27, 62), an activating receptor present on the surface of all NK and CD8+ T cells (49). In humans, NKG2D ligands include major histocompatibility complex I-related chains A and B (MICA and MICB) and several UL16 binding proteins (ULBP1 to ULBP6) that have a highly restricted expression in normal tissues but can be induced by cellular stress such as viral infection, tumor transformation, heat shock, and DNA damage (13). We showed that Nef is able to downregulate some NKG2D ligands, specifically, MICA, ULBP1, and ULBP2, through an as yet unidentified mechanism (12). Accordingly, Nef expression alone protected T cells from lysis by NK cells (12). In addition, it has been shown that Nef inhibits the expression of the ligand for the NKp44-activating receptor and lowers the susceptibility of HIV-infected cells to NK cell-mediated killing (26). The capacity of Nef to interfere with the expression of various ligands for activating NK receptors may thus play an important role in HIV-1 evasion of NK-cell mediated immunosurveillance. An additional strategy to avoid the immune response of NK cells is exerted by the late viral protein Vpu that avoids NK cell degranulation by downregulating NTB-A on the surface of infected cells (54).
Another activating receptor triggering NK cell functions is DNAM-1 (DNAX accessory molecule-1 or CD226), an adhesion molecule expressed by several cell types, including NK and CD8+ T cells (55). DNAM-1 recognizes two immunoglobulin-like molecules, PVR (poliovirus receptor, CD155, or nectin-like molecule 5) and nectin-2 (CD112), belonging to the family of nectins and nectin-like proteins that regulate cell adhesion, movement, and proliferation, as well as virus entry and immune recognition (60). Upon engagement by its ligands, DNAM-1 can enhance cytokine production and cytotoxicity by NK and T cells toward various tumors (55, 59). The importance of DNAM-1 is also underlined by the fact that some herpesviruses such as human cytomegalovirus (HCMV) have evolved a potent immune evasion strategy which implies the capacity to inhibit the cell surface expression of both DNAM-1 ligands, thus affecting the cytotoxic responses of NK cells (39, 48, 61).
In view of its important role as an immune-activating molecule, we sought to investigate PVR expression in the context of HIV-1 infection. Our interest particularly focused on the role of Nef, considering its established features of regulator of protein trafficking, immunoevasin, and pathogenicity factor.
MATERIALS AND METHODS
Cells and antibodies.
293T and Jurkat E6-1 cells were maintained in Dulbecco's modified Eagle's medium (Gibco-BRL) and RPMI 1640 medium (Euroclone), respectively, both supplemented with 10% fetal bovine serum (Euroclone), 2 mM l-glutamine (Gibco-BRL), and 100 units/ml penicillin-streptomycin (Euroclone). Primary CD4+ T cells were isolated and cultured as described previously (12). Peripheral blood mononuclear cells (PBMCs) were obtained from a donor bank.
Transfection of 293T cells was performed by a standard calcium-phosphate method.
For flow cytometric analysis, the following antibodies were used: mouse monoclonal antibody (MAb) anti-PVR (SKII.4, IgG1), anti-HLA-I (W6/32, IgG2a), fluorescein isothiocyanate (FITC)-conjugated anti-p24 (KC57; Beckman Coulter), phycoerythrin (PE)-conjugated anti-HLA-I (BD Biosciences) and anti-CD4 (Dako), and isotype control IgG1 and IgG2a (BD Biosciences). As secondary antibodies, goat anti-mouse IgG (GAM) conjugated to PE (Jackson ImmunoResearch) and Alexa 647 (Invitrogen) were used. The MAbs anti-DNAM-1 (DX11; AbD Serotec) and anti-NKG2D (149810; R&D Systems) or the isotype control IgG1 were used in cytotoxicity assays. For Western blotting, we used antibodies against Nef (sheep serum ARP444), green fluorescent protein (GFP)/yellow fluorescent protein (YFP) (MAb; Clontech), and actin (MAb; Sigma).
DNA constructs.
Proviral HIV-1 constructs (L37Q, P72A/P75A, P78L, LL165AA, DD175AA, F191A) were generated in the pNLblue Nco nef Not vector (24). Specific mutations (listed in Table 1) were introduced into the nef gene by standard site-directed mutagenesis based on recombinant overlapping PCR. The mutated NL4-3 nef gene was amplified by PCR with specific primers introducing NcoI and NotI sites at the 5′ and 3′ ends, respectively, and cloned in the NcoI/NotI sites, replacing the wild-type (wt) nef gene. A previously described nef gene (RP4-9) derived from a rapid progressor patient (9) was subcloned into pNLblue to generate wt-RP4Nef. The NL4-3 nef (either wt or G2A) was amplified by PCR with specific primers eliminating the stop codon and cloned in the HindIII/EcoRI sites in the pDsRed1-N1 vector (Clontech) to generate C-terminally DsRed-tagged Nef proteins. DNA constructs were confirmed by sequencing on both strands. The constructs obtained from other laboratories are listed in Acknowledgments.
Table 1.
Phenotype of Nef mutants expressed in the NL4-3 HIV-1 strain
| Nef mutation | Lost interaction with: | Downregulation ofa: |
||
|---|---|---|---|---|
| HLA-I | CD4 | PVR | ||
| None (wt) | None | ++ | ++ | ++ |
| 3 stop codons (ΔNef) | All | − | − | − |
| G2A | N-Myristoyl transferase, membranes | − | − | − |
| L37Q | Unknown | ++ | − | ++ |
| EEEE65AAAA | PACS-1/2, HLA-I/AP-1 complex, p21-activated kinase 2 (PAK2) | − | − | − |
| P78L | Unknown | − | − | − |
| P72A/P75A | SH3 domain of Src kinases and Vav, PAK2 | + | ++ | + |
| LL165AA | Adaptor proteins AP-1/2/3, vacuolar ATPase (VH1) | ++ | − | ++ |
| DD175AA | VH1 | ++ | − | ++ |
| F191A | PAK2 | + | ++ | + |
++, +, and −, full, intermediate, and null downregulation activity, respectively.
Viral stocks.
Stocks of NL4-3 virus (NIH Reagent Program), either wt virus, virus unable to express Nef (ΔNef) (16), or virus presenting specific mutations, were prepared by transfecting 20 μg of proviral plasmid and 3.5 μg of pCMV-VSV-G into 293T cells using the standard calcium phosphate method. At 48 h posttransfection, cell culture supernatants were collected, clarified by centrifugation, and stored at −80°C. Viral stocks were titrated by anti-p24 enzyme-linked immunosorbent assay (Innogenetics) according to the manufacturer's instructions.
HIV-1 infection.
For HIV-1 infection, Jurkat cells were resuspended at 2 × 106 cells/ml in medium with 8 μg Polybrene/ml, either alone or together with 50 ng of p24/106 cells, and centrifuged at 2,500 rpm for 90 min at 30°C. To obtain more than 90% infected cells at day 3 postinfection (p.i.) for use as targets in cytotoxicity assays, Jurkat cells were infected as described above, but 200 ng of p24/106 cells was used. Subsequently, cells were washed and resuspended in medium at 7 × 105/ml.
To infect primary CD4+ T lymphocytes, cells were exposed to 50 ng of p24/106 cells for 4 h at 37°C. Cells were washed twice and resuspended at 1.5 × 106/ml in medium supplemented with 100 IU human recombinant interleukin-2 (IL-2; Sigma-Aldrich)/ml. At 5 days p.i., cells were stimulated by adding 100 ng/ml Staphylococcus aureus enterotoxin B (SEB; Sigma-Aldrich) and irradiated allogeneic PBMCs at a 1:1 cell ratio.
Flow cytometry.
The following procedures were performed at 4°C in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin and 0.1% sodium azide, unless otherwise specified. HIV-1-infected or uninfected cells (2 × 105 Jurkat or 5 × 105 CD4+ T cells) were incubated with anti-PVR, anti-HLA-I MAb, or isotype control IgG. After washing, cells were incubated with Alexa 647-conjugated GAM (PE-conjugated GAM was used [see Fig. S1 in the supplemental material]). Alternatively, cells were incubated with PE-conjugated anti-HLA-I or anti-CD4 antibody. For simultaneous detection of intracellular p24, cells were washed, incubated with normal mouse serum diluted 1:20, and then washed, fixed, permeabilized with BD Biosciences reagents, and incubated at room temperature with FITC-conjugated anti-p24 antibody. Finally, cells were washed, resuspended in 1% paraformaldehyde (PF), and analyzed (FACSCalibur flow cytometer; BD Biosciences).
For intracellular staining of total PVR or HLA-I, cells were collected, washed, and permeabilized, prior to being submitted to the double-labeling procedure (described above) at room temperature.
Western blot analysis.
Total lysates of 293T cells were separated by 12% SDS-PAGE and analyzed by immunoblotting with appropriate antibodies and the Pierce ECL substrate (Thermo Scientific) as previously described (9).
Cytotoxicity assay.
Flow cytometry-based cytotoxicity assays were performed as described previously (38) using 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled and IL-2-activated NK cells as effector cells and Jurkat cells at day 3 p.i. as targets. Briefly, PBMCs from healthy donors were labeled with 500 nM CFSE (Sigma-Aldrich) in PBS for 10 min at 37°C, washed, and then used to purify NK cells by means of Dynabeads Untouched human NK cells (Invitrogen). NK cells were stimulated with 500 IU/ml of IL-2 for 18 h and then seeded with a constant number of target cells (50,000) at different effector-to-target cell (E:T) ratios in a 96-well plate for 4 h at 37°C. Cells were then labeled with the intercalating DNA dye 7-amino-actinomycin D (5 μg/ml; Sigma-Aldrich) for 20 min and then washed, fixed in 1% PF acid, and analyzed. For each E:T ratio, 20,000 target cells (CFSE-negative population) were acquired by fluorescence-activated cell sorting (FACS). Where indicated, effector cells were incubated with blocking antibodies for 15 min prior to incubation with target cells.
Data analysis.
Each experiment was performed at least three times. All statistical analyses (mean, standard error [SE], normality tests, Student's t test) were performed using GraphPad Prism (version 5) software. A P value of <0.05 was considered statistically significant.
RESULTS
HIV-1 downregulates PVR in a Nef-dependent manner.
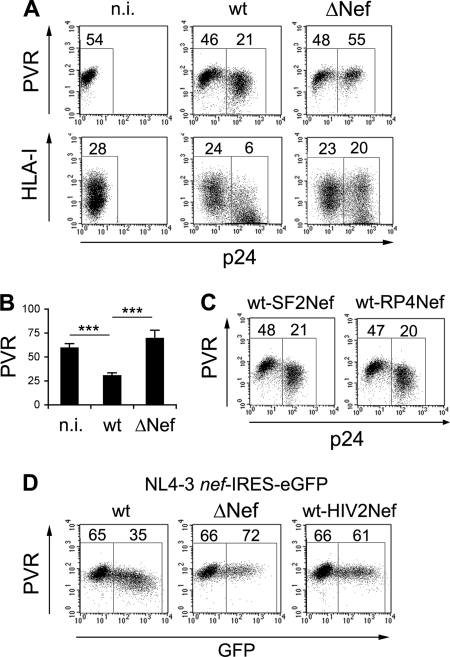
To investigate whether HIV-1 and, in particular, the Nef protein had the capacity to affect cell surface PVR levels, we infected the T lymphoblastoma Jurkat cell line that constitutively expresses PVR but not nectin-2 (59) with the wt and Nef-deficient (ΔNef) NL4-3 viral strain. Both wt and ΔNef viruses were pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G) to allow entry into target cells by endocytosis and obviate Nef for optimal viral infectivity (15). At 3 days p.i., cells were monitored for the intracellular accumulation of the viral p24 Gag capsid antigen and the cell surface expression of PVR or HLA-I, a well-known Nef target. Figure 1A and B show that, similar to the HLA-I levels, the expression of cell surface PVR was dramatically decreased (by ∼50%) in p24-positive (p24+) cells infected with wt virus compared to noninfected (n.i.) cells. Moreover, like HLA-I, PVR was not reduced in ΔNef virus-infected cells. These findings demonstrate that HIV-1 downregulates PVR in infected Jurkat cells and that this viral function is Nef dependent.
Fig 1.
PVR is downregulated by Nef in HIV-infected Jurkat cells. (A) Jurkat cells were not infected (n.i.) or infected with HIV-1 (NL4-3) wt or ΔNef isolates. After 3 days, expression of cell surface PVR or HLA-I was analyzed together with intracellular p24 expression by two-color flow cytometry. Values corresponding to geometric mean fluorescence intensity specific for PVR and HLA-I of p24-negative cells (left gate) and productively infected p24+ cells (right gate) are indicated. (B) The mean fluorescence intensity ± SE relative to PVR in n.i. and in p24+ wt- and ΔNef isolate-infected cells was determined as described for panel A in eight independent experiments. ***, P < 0.001. (C and D) Jurkat cells were infected for 3 days and analyzed as described for panel A. Data from one representative experiment out of three are shown. (C) An NL4-3 virus expressing the Nef protein of the SF2 strain (wt-SF2Nef) or derived from an HIV-infected patient (wt-RP4Nef) was used. (D) Cells were infected with NL4-3-based viruses in which the nef coding sequence was either conserved (wt), disrupted (ΔNef), or replaced with HIV-2 nef (wt-HIV2Nef), followed by an internal ribosomal entry site (IRES) and the gene encoding the enhanced GFP (NL4-3 nef-IRES-eGFP viruses). The mean fluorescence intensity of PVR on GFP-negative and GFP+ cells is reported.
To test whether or not this novel activity of Nef was a peculiar feature of the laboratory-grown NL4-3 strain, we used an NL4-3 virus in which the resident nef gene was replaced either by that of the SF2 strain (wt-SF2Nef), which more closely resembles primary variants, or by that derived from an HIV-infected patient (wt-RP4Nef). Both viruses encode fully functional Nef proteins, as shown in previous studies (9, 12, 24). The cell surface PVR levels were strongly decreased by the two recombinant viruses (Fig. 1C), indicating that the capacity to downregulate PVR is not restricted to a single viral strain but is instead a conserved function of the HIV-1 Nef protein. This Nef activity, however, was not maintained in HIV-2, a lentivirus that is related to HIV-1 but that possesses a lower pathogenic potential. In fact, expression of the HIV-2 nef gene in the NL4-3 viral background completely abrogated the downmodulation of PVR in infected cells (Fig. 1D).
Infection with HIV-1 modulates the expression of PVR in primary CD4+ T cells.
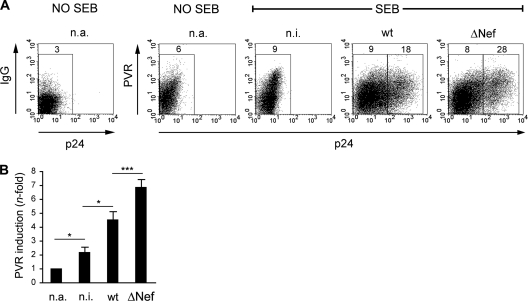
PVR is present on tumors of different origins (epithelial, neurologic, and hematopoietic) and also on various cell types under physiologic conditions (33, 46, 50). In primary CD4+ T lymphocytes, PVR expression is normally lacking but can be induced upon stimulation with mitogens (2, 11). To explore the capacity of HIV-1 to affect PVR expression in CD4+ T lymphocytes, freshly purified CD4+ T cells were infected with wt or ΔNef VSV-G-pseudotyped viruses or not infected and then cultivated in the absence of mitogenic stimuli for 5 days. During this time, although viral replication cannot occur due to the quiescent cell status, the Nef protein accumulates within wt-infected cells and exerts its proviral function by sensitizing these cells to activation, as shown elsewhere (42, 63). Next, cells were activated by stimulation with the SEB superantigen, with the exception of one aliquot of n.i. cells (not activated [n.a.]), and analyzed at various times for the expression of intracellular p24 and cell surface PVR (Fig. 2A and data not shown). Infected p24+ cells became detectable 3 days after stimulation, and, concomitantly, PVR was induced by cellular activation in n.i. cells compared to n.a. cells. Of note, compared to n.i. cells, surface PVR levels were increased in wt-infected cells and to a greater extent in cells infected with ΔNef virus (Fig. 2A and B). Thus, primary CD4+ T cells responded to HIV-1 infection by increasing surface PVR expression, but this response was countered by Nef.
Fig 2.
Surface PVR expression in primary CD4+ T cells undergoing HIV-1 infection. (A) Purified CD4+ T lymphocytes isolated from a healthy donor were not infected (n.i.) or infected with HIV-1 wt or ΔNef and then stimulated with SEB 5 days later. As control, an aliquot of n.i. cells was left unstimulated (n.a.). After 3 days, cell surface PVR and intracellular p24 were analyzed as described in the legend to Fig. 1. Isotype control staining (IgG) of n.a. cells is also shown. (B) Quantitative assessment of PVR upmodulation upon activation of CD4+ T cells infected with wt or ΔNef virus or not infected. The modulation of PVR expression under the indicated cell conditions was calculated by subtracting from each mean fluorescence intensity the value obtained for the isotype control staining and dividing the mean fluorescence intensity obtained for n.i. and wt- and ΔNef virus-infected cells by the mean fluorescence intensity obtained for n.a. cells. Values are averages ± SEs derived from six independent experiments, as shown in panel A. *, P < 0.05; ***, P < 0.001.
Common features in the downregulation of PVR and HLA-I molecules by Nef.
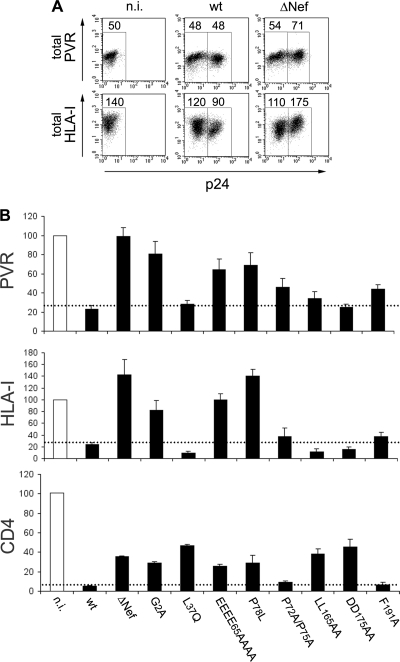
We further investigated the effect of Nef on PVR expression using the HIV-infected Jurkat cell system. We have not been able to detect PVR by Western blot analysis using commercially available antibodies (data not shown). Therefore, to evaluate the total PVR amounts, n.i., wt-infected, and ΔNef virus-infected cells were permeabilized, stained with antibodies either against PVR or against HLA-I as a control, together with anti-p24 antibody, and then analyzed by FACS. Infection with ΔNef virus but not wt virus resulted in increased total PVR levels (Fig. 3A). In line with the Nef capacity to degrade HLA-I (52), the overall HLA-I amounts were found to be reduced and increased, respectively, in wt- and ΔNef virus-infected cells compared to n.i. cells (Fig. 3A). Analogous results were obtained with immunofluorescence microscopy (data not shown). These data suggest that, similarly to its effect on HLA-I, Nef lowers the intracellular accumulation of PVR that occurs during HIV-1 infection.
Fig 3.
Mechanisms of PVR modulation by HIV-1 Nef. (A) At 3 days postinfection, noninfected (n.i.) and wt- and ΔNef virus-infected Jurkat cells were permeabilized and then analyzed by two-color flow cytometry for total PVR or HLA-I levels as well as for p24 expression. Mean fluorescence intensity values are indicated. (B) Jurkat cells were not infected or infected with HIV-1 wt, ΔNef, or expressing a mutated Nef protein, as indicated, and then analyzed 3 days later for the expression of intracellular p24 and surface PVR or HLA-I, as described in the legend to Fig. 1A. Surface CD4 was analyzed at 1 day p.i. to limit the contribution of the late viral proteins Vpu and Env to the total CD4 downregulation. Surface expression of each molecule in cells infected with the indicated viruses was calculated by setting the mean fluorescence intensity obtained for n.i. cells to 100%. Values are averages ± SEs derived from at least three independent experiments. The dotted lines indicate the maximal surface expression found on wt HIV-infected cells.
The reduced cell surface HLA-I levels in HIV-infected cells are due to the capacity of Nef to trigger cell surface HLA-I internalization and sorting to the trans-Golgi network (TGN) (5), as well as to divert newly synthesized HLA-I molecules to degradative compartments via an AP-1-dependent pathway (52). However, to reduce surface CD4 molecules, Nef uses distinct components of the endocytic machinery such as the clathrin/AP-2 pathway to lysosomes (51). Mutagenesis-based studies have established that the Nef activities of HLA-I and CD4 downregulation and the protein's capacity to interact with known cellular cofactors require specific amino acid residues and are in large part genetically separable. Therefore, to further investigate the PVR downregulation activity of Nef, we employed a panel of HIV-1 isolates expressing Nef proteins mutated at conserved residues and thus impaired with regard to specific functions, as described in previous studies (10, 24, 32, 44) (Table 1). Results showed that Nef mutations that abolished (EEEE65AAAA, P78L) or reduced (P72A/P75A, F191A) HLA-I downregulation also affected to a similar extent the capacity to downmodulate PVR (Fig. 3B and Table 1; see Fig. S1 in the supplemental material). Conversely, those mutants that specifically inactivated the function of Nef on CD4 (L37Q, LL165AA, DD175AA) retained the PVR-downregulating activity. Finally, the G2A mutation that impairs the N-terminal myristoylation of Nef necessary for its localization on membranes and for all reported Nef activities (24) also inhibited the capacity to reduce cell surface PVR expression. Overall, these data suggest that the Nef activities of PVR and HLA-I downregulation share common molecular mechanisms that are distinct from those employed to target CD4.
Nef is assisted by the Vpu late viral factor in the downregulation of PVR.
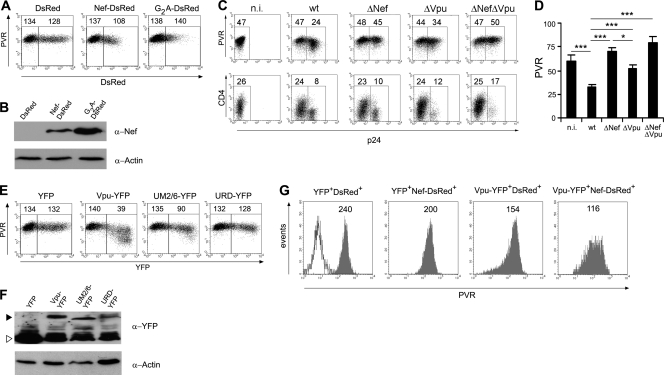
Having observed the Nef-induced downregulation of PVR in the context of HIV-infected cells, we wondered whether the Nef protein mediated this activity by itself or other viral functions were involved. To address this point, we generated a C-terminally DsRed-tagged version of NL4-3 Nef, either wt or with the G2A mutation, and expressed these proteins or DsRed alone in 293T cells by transient transfection (Fig. 4A and B). By FACS analysis, Nef-DsRed-positive (Nef-DsRed +) cells but not G2A-DsRed+ or DsRed+ cells displayed on their surface about 20% less PVR than untransfected cells, demonstrating that expression of the Nef protein alone can downregulate PVR, although less efficiently than in cells undergoing productive HIV-1 infection (Fig. 1A). The expression of Nef alone in Jurkat cells produced the same results as those found in 293T cells (data not shown). Hence, it is possible that other viral factors assist Nef to reduce cell surface PVR levels efficiently. To test this hypothesis, we examined whether the HIV-1 accessory protein Vpu, which can downregulate various cell surface proteins (i.e., CD4, tetherin, CD1d, and NTB-A [21, 41, 54]), could be a partner to Nef in targeting PVR. We thus tested the levels of surface PVR in Jurkat cells infected with HIV-1 defective for the expression of Vpu alone (ΔVpu) or of both Vpu and Nef (ΔVpu ΔNef) together with wt and ΔNef viruses (Fig. 4C and D). Results showed that in ΔVpu virus-infected cells, the surface expression of PVR was reduced, although to a lesser extent than in wt-infected cells, indicating that Vpu contributes to the viral capacity to downregulate PVR. This Vpu activity possibly requires the presence of Nef, since PVR expression in ΔNef virus-infected cells was indistinguishable from that of cells not infected or infected with ΔVpu ΔNef virus. As expected, the capacity of HIV-1 to downregulate CD4 was reduced in the absence of Nef and/or Vpu. The residual CD4 downregulation was likely mediated by the viral Env protein (57). Moreover, by measuring PVR levels in permeabilized infected cells, we found that ΔVpu virus maintained the capacity of wt virus to inhibit the intracellular accumulation of PVR (data not shown), a function that was Nef dependent (Fig. 3A). Apparently, in the context of HIV-infected cells, Nef functions in concert with Vpu to downregulate surface PVR but acts in a Vpu-independent manner to restrain intracellular PVR accumulation.
Fig 4.
Vpu contributes to the activity of Nef on surface PVR downregulation. (A) 293T cells were transfected with 0.5 μg of vector expressing DsRed, Nef-DsRed, or G2A-DsRed and analyzed 48 h later by two-color flow cytometry to measure DsRed fluorescence and cell surface PVR. Mean fluorescence intensity values relative to the value for PVR in DsRed-negative (left gate) and DsRed-positive (right gate) cells are indicated. (B) Total lysates of cells described in panel A were analyzed by Western blotting with anti-Nef and antiactin antibodies. (C) Jurkat cells were infected with HIV-1 either wt or defective for the expression of Nef, Vpu, or both proteins, as indicated, and analyzed for the surface expression of PVR or CD4 (on day 3 or 1 p.i., respectively), together with intracellular p24 accumulation. (D) The mean fluorescence intensity ± SE for PVR in n.i. and in p24+ infected cells was determined as described for panel C in seven independent experiments. *, P < 0.05; ***, P < 0.001. (E and F) 293T cells were transfected and analyzed as described for panels A and B, with the difference that the indicated YFP, Vpu-YFP, UM2/6-YFP, or URD-YFP proteins were expressed and monitored by YFP-specific fluorescence and anti-GFP/YFP Western blotting. The YFP-tagged Vpu proteins and YFP alone are indicated with a filled arrowhead and an open arrowhead, respectively. (G) 293T cells were doubly transfected with 0.5 μg of each vector in order to express DsRed or DsRed-Nef together with YFP or Vpu-YFP and then analyzed 48 h later by FACS. The expression of PVR on gated double-positive cells is indicated. The open histogram shows unlabeled cells. Results obtained with 293T cells (A, B, and E to G) are from one representative experiment out of three.
To confirm and further investigate the role of Vpu in PVR downregulation, we expressed in 293T cells a C-terminally YFP-tagged Vpu protein, either wt or with previously described mutations that disrupted the capacity to counteract tetherin (randomized transmembrane region; URD mutant) or to degrade both tetherin and CD4 (alanine substitution of S52 and S56; UM2/6 mutant) (4). Figure 4E and F show that expression of Vpu-YFP alone induced a strong downmodulation of PVR and that this activity was lost or partially reduced by the mutated Vpu variants URD-YFP and UM2/6-YFP, respectively. Similar results were obtained in transfected Jurkat cells (data not shown). Unlike in the HIV-infected Jurkat cell system, in transfected 293T cells Vpu was not able to enhance the activity of Nef on PVR, since PVR downmodulation in cells coexpressing Vpu-YFP and Nef-DsRed corresponded approximately to the combined effects measured in singly positive Vpu-YFP-positive (Vpu-YFP+) and Nef-DsRed+ cells (Fig. 4G). This could be due to the fact that a cotransfected cell system misrepresents the events occurring in HIV-infected cells in terms of the kinetics of Nef and Vpu expression and the stoichiometric ratio between the two proteins. Moreover, the existence of additional viral factors participating in Nef-induced downmodulation of PVR cannot be excluded. Nevertheless, results obtained in 293T cells clearly demonstrate that Vpu has the capacity to affect the cell surface expression of PVR.
NK-cell mediated lysis of HIV-infected cells is reduced by Nef and involves DNAM-1–PVR interaction.
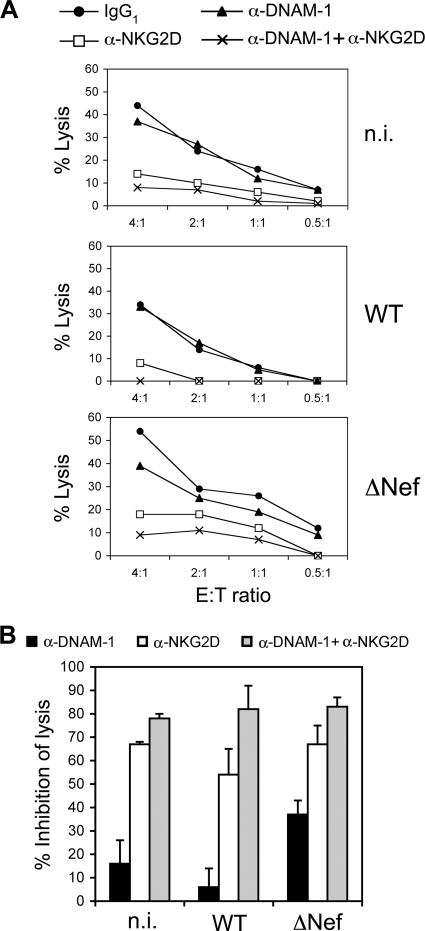
The interaction of PVR with the activating receptor DNAM-1 results in NK cell triggering and cytotoxicity (7). Therefore, by interfering with PVR expression, Nef might help HIV-1 to evade NK cell-mediated immune responses. To investigate this issue, we evaluated the DNAM-1-mediated killing of Jurkat cells not infected or infected with wt or ΔNef virus using purified IL-2-activated NK cells as effectors. Since we previously showed that HIV-1 infection of Jurkat cells modulates the expression of ligands for the activating receptor NKG2D (12), NK cell-mediated lysis was tested in the absence or in the presence of blocking MAbs specific for DNAM-1 and NKG2D receptors, either alone or in combination. First, we found that Jurkat cells infected with HIV-1 lacking Nef expression were more susceptible to NK cell-mediated lysis, since killing of cells infected with ΔNef virus (or not infected) was higher than that of wt-infected cells (Fig. 5A). Second, we also observed that lysis of cells infected with ΔNef virus but not with wt virus was reduced by MAb-mediated blocking of DNAM-1, indicating that protection of HIV-infected cells by Nef results in part from the evasion of DNAM-1-mediated responses (Fig. 5B). Blocking of NKG2D alone resulted, however, in a stronger inhibition of NK cell-mediated lysis of the three cell targets, in line with the capacity of NKG2D to deliver a potent activating signal upon binding of its ligands, even when these are partially downmodulated, as occurs in cells infected with wt virus (12). Importantly, when anti-DNAM-1 MAb was used in combination with anti-NKG2D MAb, the simultaneous blocking of both receptors impaired NK cell-mediated lysis to a greater extent than anti-NKG2D blocking alone and virtually abrogated killing of wt-infected cells. These results indicate that the susceptibility to NK cell-mediated lysis of cells infected with HIV-1 is critically controlled by both the NKG2D and DNAM-1 pathways.
Fig 5.
Involvement of DNAM-1 in the lysis of HIV-1-infected cells. IL-2-activated NK cells were tested for cytotoxicity against noninfected (n.i.) and wt- and ΔNef virus-infected Jurkat cell targets in a FACS-based assay. (A) Saturating amounts of blocking MAb to the indicated receptors or isotype control (IgG1) were preincubated with NK cells before testing at the indicated effector-to-target cell (E:T) ratio. Data are expressed as percent lysis. Representative results from one out of three independent experiments are shown. (B) Data are expressed as percent inhibition of lysis calculated according to the formula: [1 − (% of lysis with MAb)/(% of lysis with IgG1)] × 100. Results from three independent experiments (mean ± SE) at an E:T ratio of 4:1 or 3:1 are shown.
DISCUSSION
In the present study, we show for the first time that surface expression of PVR in CD4+ T cells undergoing HIV-1 infection is reduced by the viral Nef protein, assisted to a certain extent by another viral factor, Vpu. In the context of infected Jurkat T cells that constitutively express PVR, Nef downregulated surface PVR levels dramatically, and this activity was conserved by Nef proteins from two laboratory strains of HIV-1 (NL4-3, SF2) or from a virus derived from an HIV-infected patient. This function of Nef was also detected in primary CD4+ T lymphocytes infected in vitro with HIV-1 and then activated. Specifically, the cell surface expression of PVR induced by T cell activation was increased to a greater extent with Nef-deficient virus than the wild type. This phenomenon resembles the upregulation of PVR observed in fibroblasts infected with UL141-deficient strains of HCMV (61), suggesting that impairment of PVR expression may be a common beneficial strategy evolved by viruses to avoid DNAM-1-mediated immune recognition. The UL141 protein of HCMV not only induces the intracellular sequestration of PVR but also promotes, in association with an additional unknown viral factor(s), the proteasomal degradation of another DNAM-1 ligand, nectin-2 (48). In the context of HIV-infected T cells, nectin-2 seems to be irrelevant since it is not expressed on the membrane of T cell lines (59) or primary CD4+ T lymphocytes (2).
The results of our study show that Nef uses the same motifs to reduce both PVR and HLA-I molecules, implying the existence of a common downregulation mechanism. Two different models have been proposed to explain Nef-mediated HLA-I downmodulation (20). The first model, the signaling model, implies the sequential association of Nef with signaling factors, such as the PI3K kinase, leading to clathrin-independent HLA-I internalization and intracellular retention. The second model, the stoichiometric model, presumes that Nef stabilizes the association of the AP-1 clathrin adaptor protein complex with a tyrosine-based sorting signal, 320YSQA, in the cytoplasmic tail of nascent HLA-A and HLA-B molecules that are then redirected to degradative compartments in a PI3K-independent manner. Interestingly, PVR was shown to be internalized from the cell surface and degraded through a clathrin-dependent pathway (30). Moreover, the cytoplasmic tail of PVR contains a sequence, 398YSAV, that matches the tyrosine-based YXXΦ sorting motif recognized by AP complexes (6). It will be interesting to determine whether the 398YSAV sequence of PVR indeed functions as a sorting signal and whether it is required for Nef-mediated downregulation, as for the 320YSQA motif in HLA-A/B molecules (17).
While Nef expression alone suffices to reduce the levels of intracellular and cell surface HLA-I, Nef acts individually to diminish intracellular PVR but requires the presence of Vpu for optimal downregulation of cell surface PVR. Vpu is an integral membrane protein expressed during the late stage of the HIV-1 life cycle and shares with Nef the capacity to downregulate CD4 (21). Specifically, Vpu mediates polyubiquitination and proteasomal degradation of newly synthesized CD4 molecules in the endoplasmic reticulum. In addition, in the TGN, Vpu sequesters tetherin, a cellular restriction factor for HIV-1, and targets this protein for ubiquitination, followed by proteosomal and/or lysosomal degradation. Whether these pathways mediate the contribution of Vpu to Nef-induced PVR downregulation will have to be determined. Using specific Vpu mutants, we found that, although the transmembrane region of Vpu is important for targeting both PVR and tetherin, downmodulation of PVR by Vpu differs from the protein activities on tetherin and CD4 degradation, in that the S52 and S56 residues are largely dispensable. Interestingly, screening of a large number of primate and human lentiviruses revealed that, during the cross-species viral transmission, the nef and vpu genes have evolved by reciprocally shaping their functions (53). In line with the existence of an evolutionary link between Nef and Vpu functions, we found that HIV-2 lacking a vpu gene expresses a Nef protein that is inactive on PVR. This observation suggests that the evolutionary pressure on HIV-1 to maintain the PVR-downmodulating activity did not act on HIV-2, adding to the number of differences already noted between the two viruses.
The ability of Nef to downregulate PVR discussed here may, in combination with the protein's capacity to downregulate NKG2D ligands, provide HIV-1 with an additional means to counter the antiviral function of NK cells. Indeed, we show that infected Jurkat cells are more resistant to NK cell lysis than uninfected cells or cells infected with a Nef-deficient virus. Using anti-DNAM-1 blocking MAb, we observed that Nef, in line with its capacity to downmodulate PVR, protects HIV-infected cells from NK cell-mediated killing at least in part by preventing DNAM-1-mediated responses. By blocking NKG2D, we found that this receptor has a stronger impact than DNAM-1 on NK cell-mediated killing of HIV-infected T cells with or without Nef expression. This accords with various studies showing that NKG2D exerts a stronger effect than DNAM-1 in the context of NK cell-mediated killing of various tumors expressing ligands for both receptors (8, 14, 22, 47). However, we found that the simultaneous blocking of both NKG2D and DNAM-1 resulted in a stronger reduction of NK cell-mediated lysis of HIV-infected cells than individual NKG2D blocking, clearly indicating that both pathways are involved. Notably, when the NKG2D pathway was inhibited, additional blocking of DNAM-1 strongly impaired, if not completely abrogated, the capacity of NK cells to kill Jurkat cells infected with wt HIV-1. Therefore, given that NKG2D is downmodulated on NK cells of HIV-infected patients (28, 43), Nef-mediated targeting of the PVR/DNAM-1 pathway might have important functional consequences in vivo. Conflicting results as to the expression of DNAM-1 on the surface of NK cells have been reported; it was found to be either not affected (40) or increased (64) in HIV-infected individuals. Further work is clearly needed to establish whether and how Nef expression allows HIV-1 evasion of NK cell responses mediated by NKG2D and DNAM-1 in infected patients. An additional aspect to be explored concerns the potential impact of Nef-induced PVR downregulation on the antiviral immune responses mediated by two other receptors engaged by PVR, tactile (CD96) (29) and TIGIT (56), both expressed by NK as well as T cells.
Notably, Nef displays another activity that allows avoidance of NK cell-mediated lysis and consists of downregulating the ligand for the natural cytotoxicity receptor NKp44 (26). Moreover, a recent study showed that Vpu downregulates the signaling molecule NTB-A, thus leading to reduced NTB-A engagement on the surface of NK cells and decreased NK cell degranulation and cytotoxicity against HIV-infected cells (54). On the basis of the data shown in the present study, the capacity of Vpu to protect infected cells from attack by NK cells could also result from the cooperative function of Vpu in Nef-mediated PVR downregulation. Overall, there is increasing evidence to suggest that HIV-1 has evolved a complex strategy to evade NK cell responses. Such a multifaceted effort to avoid triggering NK cell activity, together with the various alterations of NK cell phenotype and function observed in viremic HIV-infected patients (1, 25), suggests that NK cells play an important role in the control of HIV-1 infection. Hence, understanding the mechanisms evolved by the virus to evade the immune responses of NK cells may lead to new strategies aimed at harnessing the capacity of these cells to mediate anti-HIV activity and improving immune control in HIV-infected individuals.
Finally, we believe that the suppression of PVR surface expression by the HIV-1 Nef protein may have implications that go beyond the regulation of NK cell function. One potential consequence could be avoidance of the interaction of PVR with the DNAM-1 receptor expressed by CD8+ T cells, an interaction that is important for CD8+ T cell proliferation and antigen-specific responses (31). Colonna and colleagues have recently shown that DNAM-1 is downregulated in exhausted HIV-1-specific CD8+ T cells derived from chronically infected patients with high viral loads and suggested that this aberrant phenotype may result from the interaction of CD8+ T cells with PVR-expressing HIV-infected T cells (11). The induction of PVR by HIV-1 in primary CD4+ T cells reported here supports this hypothesis, although in this cell system Nef constrains PVR increase. To avoid DNAM-1-mediated immune responses, it is possible that the virus, through the activity of Nef, limits the expression of PVR to levels that are still high enough to cause DNAM-1 downregulation on HIV-1-specific CD8+ T cells in the late phases of chronic infection. Furthermore, since PVR, by interacting with nectin-3 and vitronectin, is critically involved in the regulation of cell-to-cell adhesion and cellular movement (60), it is tempting to speculate that Nef-induced PVR downregulation would affect the capacity of infected CD4+ T cells to interact with other cells or to migrate with positive consequences for HIV-1 replication and spread. Several reports have demonstrated a key role for Nef in the aberrant formation of immunological synapses between HIV-infected T lymphocytes and antigen-presenting cells that may favor viral replication by avoiding activation-induced cell death (23). In addition, Nef restricts the chemotaxis and transendothelial migration of infected T cells (45, 58), a phenomenon that should predictably prevent T cells from homing to lymph nodes and establishing effective antiviral humoral responses. Whether the PVR downregulation activity disclosed here is involved in these pathogenic functions of Nef deserves to be investigated in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following for kindly providing reagents: M. Colonna, Washington University, St. Louis, MO (anti-PVR SKII.4 antibody); O. T. Fackler, University of Heidelberg, Heidelberg, Germany (pNLblue Nco nef Not, wt-SF2Nef, G2A); F. Kirchhoff, University of Ulm, Ulm, Germany (ΔVpu, ΔVpu ΔNef, NL4-3 nef-IRES-eGFP viruses containing a nef gene either wt, disrupted, or derived from HIV-2 60415K); M. Schindler, University of Munich, Munich, Germany (pVpu-, pURD-, and UM2/6-YFP, pEYFP); M. Harris, University of Leeds, Leeds, United Kingdom (anti-Nef serum ARP444); M. Federico, Istituto Superiore di Sanità, Rome, Italy (pCMV-VSV-G); and E. Affabris, University Roma Tre, Rome, Italy (EEEE65AAAA). We also thank Alessandra Soriani for helpful discussions.
The work was supported by grants from the Italian Ministry of Health, Programma Nazionale di Ricerca sull AIDS, in collaboration with ISS and Ricerca Corrente 2010, cofunded by the Italian 5 × 1000 contribution 2008 to M.D., and by grants from the Italian Association for Cancer Research (AIRC), the Italian Ministry of University and Research (MIUR), and the Sapienza University of Rome, Rome, Italy, to A.S. and C.C.
Footnotes
Published ahead of print 1 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Alter G, Altfeld M. 2009. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 265:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardolino M, et al. 2011. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T-cell interaction. Blood 117:4778–4786 [DOI] [PubMed] [Google Scholar]
- 3. Arien KK, Verhasselt B. 2008. HIV Nef: role in pathogenesis and viral fitness. Curr. HIV Res. 6:200–208 [DOI] [PubMed] [Google Scholar]
- 4. Banning C, et al. 2010. A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells. PLoS One 5:e9344 doi:10.1371/journal.pone.0009344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853–866 [DOI] [PubMed] [Google Scholar]
- 6. Bonifacino JS, Traub LM. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447 [DOI] [PubMed] [Google Scholar]
- 7. Bottino C, et al. 2003. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 198:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlsten M, et al. 2007. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 67:1317–1325 [DOI] [PubMed] [Google Scholar]
- 9. Casartelli N, Di Matteo G, Potesta M, Rossi P, Doria M. 2003. CD4 and major histocompatibility complex class I downregulation by the human immunodeficiency virus type 1 Nef protein in pediatric AIDS progression. J. Virol. 77:11536–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casartelli N, et al. 2006. The Pro78 residue regulates the capacity of the human immunodeficiency virus type 1 Nef protein to inhibit recycling of major histocompatibility complex class I molecules in an SH3-independent manner. J. Gen. Virol. 87:2291–2296 [DOI] [PubMed] [Google Scholar]
- 11. Cella M, et al. 2010. Loss of DNAM-1 contributes to CD8+ T-cell exhaustion in chronic HIV-1 infection. Eur. J. Immunol. 40:949–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerboni C, et al. 2007. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J. Gen. Virol. 88:242–250 [DOI] [PubMed] [Google Scholar]
- 13. Champsaur M, Lanier LL. 2010. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 235:267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan CJ, et al. 2010. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J. Immunol. 184:902–911 [DOI] [PubMed] [Google Scholar]
- 15. Chazal N, Singer G, Aiken C, Hammarskjold ML, Rekosh D. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chowers MY, et al. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen GB, et al. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671 [DOI] [PubMed] [Google Scholar]
- 18. Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401 [DOI] [PubMed] [Google Scholar]
- 19. Deacon NJ, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988–991 [DOI] [PubMed] [Google Scholar]
- 20. Dikeakos JD, et al. 2010. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol. Biol. Cell 21:3279–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dube M, Bego MG, Paquay C, Cohen EA. 2010. Modulation of HIV-1-host interaction: role of the Vpu accessory protein. Retrovirology 7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El-Sherbiny YM, et al. 2007. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 67:8444–8449 [DOI] [PubMed] [Google Scholar]
- 23. Fackler OT, Alcover A, Schwartz O. 2007. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7:310–317 [DOI] [PubMed] [Google Scholar]
- 24. Fackler OT, et al. 2006. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology 351:322–339 [DOI] [PubMed] [Google Scholar]
- 25. Fauci AS, Mavilio D, Kottilil S. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5:835–843 [DOI] [PubMed] [Google Scholar]
- 26. Fausther-Bovendo H, et al. 2009. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS 23:1077–1087 [DOI] [PubMed] [Google Scholar]
- 27. Fogli M, et al. 2008. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 4:e1000101 doi:10.1371/journal.ppat.1000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu GF, et al. 2010. Differences in natural killer cell quantification and receptor profile expression in HIV-1 infected Chinese children. Cell. Immunol. 265:37–43 [DOI] [PubMed] [Google Scholar]
- 29. Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. 2004. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J. Immunol. 172:3994–3998 [DOI] [PubMed] [Google Scholar]
- 30. Fujito T, et al. 2005. Inhibition of cell movement and proliferation by cell-cell contact-induced interaction of Necl-5 with nectin-3. J. Cell Biol. 171:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilfillan S, et al. 2008. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 205:2965–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giolo G, et al. 2007. Internalization and intracellular retention of CD4 are two separate functions of the human immunodeficiency virus type 1 Nef protein. J. Gen. Virol. 88:3133–3138 [DOI] [PubMed] [Google Scholar]
- 33. Kakehi S, Nakahama K, Morita I. 2007. Expression and possible role of PVR/CD155/Necl-5 in osteoclastogenesis. Mol. Cell. Biochem. 301:209–217 [DOI] [PubMed] [Google Scholar]
- 34. Kestler HW, III, et al. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662 [DOI] [PubMed] [Google Scholar]
- 35. Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228–232 [DOI] [PubMed] [Google Scholar]
- 36. Kirchhoff F, Schindler M, Specht A, Arhel N, Munch J. 2008. Role of Nef in primate lentiviral immunopathogenesis. Cell. Mol. Life Sci. 65:2621–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lanier LL. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lecoeur H, Fevrier M, Garcia S, Riviere Y, Gougeon ML. 2001. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J. Immunol. Methods 253:177–187 [DOI] [PubMed] [Google Scholar]
- 39. Magri G, et al. 2011. NKp46 and DNAM-1 NK-cell receptors drive the response to human cytomegalovirus-infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood 117:848–856 [DOI] [PubMed] [Google Scholar]
- 40. Mavilio D, et al. 2006. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J. Exp. Med. 203:2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moll M, Andersson SK, Smed-Sorensen A, Sandberg JK. 2010. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood 116:1876–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neri F, Giolo G, Potesta M, Petrini S, Doria M. 2011. The HIV-1 Nef protein has a dual role in T cell receptor signaling in infected CD4+ T lymphocytes. Virology 410:316–326 [DOI] [PubMed] [Google Scholar]
- 43. Nolting A, et al. 2010. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology 406:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Neill E, et al. 2006. Dynamic evolution of the human immunodeficiency virus type 1 pathogenic factor, Nef. J. Virol. 80:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park IW, He JJ. 2009. HIV-1 Nef-mediated inhibition of T cell migration and its molecular determinants. J. Leukoc. Biol. 86:1171–1178 [DOI] [PubMed] [Google Scholar]
- 46. Pende D, et al. 2006. Expression of the DNAM-1 ligands, nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood 107:2030–2036 [DOI] [PubMed] [Google Scholar]
- 47. Pende D, et al. 2005. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the poliovirus receptor (CD155) and nectin-2 (CD112). Blood 105:2066–2073 [DOI] [PubMed] [Google Scholar]
- 48. Prod'homme V, et al. 2010. Human cytomegalovirus UL141 promotes efficient downregulation of the natural killer cell activating ligand CD112. J. Gen. Virol. 91:2034–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raulet DH. 2003. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 3:781–790 [DOI] [PubMed] [Google Scholar]
- 50. Reymond N, et al. 2004. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J. Exp. Med. 199:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roeth JF, Collins KL. 2006. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70:548–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167:903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sauter D, et al. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shah AH, et al. 2010. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 8:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shibuya A, et al. 1996. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 4:573–581 [DOI] [PubMed] [Google Scholar]
- 56. Stanietsky N, et al. 2009. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. U. S. A. 106:17858–17863 doi:10.1073/pnas.0903474106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stevenson M, Meier C, Mann AM, Chapman N, Wasiak A. 1988. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell 53:483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stolp B, et al. 2009. HIV-1 Nef interferes with host cell motility by deregulation of cofilin. Cell Host Microbe 6:174–186 [DOI] [PubMed] [Google Scholar]
- 59. Tahara-Hanaoka S, et al. 2004. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int. Immunol. 16:533–538 [DOI] [PubMed] [Google Scholar]
- 60. Takai Y, Miyoshi J, Ikeda W, Ogita H. 2008. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 9:603–615 [DOI] [PubMed] [Google Scholar]
- 61. Tomasec P, et al. 2005. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat. Immunol. 6:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ward J, et al. 2007. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 110:1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu Y, Marsh JW. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503–1506 [DOI] [PubMed] [Google Scholar]
- 64. Ye X, et al. 2006. Expression of human CD226 on T cells and natural killer cells and of soluble CD226 in plasma of HIV-1-infected Chinese patients. Viral Immunol. 19:576–581 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.