Abstract
Despite its clinical importance, the molecular biology of HIV-1 latency control is at best partially understood, and the literature remains conflicting. The most recent description that latent HIV-1 is integrated into actively expressed host genes has further confounded the situation. This lack of molecular understanding complicates our efforts to identify therapeutic compounds or strategies that could reactivate latent HIV-1 infection in patients, a prerequisite for the eradication of HIV-1 infection. Currently, many therapeutic development efforts operate under the assumption that a restrictive histone code could govern latent infection and that either dissipation of the histone-based restrictions or NF-κB activation could be sufficient to trigger HIV-1 reactivation. We here present data that suggest an additional, higher level of molecular control. During a high-content drug screening effort, we identified AS601245 as a potent inhibitor of HIV-1 reactivation in latently infected primary T cells and T cell lines. In either system, AS601245 inhibited HIV-1 reactivation despite high levels of induced NF-κB activation. This finding suggests the presence of a gatekeeper kinase activity that controls latent HIV-1 infection even in the presence of high levels of NF-κB activity. Potential therapeutic stimuli that do not target this gatekeeper kinase will likely fail to trigger efficient system-wide HIV-1 reactivation.
INTRODUCTION
Current antiretroviral treatment for HIV-1 infection (ART) can efficiently suppress HIV-1 replication; however, even successful long-term ART cannot eradicate infection. Following therapy cessation, the virus rapidly rebounds. This viral reemergence is thought to be driven by the presence of a reservoir of latently HIV-1-infected resting CD4+ memory T cells (4, 8, 16, 23, 66, 82). In these cells, which constitute a key part of our immunological memory, the virus is integrated in a transcriptional inactive state and can, due to the long half-life of memory T cells, persist in the face of ART. Obliteration of this pool of latently infected T cells will be a prerequisite for any HIV-1 treatment strategy with curative intent. As latently HIV-1-infected CD4+ memory T cells have no specific phenotype, the cells cannot be directly targeted (7). Therapeutic strategies that systemically reactivate latent infection events are currently considered the only means to target this viral reservoir.
There are two main lines of thought on how HIV-1 reactivation could be achieved. Under the assumption that latent HIV-1 infection is controlled by the same molecular mechanisms that control inducible cellular promoters, histone deacetylase (HDAC) inhibitors were used to trigger reactivation by resolving a restrictive histone code that was described to be associated with the latent HIV-1 promoter. By this means, reactivation should be achieved without triggering cellular activation. Although evidence was presented by some that HDAC inhibitors can reactivate latent HIV-1 in cell culture (31, 38, 73, 81), others could not confirm these results (5, 20, 79). Also, the reported effect of valproic acid on the latent reservoir in patients (43) was disputed by others (62–64), and later the findings that the HDAC inhibitor valproic acid could influence the size of the latent reservoir in patients were revised by the authors in a second publication (2).
Other therapeutic attempts to purge the latent HIV-1 reservoir were based on early findings that describe the importance of NF-κB activity for HIV-1 expression. NF-κB-activating agents, such as the phorbol esters phorbol myristate acetate (PMA) and prostratin or the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), were reported to potently reactivate latent HIV-1 infection in a series of T cell lines, in cells of the monocytic lineage, and in some in vitro models of HIV-1 latency in primary T cells (24, 25, 76). NF-κB activation was considered a necessary and sufficient stimulus to trigger HIV-1 reactivation. For clinical translation, this approach will require the dissociation of HIV-1 activation from cellular gene activation, as the responsiveness of many inflammatory cytokines to NF-κB activation exposes patients to the risk of a cytokine storm induced by NF-κB-activating agents (1). Attempts to translate this idea into the clinical situation were made using interleukin 2 (IL-2) or the anti-CD3 monoclonal antibody (MAb) OKT3 to intensify ART but were ultimately not successful in eradicating the pool of latent HIV-1 infection (14, 15, 41). It remains unclear as to exactly why these therapeutic attempts failed. Possibly, as these stimuli also trigger a cytokine response, it may have been impossible to apply them at a sufficiently high concentration (27). However, there is also the possibility that NF-κB activation by itself is insufficient to trigger HIV-1 reactivation due to another layer of molecular control, a scenario that is supported by the finding that TNF-α stimulation activates NF-κB in latently HIV-1-infected T cells but fails to trigger HIV-1 reactivation (71).
Dissecting the molecular control mechanisms for latent HIV-1 infection will be important to improve our ability to specifically target latent HIV-1 infection in future therapeutic attempts.
During a drug screen for inhibitors of latency establishment, we identified AS601245 (Jun N-terminal protein kinase [JNK] inhibitor V) (11) as a kinase inhibitor that could prevent HIV-1 reactivation in T cell lines and primary T cells. Viral reactivation was prevented despite potent NF-κB activity that was induced by phorbol esters or CD3/CD28 antibody costimulation. Therefore, inhibition of reactivation could not be explained by NF-κB blockade. Our findings thus suggest the presence of a kinase activity that is targeted by AS601245 and that even supersedes NF-κB effects on the latent HIV-1 promoter.
As an extension, our findings emphasize that triggering just NF-κB activity is not sufficient to trigger HIV-1 reactivation. The presence of a gatekeeper kinase activity or pathway, which is suggested by the inhibitory effect of AS601245, may explain why stimuli, such as TNF-α, anti-CD3 MAbs, or IL-2, that are known to trigger NF-κB activity in primary T cells fail to trigger HIV-1 reactivation and were clinically unsuccessful. Our findings thus suggest that more complex HIV-1 eradication treatment strategies that target latent HIV-1 infection at several levels of molecular control likely need to replace current efforts that are based on the magic bullet approach, where a single drug is believed to exert the full required therapeutic activity.
MATERIALS AND METHODS
Cell culture, plasmids, and reagents.
All T cell lines were maintained in RPMI 1640 supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). The latently infected J89GFP T cells, CG3, CA5, and EF7 T cells have been described earlier (20, 42). In CG3 T cells, the latent HIV-1 integration event was located in an intergenic region between the TIGD5 gene and the PYCRL gene. In CA5 T cells, the virus was found integrated into the RMB12/CPNE1 gene in the same-sense orientation relative to the transcriptional direction of the host gene. In EF7 cells, the virus was found integrated into the WHSC1 gene in the converse-sense orientation relative to the transcriptional direction of the host gene. NOMI reporter T cells are described in references 36 and 47. FBS was obtained from HyClone (Logan, UT) and was tested on a panel of latently infected cells to ensure that the utilized FBS batch did not spontaneously trigger HIV-1 reactivation (35, 42). The phorbol ester 13-phorbol-12-myristate acetate (PMA), N′-N′-hexamethylene-bisacetamide (HMBA), and the inhibitors parthenolide, flavopiridol, aloisine A, and roscovitine were purchased from Sigma. Recombinant human TNF-α was obtained from R&D. SB202190 (p38 MAPK), U0126 (extracellular signal-regulated kinase [ERK]), SP600125 (JNK), TDZD-8 and AR-A014418 (GSK3β), Akt inhibitor VIII, and Ly29402 (PI3 kinase) were purchased from Calbiochem. All antibodies were purchased from BD Pharmingen.
Glycerol gradient sedimentation analysis.
A total of 1 × 106 J89GFP or CA5 T cells were left untreated or pretreated with 10 μM AS601245 for 1 h and then stimulated with TNF-α (3 ng/ml). Cells were washed twice with cold phosphate-buffered saline (PBS) and then lysed for 30 min on ice in lysis buffer (0.5% Triton X-100, 20 mM HEPES [pH 7.9], 150 mM NaCl, 20 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol [DTT], 0.2 mM EDTA, and protease inhibitor cocktail [P8340; Sigma]), followed by centrifugation at 14,000 rpm for 10 min. For each experimental condition, the same amount of protein lysate was fractionated on 5 ml of a 10 to 45% glycerol gradient in lysis buffer in an SW-Ti55 rotor for 16 h at 45,000 rpm. Fractions were resolved on 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The antibody used for Western blotting was rabbit anti-Cdk9 (catalog no. sc-484; Santa Cruz Biotechnology).
Western blotting.
Cells were harvested by centrifugation, washed once with PBS buffer, and lysed in RIPA buffer (Cell Signaling) according to the manufacturer's instructions. Protein concentration of the lysates was determined by the bicinchoninic acid (BCA) method according to the manufacturer's recommendations (Pierce). About 20 to 40 μg of protein per sample was separated on precasted 10% Mini-Protean TGX gels (Bio-Rad) and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane using an iBlot gel transfer system (Invitrogen). Western blot analysis was performed according to standard protocols. Total JNK and phospho-JNK proteins were detected with specific monoclonal antibodies (catalog no. 9251 and 9252; Cell Signaling). A horseradish peroxidase-conjugated mouse anti-rabbit polyclonal antibody (catalog no. 2629; Cell Signaling) was used as the secondary antibody. The blot was developed using the Western Lightning Ultra chemiluminescent substrate from Perkin Elmer, Inc., and detected in an EpiChemi3 darkroom (UVP BioImaging Systems).
TransAM assays for NF-κB and AP-1 activity.
NF-κB p50 and p65 activities in nuclear extracts of cells were determined using TransAM assays (Active Motif). All experiments were performed according to the manufacturer's instructions. TransAM assays measure the ability of activated NF-κB to bind to an NF-κB consensus sequence in solution, with a 5- to 10-fold-higher sensitivity than gel shift assays. To determine whether the activation of AP-1 family members that have been reported to serve as JNK substrates or that are relevant for HIV-1 expression would be inhibited by AS601245, we utilized TransAM assays (Active Motif). These DNA binding enzyme-linked immunosorbent assays (ELISAs) allowed us to determine how activation of c-Fos, FosB, Fra-1, c-Jun, JunB, or JunD and the ability of these AP-1 factors to bind to their DNA recognition sequence would be influenced by AS601245. All experiments were performed according to the manufacturers' instructions.
Flow cytometry.
Infection levels in the cell cultures were monitored by flow cytometric (FCM) analysis of green fluorescent protein (GFP) expression. FCM analysis was performed on a GUAVA EasyCyte (GUAVA Technologies, Inc.), a FACSCalibur, or an LSRII (BD). Cell sorting experiments were performed using a FACSAria flow cytometer (BD). Data analysis was performed using either CellQuest (BD) or GUAVA Express (GUAVA Technologies, Inc.) software.
RESULTS
Identification of AS601245 as an inhibitor of HIV-1 reactivation.
During a high-content drug screen, we identified AS601245 [1,3-benzothiazol-2-yl-(2-{[2-(3-pyridinyl)ethyl]amino}-4-pyrimidinyl)-acetonitrile; JNK inhibitor V] (11) as an inhibitor of HIV-1 reactivation. In vivo, AS601245 has been shown to have neuroprotective properties and reduces damage to neurites and activation of astrocytes without detrimental side effects (10, 11). However, AS601245 has never been investigated as an inhibitor that could influence HIV-1 infection.
To determine the inhibitory capacity of AS601245 on reactivation of latent HIV-1 infection, we performed dose matrix experiments in which AS601245 was titrated against several HIV-1-reactivating stimuli, including PMA, TNF-α, and HIV-1-reactivating factor (HRF) (78). AS601245 inhibited reactivation by either stimulus in a concentration-dependent manner. Figure 1A depicts the inhibitory effect of AS601245 on reactivation of latent HIV-1 infection in CA5 T cells that were stimulated with increasing concentrations of TNF-α. In this system, the 50% inhibitory concentration (IC50) for AS601245 would be 2.5 μM. As PMA, TNF-α, and HRF are using different signal transduction pathways, it is likely that AS601245 blocks a key step in a process involved in HIV-1 reactivation and does not inhibit a signal transduction event.
Fig 1.
AS601245 inhibits reactivation of latent HIV-1 infection. (A) In a dose matrix experiment, TNF-α as the HIV-1-reactivating agent and AS601245 were titrated on the latently HIV-1-infected CA5 T cells. Levels of HIV-1 reactivation were determined 24 h poststimulation using flow cytometric analysis (% GFP-positive cells). TNF-α concentrations (ng/ml) are indicated. (B) To determine whether the inhibitory effect of AS601245 on HIV-1 reactivation was dependent on the type of viral integration, we determined the influence of increasing concentrations of AS601245 on PMA-mediated HIV-1 reactivation (10 ng/ml) in latently HIV-1-infected T cells in which HIV-1 was integrated in the same sense orientation relative to the transcriptional orientation of the host gene (CA5 cells), T cells in which HIV-1 was integrated in the converse sense orientation relative to the host gene (EF7 cells), and T cells in which HIV-1 was integrated into an intergenic region (CG3 cells).
In CA5 T cells, the latent virus is integrated into an exon of the RMB12/CPNE1 gene. Viral transcription and host gene transcription occur in the same direction. To ensure that AS601245 acts as a general inhibitor of HIV-1 reactivation, we tested the compound on two additional T cell lines for which we have characterized the site of integration. In EF7 T cells, the virus is integrated in an intron of the WHSC1 gene. Integration has occurred in the converse sense orientation relative to the orientation of host gene transcription. In CG3 T cells, the latent virus is integrated into an intergenic region between the TIGD5 and the PYCRL genes. In these experiments, we found that AS601245 inhibited HIV-1 reactivation independent of the integration site characteristics of the latent virus (Fig. 1B).
AS601245 prevents reactivation of latent HIV-1 infection in primary T cells.
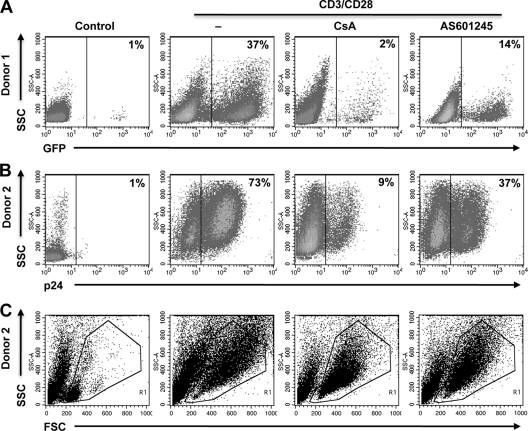
We next tested whether AS601245 would also exert its inhibitory activity on reactivation of latent HIV-1 infection in primary T cells. For this purpose, latently infected cultured central memory T cells were prepared from primary naïve cells as previously described (5, 6). Reactivation of latent HIV-1 infection was then triggered by antibody-mediated CD3/CD28 costimulation. As with the stimuli used in the above-described experiments, CD3/CD28 costimulation has been reported to stimulate NF-κB activity in primary T cells but also activates the nuclear factor of activated T cells (NFAT) pathway. As shown in Fig. 2, AS601245 also inhibited HIV-1 reactivation in this primary T cell model of latent HIV-1 infection. Experiments from two different donors, either using a GFP reporter virus (Fig. 2A) or using intracellular p24 staining (Fig. 2B) to detect HIV-1 reactivation under the various experimental conditions, are presented. In experiment 1, when T cells were infected with a GFP reporter virus, CD3/CD28 costimulation triggered HIV-1 reactivation in 37% of the cells. Cyclosporine, an inhibitor of NFAT activation, was used as a control inhibitor and, as expected, mostly abrogated CD3/CD28-mediated HIV-1 reactivation (2% GFP-positive cells). In the presence of 10 μM AS601245 reactivation, levels were reduced to 14%. Reactivation levels were determined 72 h poststimulation. Similar results were obtained when p24 staining was utilized to detect reactivation in the presence or absence of AS601245 in primary T cells infected with wild-type (wt) HIV-1 (NL43; Fig. 2B). At the utilized concentrations of AS601245, cell viability was not affected. As seen in the corresponding forward scatter (FSC)/side scatter (SSC) dot plots (Fig. 2C), AS601245 at 10 μM did not affect cell viability and did not affect the ability of the CD3/CD28 MAb combination to trigger cell activation. In the presence of 10 μM AS601245, CD3/CD28-stimulated primary T cells still transformed to a larger and more granular cell phenotype relative to the resting cell phenotype seen in the untreated control cells. These data suggest that the kinase activity targeted by AS601245 controls latent HIV-1 infection in both T cell lines and primary T cells, without impairing overall T cell function.
Fig 2.
AS601245 inhibits HIV-1 reactivation in primary T cells. Latently HIV-1-infected primary T cells were generated as previously described (5). (A) PBMCs from donor 1 were infected with a GFP reporter virus. Active background infection (Control) and the level of reactivated HIV-1 were determined by flow cytometric analysis for GFP expression. HIV-1 reactivation was achieved by antibody-mediated CD3/CD28 costimulation. Cyclosporine (CsA), a reported inhibitor of CD3/CD28-mediated HIV-1 reactivation, was used as a specificity control. The inhibitory effect of AS601245 (AS) at 10 μM on HIV-1 reactivation is shown in the last dot plot. (B) The same experiments were performed using HIV-1 HXB2, and infection levels were detected by intracellular staining for Gag p24 protein. (C) FSC/SSC (cell size/granularity) plots reveal the resting phenotype prior to stimulation and the transition to an activated cell phenotype following CD3/CD28 stimulation in the presence or absence of cyclosporine or AS601245.
AS601245 suppresses HIV-1 reactivation despite high levels of induced NF-κB activity.
All of the utilized HIV-1-reactivating stimulators converge in the NF-κB pathway. As other reported inhibitors of HIV-1 reactivation exerted their inhibitory function by preventing NF-κB activation, a key transcription factor for HIV-1 expression, we tested the ability of AS601245 to prevent induced NF-κB activation (80). For this purpose, we stimulated the latently HIV-1-infected CA5 reporter T cells with PMA, TNF-α, or HRF, either in the presence or absence of optimal concentrations of AS601245, and determined the kinetic p50 and p65 activity profiles over the first 60 min, when peak activation is expected, using TransAM NF-κB assays. Optimal concentration was defined as maximum inhibitory on-target effect with no or minimal cytopathic effect.
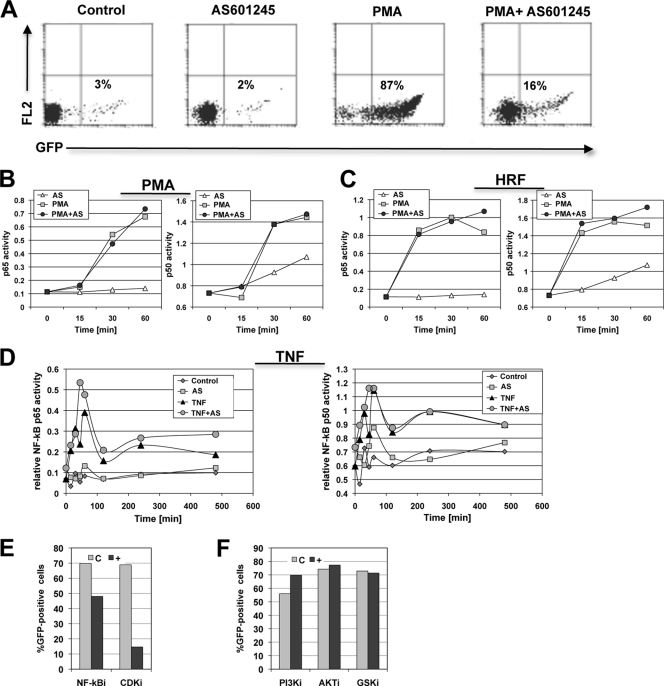
As shown in Fig. 3A, PMA-induced HIV-1 reactivation was fully suppressed by AS601245. Surprisingly, we found that NF-κB activation was not inhibited by AS601245. AS601245 would thus prevent HIV-1 reactivation despite high levels of induced NF-κB activity. The initial increase in NF-κB p50 and p65 activity triggered by PMA or HRF stimulation in the presence or absence of AS601245 over the first 60 min following stimulation is shown in Fig. 3B and C. An extended kinetic of NF-κB activity following TNF-α stimulation in the presence or absence of AS601245 is depicted in Fig. 3D. Again, no inhibition of TNF-α-induced NF-κB activity by AS601245 was observed during the 500-min experimental period.
Fig 3.
AS601245 prevents HIV-1 reactivation without inhibiting NF-κB. (A) Latently HIV-1-infected CA5 T cells were left untreated or pretreated with AS601245 for 1 h. Then the cells were left either untreated or stimulated with PMA to trigger HIV-1 reactivation as indicated. Levels of HIV-1 reactivation were determined by flow cytometric analysis for GFP expression 24 h after PMA stimulation. (B) From the cultures depicted in panel A, samples were taken at the indicated time points (0 to 60 min) and used to determine the level of induced NF-κB activity in the presence or absence of AS601245 by using TransAM assays. (C) Experiments similar to those described in the legend to panel B using HRF as the HIV-1-reactivating stimulus. (D) CA5 T cells were stimulated with TNF-α, and NF-κB activity was followed over a 500-min period to determine any long-term effects of AS601245 on TNF-α-induced NF-κB activity oscillation. (E) Inhibitory effect of the NF-κB inhibitor aloisine A and the CDK inhibitor roscovitine on HIV-1 reactivation in CA5 T cells. (F) Effect of the PI3 kinase inhibitor Ly294002, AKT inhibitor VIII, or the GSK inhibitor TDZD-8 on HIV-1 reactivation in CA5 T cells. All experiments were done as dose matrix experiments, and the depicted conditions represent maximum achievable inhibition at a cell viability of >50%.
These results demonstrate that NF-κB activation, measured by the ability of p50 and p65 to bind to its DNA recognition sequence, is not impaired by AS601245. Reported NF-κB inhibitors, such as aloisine A or the cyclin-dependent kinase (CDK) inhibitor roscovitine, somewhat inhibited HIV-1 reactivation, although to a lesser degree than AS601245 (Fig. 3E). Inhibitors of pathways known to affect NF-κB phosphorylation, such as the GSK3β or AKT/PI3 kinase pathway, had no effect on HIV-1 reactivation (Fig. 3F). AS601245 thus indeed controls latent HIV-1 infection even in the presence of high levels of NF-κB activity.
AS601245 effect on HIV-1 latency establishment.
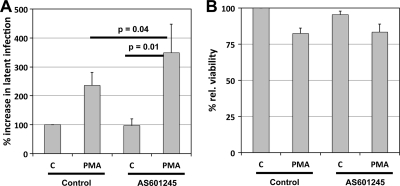
We next tested the influence of AS601245 on HIV-1 latency establishment using a previously published experimental system (20). Briefly, we infected Jurkat T cells with a GFP reporter virus at different multiplicities of infection (MOIs) (6 to 47% active infection on day 3 postinfection). Reverse transcriptase (RT) inhibitors were added 24 h postinfection to prevent the formation of preintegration latency (20, 54). The cells were infected either in the absence or presence of 10 μM AS601245. As the infection cultures were continued past day 17, when a stable population of latently infected cells is usually established, we consistently observed an appreciable increase in the size of the latently infected cell reservoir for cell cultures treated with AS601245 compared to that of the untreated control cultures (Fig. 4). AS601245 in these experiments doubled or tripled the pool of latently HIV-1-infected T cells, while cell viability was only marginally affected (Fig. 4B). AS601245 thus not only blocks reactivation but can force de novo HIV-1 infection events into a latent state.
Fig 4.
AS601245 forces HIV-1 into latent infection. To determine whether AS601245 would promote the establishment of latent HIV-1 infection, Jurkat T cells were infected with a GFP reporter virus (NLENG1) at different MOIs (6 to 47% active infection on day 3) either in the absence (Control) or presence of AS601245. (A) On day 17 postinfection, each culture was split, and the subcultures were either left untreated (C) or were stimulated with PMA to trigger (re)activation of silent HIV-1 infection events. The level of active HIV-1-expressing cells in either culture was then determined after 24 h as the percentage of GFP-expressing cells. For each culture condition, the difference in the percentage of GFP-positive cells in the control culture and the corresponding PMA-induced cultures is equal to the size of the formed reservoir of latently HIV-1-infected T cells. To account for the differences in initial infection, results are represented as relative infection levels. Results represent the means ± standard deviations from four independent experiments. To determine the significance of the observed differences, we used Student's t test. (B) Cell viability for the respective experimental conditions.
AS601245 effect on cellular gene expression.
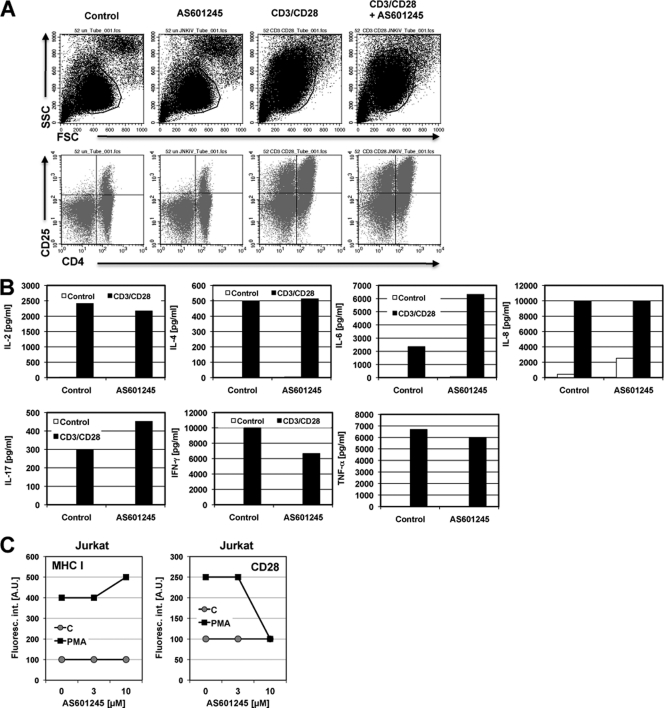
To confirm that AS601245 would not act as an unspecific inhibitor of transcription, we next investigated the effect of AS601245 on baseline and CD3/CD28 stimulation-induced expression of a series of relevant T cell molecules (CD4, CD25, CD54, major histocompatibility complex class I [MHC-I], MHC-II) using flow cytometric analysis. In peripheral blood mononuclear cells (PBMCs), AS601245 (10 μM) neither changed baseline expression of CD25 nor prevented CD3/CD28 stimulation-induced upregulation of CD25 (Fig. 5A). Similarly, AS601245 did not influence baseline or induced CD54 (ICAM-1) expression (data not shown). MHC-I and MHC-II expressions were not influenced by the presence of AS601245 (data not shown). As seen before, AS601245 did not affect differentiation of the primary T cells into an activated phenotype, as visible in the FSC/SSC plots (Fig. 5A).
Fig 5.
Effect of AS601245 on cellular gene expression. (A) PBMCs were left untreated (Control), treated with 10 μM AS601245, or activated with an anti-CD3/CD28 MAb combination in the absence or presence of 10 μM AS601245. After 24 h, the influence of AS601245 on cell activation as defined by the FSC/SSC phenotype and CD25 expression levels was determined by flow cytometric analysis. (B) The influence of AS601245 (10 μM) on CD3/CD28-induced cytokine expression (IL-2, IL-4, IL-6, IL-8, IL-17, TNF-α, and IFN-γ) was determined using BioPlex analysis. (C) Baseline and PMA-induced MHC-I and CD28 expression in Jurkat T cells was determined 48 h after the addition of AS601245 and PMA using flow cytometric analysis.
We further tested the effect of AS601245 on activation-induced cytokine secretion. At 24 h after CD3/CD28 stimulation of PBMCs from two donors, we harvested supernatants and analyzed for the presence of IL-2, IL-4, IL-6, IL-8, IL-17, TNF-α, and gamma interferon (IFN-γ). In both donors, we found no or low-level inhibition of induced IL-2, IL-4, IL-8, IFN-γ, and TNF-α expression. For both donors, induced IL-6 expression was boosted in the presence of 10 μM AS601245. AS601245 boosted IL-17 expression for one of the donors (Fig. 5B).
In Jurkat T cells, we obtained similar results for the constitutive and induced expressions of cell surface markers. For example, following PMA stimulation of Jurkat T cells, AS601245 had no effect on MHC-I upregulation (Fig. 5C). PMA-induced CD28 upregulation was unaffected by 3 μM AS601245 but inhibited at 10 μM AS601245 (Fig. 5C). Taken together, these data provide evidence that AS601245 is not a general transcription inhibitor but seems to relatively selectively prevent reactivation of latent HIV-1 infection.
AS601245 effect on JNK and JNK substrate activation.
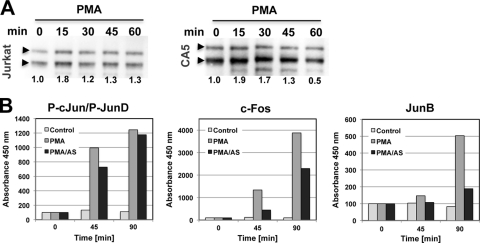
AS601245 has been reported as a specific inhibitor of JNK activity. JNK would be an interesting candidate target to explain our observations, as AP-1, which has been described as essential for efficient HIV-1 transcription, is a well-described JNK substrate (26, 58–60, 65). Mutations of the three AP-1 sites in the enhancer element of the HIV-1 long terminal repeat (LTR) have been demonstrated to substantially decrease HIV-1 expression (26, 59, 60). Therefore, it is conceivable that the observed inhibitory activity of AS601245 on HIV-1 reactivation could be exerted through modified AP-1 interaction with these crucial transcription factor binding sites. To see whether JNK would indeed be the molecular target of AS601245 in the context of HIV-1 reactivation, we initially investigated whether and how the HIV-1-reactivating stimuli used would trigger JNK activation. With PMA being the strongest HIV-1-reactivating stimulus in this system, we used this activator to study the effect of AS601245 on JNK and JNK substrate activation. As seen in Fig. 6A, the effects of PMA stimulation on JNK activation, as measured by changes in the level of phosphorylated JNK, were relatively small in both the parental Jurkat cell population and the latently HIV-1-infected CA5 T cells. No inhibitory effect of AS601245 on PMA-induced JNK phosphorylation was observed. As AS601245 has been reported to act as an ATP competitive inhibitor, which means it would not inhibit JNK phosphorylation but would inhibit JNK substrate phosphorylation, this was expected. We next investigated whether AS601245 would inhibit the induction of phosphorylation of AP-1 proteins that are reportedly JNK substrates. PMA led to c-Jun, c-Fos, and JunB activation in the latently HIV-1-infected CA5 T cells (Fig. 6B). AS601245 addition delayed PMA induced c-Jun activation and reduced c-Fos and JunB activation by 50% or 70%, respectively. In support of the idea that AP-1 binding to the LTR is one target of AS601245 as an inhibitor of HIV-1 reactivation, we found that a second JNK inhibitor, SP600215, also inhibited HIV-1 reactivation but with less efficiency (data not shown). JNK specificity of the inhibitory effect is further suggested by our finding that inhibitors of the mitogen-activated protein kinase family (MAPK), such as the ERK inhibitor U0126 or the p38 inhibitor SB202190, exhibited no inhibitory activity on HIV-1 reactivation. Also, as mentioned earlier, the GSK3β inhibitors TDZD-8 and AR-A014418, Akt inhibitor VIII, or the PI3-kinase inhibitor Ly29402 did not affect HIV-1 reactivation (Fig. 3F). The negative data on several key activation pathways for T cells support the notion that AS601245 acts to prevent HIV-1 reactivation by inhibiting the JNK pathway and not by an as-yet-undescribed, unspecific side effect.
Fig 6.
Effect of AS601245 on JNK and JNK substrate activation. (A) Jurkat T cells and the latently HIV-1-infected CA5 T cells were stimulated with PMA, and changes in the JNK2 (upper bands) and JNK1 (lower bands) phosphorylation levels were determined by Western blotting using a phospho-JNK MAb. (B) CA5 T cells were stimulated with PMA in the absence or presence of AS601245, and activation of c-Jun, c-Fos, and JunB as possible JNK substrates was quantified using a TransAM assay (data are representative of results from two independent experiments).
AS601245 inhibits P-TEFb release from its complex with HEXIM-1.
As our data indicated that the inhibitory effect of AS601245 on reactivation of latent HIV-1 infection likely occurred by preventing efficient transcriptional elongation induced by stimulation, we investigated the possibility that AS601245 would also prevent the release of positive transcription elongation factor (P-TEFb) from its inactive complex with HEXIM-1. Previous reports have described the presence of paused RNAP II at the latent HIV-1 LTR (48–50). This paused RNAP II complex is characterized by the inclusion of the negative elongation factor (NELF) (40, 70, 83). P-TEFb association to RNAP II and NELF removal is essential to trigger efficient elongation. The binding of P-TEFb (a complex of cyclin T1 and CDK9) to the RNAP II complex associated with the HIV-1 LTR has been demonstrated as essential for efficient transcriptional elongation (45, 84). Conversely, restriction of P-TEFb has been associated with HIV-1 latency (71). HMBA-mediated release of P-TEFb from its complex with HEXIM-1 has previously been reported to trigger HIV-1 reactivation (13, 18).
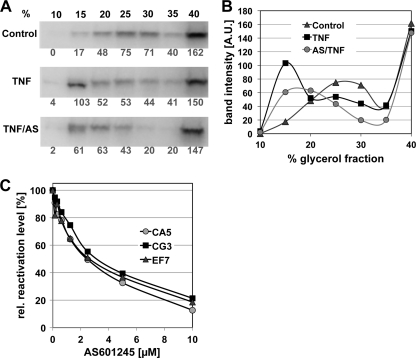
To explore an effect of AS601245 on P-TEFb release from the inactive complex with HEXIM-1, we stimulated CA5 T cells with TNF-α either in the absence of AS601245 or following 1 h of pretreatment with the compound. Cells were harvested from all conditions at different time points following TNF-α addition. Cell lysates were then separated on a glycerol gradient (10 to 45%) to reveal possible changes in the composition of the P-TEFb/HEXIM-1 complex. Release of P-TEFb from the inactive complex with HEXIM-1 (large complex) that is found in the glycerol gradient fractions with higher glycerol content is indicated by a shift to a smaller complex (CDK9/CycT1) found in the gradient fractions with lower glycerol content. Each gradient fraction was separated on an SDS-PAGE gel and subjected to Western blotting and antibody staining.
The results of these experiments using CA5 cells are presented in Fig. 7. Staining with anti-CDK9 antibody revealed that TNF-α treatment had not triggered visible P-TEFb release from the HEXIM-1 complex after 1 h (data not shown); however, P-TEFb release from the inactive complex could be observed by a shift of CDK9 presence to low-molecular-weight fractions 6 h after TNF-α stimulation (Fig. 7A). Pretreatment of CA5 with 10 μM AS601245 for 1 h prior to TNF-α stimulation inhibited the TNF-α-induced release of P-TEFb from its complex with HEXIM-1 at this time point. These experiments suggest that AS601245 also affects the release of P-TEFb from its inactive complex with HEXIM-1, which would be a prerequisite for efficient elongation of transcription by the paused RNAP II complex found at the latent HIV-1 LTR (40, 51, 83). The possibility that AS601245 could act by preventing P-TEFb release from its inactive complex with HEXIM-1 is also supported by the finding that HMBA-induced reactivation of latent HIV-1 infection, which is believed to be triggered by the HMBA-induced release of P-TEFb from its complex with HEXIM-1 (13, 18), is inhibited by AS601245 (Fig. 7C) in CA5, CG3, and EF7 T cells.
Fig 7.
AS601245 inhibits TNF-α-stimulated P-TEFb release. To test the ability of AS601245 to act as an inhibitor of HIV-1 reactivation by inhibiting the release of P-TEFb from its inactive complex with HEXIM-1, we performed glycerol gradient analysis to determine the effect of AS601245 on the P-TEFb/HEXIM-1 complex composition in the latently infected CA5 T cells. CA5 T cells were left untreated or treated with AS601245 (10 μM) for 1 h. Cells were then left unstimulated or HIV-1 reactivation was triggered with TNF-α (3 ng/ml). Cell lysates were separated on a glycerol gradient (10 to 45%). Each gradient fraction was separated on a 10% SDS-PAGE gel and transferred by Western blotting. Blots were stained with anti-CDk9 MAb, a component of the P-TEFb complex. (A) Release of P-TEFb from the inactive complex with HEXIM-1 (large complex), which is found in the glycerol gradient fractions with higher glycerol content, is indicated by a shift to a smaller complex (CDK9/CycT1) found in the gradient fractions with lower glycerol content. Changes in the band intensity in the P-TEFb/HEXIM-1 complex composition relative to that of control cells for CA5 T cells stimulated with TNF-α or stimulated with TNF-α following pretreatment with AS601245 are shown. All blots were stained with an anti-CDK9 antibody to reveal drug-induced shifts in the complex composition. Band densities were then determined using ImageJ. (B) CDK9 band intensities of the glycerol fractions for control cells, TNF-α-activated cells, and AS601245 pretreated/TNF-α-activated cells (6 h). The data are representative of results from two independent experiments. (C) Concentration-dependent inhibition of HMBA-induced HIV-1 reactivation by AS601245 in the latently infected CA5, CG3, and EF7 T cells.
DISCUSSION
Depletion of latent HIV-1 infection from its cellular reservoirs will have to be an essential part of any potential future HIV-1 eradication therapy. As latently HIV-1-infected T cells have no distinct phenotype that would allow the targeting of these cells directly (7), system-wide reactivation of latent HIV-1 infection currently seems the only way to achieve this goal. Following reactivation, the cytopathic effect of the active virus infection is expected to destroy the host cells. Alternatively, due to the presence of the gp41/gp120 complex on the cell surface of the cells harboring reactivated infection events, active therapeutic destruction with either gp41/gp120-specific immunotoxins (3, 12) or radioimmunotherapy could be achieved (19). How therapeutic, system-wide reactivation of latent HIV-1 infection can be achieved is unclear at this time. In fact, there is no consensus on how latent HIV-1 infection is actually governed at the molecular level.
We here report that latent HIV-1 infection is controlled in part by a kinase activity that is targeted by AS601245, a small molecule reported to act as a JNK inhibitor. Unlike other pharmacological inhibitors that inhibit HIV-1 reactivation by preventing NF-κB activation (80), AS601245 prevented reactivation even in the presence of a high level of NF-κB activity. The direct demonstration that the status of latent infection is controlled by a gatekeeper kinase activity has implications for how therapeutic strategies to reactivate latent HIV-1 infection will need to be designed.
Early on, stimuli that act as NF-κB activators, such a PMA, prostratin, or TNF-α, were found to act as potent HIV-1-reactivating agents in many cellular models of latent HIV-1 infection (24, 25, 53, 76). It was believed that NF-κB activation was both a necessary and sufficient requirement to trigger HIV-1 reactivation. The problem with translating this approach into the clinical setting is to identify stimuli that would produce sufficient levels of NF-κB activity to reactivate latent HIV-1 infection in resting CD4+ memory T cells that are considered the primary in vivo host cell type for latent infection but would not produce a cytokine storm, as many cytokine promoters are also NF-κB responsive (27, 44, 75). Dissociation of HIV-1 reactivation from cellular gene activation is a prerequisite for such a therapeutic approach (78).
Based on the idea that NF-κB augmentation could trigger HIV-1 reactivation, attempts to clinically translate these findings using IL-2 or the FDA-approved anti-CD3 MAb OKT3 were made to intensify highly active antiretroviral therapy (HAART) (14, 15, 41). These therapeutic attempts did not achieve viral eradication. One possible explanation for the failure of these strategies was that the therapeutically justifiable dose of IL-2 or OKT3 was insufficient to provide the required level of systemic NF-κB activation in the memory T cell population harboring latent HIV-1 infection (27).
Our data provide an alternative explanation for the inability of these NF-κB-inducing stimuli to trigger HIV-1 reactivation. IL-2 or anti-CD3 MAb stimulation, other than combined anti-CD3/CD28 stimulation, may simply fail to control the gatekeeper kinase activity that is targeted by AS601245. However, NF-κB activation in the absence of this kinase activity does not permit efficient HIV-1 reactivation. We were not able to test a possible influence of AS601245 on reactivation triggered by histone deacetylase inhibitors, as in our experimental system this class of compounds/drugs fails to trigger HIV-1 reactivation. Failure of HDAC inhibitors to trigger latent HIV-1 infection has been reported for the latently HIV-1-infected T cell lines (20) and the latently infected primary T cell system used in our experiments (5). We could demonstrate that AS601245 targets a molecular key mechanism for HIV reactivation, as it also inhibited HMBA-induced HIV-1 reactivation, which is thought to primarily act by releasing P-TEFb from its complex with HEXIM-1 (13, 18).
Our experiments have identified two molecular targets of AS601245, AP-1 activation and P-TEFb release from its inactive complex with HEXIM-1. Both have been described as essential for HIV-1 transcription and would be downstream of the postulated gatekeeper kinase activity.
AS601245 clearly affected the activation of AP-1 family members, a dimeric protein consisting of members of the Jun or Fos protein family. AP-1 proteins bind a palindromic DNA sequence known as the tetradecanoyl phorbol acetate (TPA)-responsive elements (TRE) at positions +95 and +160, downstream of the transcriptional start site (9, 57, 58, 72). Interestingly, in the context of the HIV-1 LTR, AP-1 has been described to act as an activator or a repressor of transcription, depending on the components of the AP-1 dimer (21, 32). Once bound to the promoter, c-Fos/c-Jun heterodimers can recruit the SWI/SNF chromatin remodeling complex to activate transcription, whereas homodimers or heterodimers consisting of other family members lack this ability (34). In addition to directly regulating HIV-1 gene expression, AP-1 inhibition could alter the activity of other transcription factors. For example, C/EBPα heterodimers with c-Jun or c-Fos act as potent activators of transcription (28). Heterodimers of c-Fos or c-Jun with C/EBPβ have been described to reduce C/EBP-mediated transactivation (33). As there have been several C/EBP binding sites reported for the HIV-1 LTR, interference of AS601245 with AP-1 protein–C/EBP complex formation could further add to the inhibitory effect of AS601245 on HIV-1 reactivation. AP-1 has further been described to interact with NF-κB and the enhancer element of the LTR. This interaction was described to result in synergistic activation of the LTR and has been proposed as a mechanism that can trigger HIV-1 reactivation (37, 80). Inhibition of AP-1 activation, even if selective for certain family members, can thus prevent initiation of efficient transcription of the latent HIV-1 LTR.
This is particularly interesting in the context of the selectivity of AS601245 for HIV-1 reactivation and the absence of an AS601245 effect on T cell activation and cytokine gene induction. The functional disparity of the AS601245 effect on HIV-1 reactivation and T cell activation/cytokine gene induction could be a result of the differential NF-κB/Rel factor binding requirements of the CD28-responsive element (CD28RE) in the HIV-1 LTR and various CD28RE-controlled cellular gene promoters (e.g., IL-2, TNF-α, IL-6, IL-8). The CD28RE is a combinatorial binding site for NF-κB and AP-1. Its essential role in gene induction was first demonstrated by the requirement for CD3/CD28 signal integration for IL-2 gene expression (30, 44, 46, 61). A similar CD28RE has been identified in the HIV-1 LTR, and accordingly, CD3/CD28 signal integration is also required for optimal activation of the HIV-1 LTR (67, 68). Trushin et al. previously demonstrated that PKCθ is a central integrative factor for both phorbol ester and TCR/CD28-mediated HIV-1 reactivation in a T cell line (J1.1 cells) (69). NF-κB and AP-1 have been identified as the primary targets of PKCθ, and selective inhibition of certain AP-1 factors by AS601245 could thus differentially influence HIV-1 and cytokine gene expression, in particular, as a functional disparity between cytokine CD28RE and the HIV-1 LTR CD28RE has been described (17).
We further demonstrated that AS601245 prevents the release of P-TEFb from its inactive complex with HEXIM-1. P-TEFb is an essential component of the actively elongating RNA RNAP II complex (84). On the latent HIV-1 LTR, paused RNAP II complex has been described, in which P-TEFb was replaced by negative elongation factor (NELF) (55, 83). P-TEFb restriction has been reported to contribute to HIV-1 latency (71). The HIV-1 Tat-triggered release of P-TEFb from the inactive complex with HEXIM-1 has been described to contribute to active viral transcription (29, 52). The importance of the P-TEFb/HEXIM-1 interaction in the context of latent HIV-1 infection is further supported by the finding that N′-N′-hexamethylene-bisacetamide (HMBA), a differentiating agent, has been reported to trigger HIV-1 reactivation by releasing P-TEFb from its complex with HEXIM-1 (18). The HIV-1-reactivating activity of HMBA that is observed in our experimental systems is also inhibited by AS601245 (Fig. 7C).
AS601245 thus seems to simultaneously interfere with HIV-1 reactivation at several levels, which could explain its exceptional potency as an inhibitor of HIV-1 reactivation.
Other inhibitors of HIV-1 reactivation that we identified, such as aloisine A or roscovitine, which seem to interfere only with one component (e.g., NF-κB, CDK) of the cellular machinery required to trigger reactivation, were not as potent.
The inhibitory effect of AS601245 demonstrates that reactivation of latent HIV-1 infection is not a strict function of low NF-κB activity levels and reveals the existence of molecular mechanisms that control and even supersede NF-κB activity. Directly targeting these mechanisms by pharmacological perturbation may reduce the activation threshold required to trigger HIV-1 reactivation. Our findings thus suggest that combinations of drugs that target control mechanisms with low-level activating agents that would allow the dissociating of HIV-1 reactivation from cellular activation may be the most promising way forward for the development of HIV-1 reactivation therapies. However, previous attempts to target latent HIV-1 infection, including those using HDAC inhibitors, were guided by the “one-target-one-drug” hypothesis, which is de facto based on the premise that the “perfect” chemical probe is sufficient to act on a single target (“a magic bullet”). Failure to consider the complex nature of latent HIV-1 infection at the molecular level may explain the lack of clinical success of these approaches (2, 41, 43, 62–64, 74).
More current research on the molecular mechanisms controlling HIV-1 latency indicates that multiple components should be triggered in a coordinated fashion to induce HIV-1 reactivation in the absence of sustained T cell activation (77). The idea to combine drugs (multidrug combinations for networked systems) (39) has already been applied in some previous studies looking into the ability of drug combinations to reactivate latent HIV-1 infection in experimental systems (22, 56). Our findings provide support for the concept of using multiple drugs to therapeutically reactivate latent HIV-1 infection.
ACKNOWLEDGMENTS
This work was funded in part by NIH grant R01AI064012 to O.K. and R01AI087508 to V.P.
Takao Shishido performed this research at the University of Alabama at Birmingham as a visiting scientist from Shionogi & Co., Ltd., Japan. Parts of the work were performed in the UAB CFAR BSL-3 facilities and by the UAB CFAR Flow Cytometry Core/Joint UAB Flow Cytometry Core, which are funded in part by NIH/NIAID P30 AI027767 and by NIH 5P30 AR048311.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Abramowicz D, Crusiaux A, Goldman M. 1992. Anaphylactic shock after retreatment with OKT3 monoclonal antibody. N. Engl. J. Med. 327:736. [DOI] [PubMed] [Google Scholar]
- 2. Archin NM, et al. 2010. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One 5:e9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berger EA, Pastan I. 2010. Immunotoxin complementation of HAART to deplete persisting HIV-infected cell reservoirs. PLoS Pathog. 6:e1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blankson JN, et al. 2000. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 182:1636–1642 [DOI] [PubMed] [Google Scholar]
- 5. Bosque A, Planelles V. 2009. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood 113:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosque A, Planelles V. 2011. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods 53:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brooks DG, et al. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413–423 [DOI] [PubMed] [Google Scholar]
- 8. Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. 1991. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canonne-Hergaux F, Aunis D, Schaeffer E. 1995. Interactions of the transcription factor AP-1 with the long terminal repeat of different human immunodeficiency virus type 1 strains in Jurkat, glial, and neuronal cells. J. Virol. 69:6634–6642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carboni S, et al. 2008. AS601245, a c-Jun NH2-terminal kinase (JNK) inhibitor, reduces axon/dendrite damage and cognitive deficits after global cerebral ischaemia in gerbils. Br. J. Pharmacol. 153:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carboni S, et al. 2004. AS601245 (1,3-benzothiazol-2-yl (2-[[2-(3-pyridinyl) ethyl] amino]-4 pyrimidinyl) acetonitrile): a c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J. Pharmacol. Exp. Ther. 310:25–32 [DOI] [PubMed] [Google Scholar]
- 12. Chaudhary VK, et al. 1988. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature 335:369–372 [DOI] [PubMed] [Google Scholar]
- 13. Choudhary SK, Archin NM, Margolis DM. 2008. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4(+) T cells. J. Infect. Dis. 197:1162–1170 [DOI] [PubMed] [Google Scholar]
- 14. Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chun TW, et al. 1999. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 5:651–655 [DOI] [PubMed] [Google Scholar]
- 16. Chun TW, et al. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1:1284–1290 [DOI] [PubMed] [Google Scholar]
- 17. Civil A, Rensink I, Aarden LA, Verweij CL. 1999. Functional disparity of distinct CD28 response elements toward mitogenic responses. J. Biol. Chem. 274:34369–34374 [DOI] [PubMed] [Google Scholar]
- 18. Contreras X, Barboric M, Lenasi T, Peterlin BM. 2007. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 3:1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dadachova E, et al. 2006. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Med. 3:e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duverger A, et al. 2009. Determinants of the establishment of human immunodeficiency virus type 1 latency. J. Virol. 83:3078–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eferl R, Wagner EF. 2003. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer 3:859–868 [DOI] [PubMed] [Google Scholar]
- 22. Finnegan A, et al. 1996. IL-10 cooperates with TNF-alpha to activate HIV-1 from latently and acutely infected cells of monocyte/macrophage lineage. J. Immunol. 156:841–851 [PubMed] [Google Scholar]
- 23. Finzi D, et al. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 24. Folks TM, et al. 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. U. S. A. 86:2365–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. 1987. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238:800–802 [DOI] [PubMed] [Google Scholar]
- 26. Franza BR, Jr, Rauscher FJ, III, Josephs SF, Curran T. 1988. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science 239:1150–1153 [DOI] [PubMed] [Google Scholar]
- 27. Fraser C, et al. 2000. Reduction of the HIV-1-infected T-cell reservoir by immune activation treatment is dose-dependent and restricted by the potency of antiretroviral drugs. AIDS 14:659–669 [DOI] [PubMed] [Google Scholar]
- 28. Friedman AD. 2007. C/EBPalpha induces PU.1 and interacts with AP-1 and NF-kappaB to regulate myeloid development. Blood Cells Mol. Dis. 39:340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujinaga K, et al. 1998. The ability of positive transcription elongation factor B to transactivate human immunodeficiency virus transcription depends on a functional kinase domain, cyclin T1, and Tat. J. Virol. 72:7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh P, Tan TH, Rice NR, Sica A, Young HA. 1993. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc. Natl. Acad. Sci. U. S. A. 90:1696–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He G, Ylisastigui L, Margolis DM. 2002. The regulation of HIV-1 gene expression: the emerging role of chromatin. DNA Cell Biol. 21:697–705 [DOI] [PubMed] [Google Scholar]
- 32. Hess J, Angel P, Schorpp-Kistner M. 2004. AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 117:5965–5973 [DOI] [PubMed] [Google Scholar]
- 33. Hsu W, Kerppola TK, Chen PL, Curran T, Chen-Kiang S. 1994. Fos and Jun repress transcription activation by NF-IL6 through association at the basic zipper region. Mol. Cell. Biol. 14:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ito T, et al. 2001. Identification of SWI.SNF complex subunit BAF60a as a determinant of the transactivation potential of Fos/Jun dimers. J. Biol. Chem. 276:2852–2857 [DOI] [PubMed] [Google Scholar]
- 35. Jones J, et al. 2007. High throughput drug screening for human immunodeficiency virus type 1 reactivating compounds. Assay Drug Dev. Technol. 5:181–189 [DOI] [PubMed] [Google Scholar]
- 36. Jones J, Whitford W, Wagner F, Kutsch O. 2007. Optimization of HIV-1 infectivity assays. Biotechniques 43:589–594 [DOI] [PubMed] [Google Scholar]
- 37. Karin M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483–16486 [DOI] [PubMed] [Google Scholar]
- 38. Keedy KS, et al. 2009. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J. Virol. 83:4749–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keith CT, Borisy AA, Stockwell BR. 2005. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 4:71–78 [DOI] [PubMed] [Google Scholar]
- 40. Kim YK, et al. 2006. Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. EMBO J. 25:3596–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kulkosky J, et al. 2002. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J. Infect. Dis. 186:1403–1411 [DOI] [PubMed] [Google Scholar]
- 42. Kutsch O, Benveniste EN, Shaw GM, Levy DN. 2002. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J. Virol. 76:8776–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lehrman G, et al. 2005. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet 366:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maggirwar SB, Harhaj EW, Sun SC. 1997. Regulation of the interleukin-2 CD28-responsive element by NF-ATp and various NF-kappaB/Rel transcription factors. Mol. Cell. Biol. 17:2605–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mancebo HS, et al. 1997. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 11:2633–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McGuire KL, Iacobelli M. 1997. Involvement of Rel, Fos, and Jun proteins in binding activity to the IL-2 promoter CD28 response element/AP-1 sequence in human T cells. J. Immunol. 159:1319–1327 [PubMed] [Google Scholar]
- 47. Ochsenbauer-Jambor C, Jones J, Heil M, Zammit KP, Kutsch O. 2006. T-cell line for HIV drug screening using EGFP as a quantitative marker of HIV-1 replication. Biotechniques 40:91–100 [DOI] [PubMed] [Google Scholar]
- 48. Palangat M, Hittinger CT, Landick R. 2004. Downstream DNA selectively affects a paused conformation of human RNA polymerase II. J. Mol. Biol. 341:429–442 [DOI] [PubMed] [Google Scholar]
- 49. Palangat M, Landick R. 2001. Roles of RNA:DNA hybrid stability, RNA structure, and active site conformation in pausing by human RNA polymerase II. J. Mol. Biol. 311:265–282 [DOI] [PubMed] [Google Scholar]
- 50. Palangat M, Meier TI, Keene RG, Landick R. 1998. Transcriptional pausing at +62 of the HIV-1 nascent RNA modulates formation of the TAR RNA structure. Mol. Cell 1:1033–1042 [DOI] [PubMed] [Google Scholar]
- 51. Pearson R, et al. 2008. Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J. Virol. 82:12291–12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng J, Zhu Y, Milton JT, Price DH. 1998. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 12:755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perez VL, et al. 1991. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J. Immunol. 147:3145–3148 [PubMed] [Google Scholar]
- 54. Pierson TC, et al. 2002. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 76:8518–8531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ping YH, Rana TM. 2001. DSIF and NELF interact with RNA polymerase II elongation complex and HIV-1 Tat stimulates P-TEFb-mediated phosphorylation of RNA polymerase II and DSIF during transcription elongation. J. Biol. Chem. 276:12951–12958 [DOI] [PubMed] [Google Scholar]
- 56. Quivy V, et al. 2002. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J. Virol. 76:11091–11103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rabbi MF, Saifuddin M, Gu DS, Kagnoff MF, Roebuck KA. 1997. U5 region of the human immunodeficiency virus type 1 long terminal repeat contains TRE-like cAMP-responsive elements that bind both AP-1 and CREB/ATF proteins. Virology 233:235–245 [DOI] [PubMed] [Google Scholar]
- 58. Roebuck KA, Brenner DA, Kagnoff MF. 1993. Identification of c-Fos-responsive elements downstream of TAR in the long terminal repeat of human immunodeficiency virus type-1. J. Clin. Invest. 92:1336–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roebuck KA, Gu DS, Kagnoff MF. 1996. Activating protein-1 cooperates with phorbol ester activation signals to increase HIV-1 expression. AIDS 10:819–826 [DOI] [PubMed] [Google Scholar]
- 60. Roebuck KA, Rabbi MF, Kagnoff MF. 1997. HIV-1 Tat protein can transactivate a heterologous TATAA element independent of viral promoter sequences and the trans-activation response element. AIDS 11:139–146 [DOI] [PubMed] [Google Scholar]
- 61. Shapiro VS, Truitt KE, Imboden JB, Weiss A. 1997. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol. Cell. Biol. 17:4051–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Siliciano JD, et al. 2007. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J. Infect. Dis. 195:833–836 [DOI] [PubMed] [Google Scholar]
- 63. Siliciano JM, Siliciano RF. 2005. Targeting HIV reservoirs with valproic acid. Hopkins HIV Rep. 17:8–9 [PubMed] [Google Scholar]
- 64. Smith SM. 2005. Valproic acid and HIV-1 latency: beyond the sound bite. Retrovirology 2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stein B, et al. 1993. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stevenson M. 1997. Molecular mechanisms for the regulation of HIV replication, persistence and latency. AIDS 11(Suppl. A):S25–S33 [PubMed] [Google Scholar]
- 67. Tong-Starkesen SE, Luciw PA, Peterlin BM. 1989. Signaling through T lymphocyte surface proteins, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J. Immunol. 142:702–707 [PubMed] [Google Scholar]
- 68. Tong-Starksen SE, Luciw PA, Peterlin BM. 1987. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc. Natl. Acad. Sci. U. S. A. 84:6845–6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Trushin SA, et al. 2005. Human immunodeficiency virus reactivation by phorbol esters or T-cell receptor ligation requires both PKCalpha and PKCtheta. J. Virol. 79:9821–9830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tyagi M, Karn J. 2007. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 26:4985–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tyagi M, Pearson RJ, Karn J. 2010. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J. Virol. 84:6425–6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van Lint C, et al. 1997. Transcription factor binding sites downstream of the human immunodeficiency virus type 1 transcription start site are important for virus infectivity. J. Virol. 71:6113–6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Van Lint C, Emiliani S, Ott M, Verdin E. 1996. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 15:1112–1120 [PMC free article] [PubMed] [Google Scholar]
- 74. van Praag RM, et al. 2001. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J. Clin. Immunol. 21:218–226 [DOI] [PubMed] [Google Scholar]
- 75. Verweij CL, Geerts M, Aarden LA. 1991. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J. Biol. Chem. 266:14179–14182 [PubMed] [Google Scholar]
- 76. Williams SA, et al. 2004. Prostratin antagonizes HIV latency by activating NF-kappaB. J. Biol. Chem. 279:42008–42017 [DOI] [PubMed] [Google Scholar]
- 77. Williams SA, et al. 2006. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 25:139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wolschendorf F, et al. 2010. Hit-and-run stimulation: a novel concept to reactivate latent HIV-1 infection without cytokine gene induction. J. Virol. 84:8712–8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang HC, et al. 2009. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J. Clin. Invest. 119:3473–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang X, Chen Y, Gabuzda D. 1999. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. J. Biol. Chem. 274:27981–27988 [DOI] [PubMed] [Google Scholar]
- 81. Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. 2004. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS 18:1101–1108 [DOI] [PubMed] [Google Scholar]
- 82. Zack JA, et al. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213–222 [DOI] [PubMed] [Google Scholar]
- 83. Zhang Z, Klatt A, Gilmour DS, Henderson AJ. 2007. Negative elongation factor NELF represses human immunodeficiency virus transcription by pausing the RNA polymerase II complex. J. Biol. Chem. 282:16981–16988 [DOI] [PubMed] [Google Scholar]
- 84. Zhu Y, et al. 1997. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 11:2622–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]