Abstract
Rift Valley fever virus (RVFV) causes outbreaks of severe disease in people and livestock throughout Africa and the Arabian Peninsula. The potential for RVFV introduction outside the area of endemicity highlights the need for fast-acting, safe, and efficacious vaccines. Here, we demonstrate a robust system for the reverse genetics generation of a RVF virus replicon particle (VRPRVF) vaccine candidate. Using a mouse model, we show that VRPRVF immunization provides the optimal balance of safety and single-dose robust efficacy. VRPRVF can actively synthesize viral RNA and proteins but lacks structural glycoprotein genes, preventing spread within immunized individuals and reducing the risk of vaccine-induced pathogenicity. VRPRVF proved to be completely safe following intracranial inoculation of suckling mice, a stringent test of vaccine safety. Single-dose subcutaneous immunization with VRPRVF, although it is highly attenuated, completely protected mice against a virulent RVFV challenge dose which was 100,000-fold greater than the 50% lethal dose (LD50). Robust protection from lethal challenge was observed by 24 h postvaccination, with 100% protection induced in as little as 96 h. We show that a single subcutaneous VRPRVF immunization initiated a systemic antiviral state followed by an enhanced adaptive response. These data contrast sharply with the much-reduced survivability and immune responses observed among animals immunized with nonreplicating viral particles, indicating that replication, even if confined to the initially infected cells, contributes substantially to protective efficacy at early and late time points postimmunization. These data demonstrate that replicon vaccines successfully bridge the gap between safety and efficacy and provide insights into the kinetics of antiviral protection from RVFV infection.
INTRODUCTION
Rift Valley fever virus (RVFV) causes sporadic but devastating outbreaks of severe human disease and widespread morbidity and mortality in livestock. RVFV is a mosquito-borne virus of the Bunyaviridae family (genus Phlebovirus), and the timing of outbreaks is often closely associated with the emergence of floodwater Aedes species mosquitoes following periods of extensive heavy rainfall (33). Although so far confined to Africa and the Arabian Peninsula, RVFV has the potential to spread to other parts of the world, given the presence and changing distribution of competent vectors throughout Europe and the Americas (10, 14, 37). Livestock (sheep, cattle, goats) are particularly susceptible to RVFV disease; outbreaks are characterized by widespread abortion storms and neonatal mortality approaching 100% (36). Infection in adult animals is associated with lower mortality, but the loss of a large proportion of young animals has a serious economic impact. Humans usually become infected after handling aborted materials or other infected animal tissues or through the bite of an infected mosquito. Although generally self-limiting, human infections can manifest as a serious febrile illness marked by myalgia, arthralgia, photophobia, and severe headache; in a small proportion of individuals, RVFV disease can progress to hepatitis, delayed-onset encephalitis or retinitis, or a hemorrhagic syndrome. Case fatality in severely afflicted individuals can be as high as 20% (4). Currently, there are no specific treatments for RVFV infection recommended for animals or people.
RVFV has a tripartite negative-sense single-stranded RNA genome. The large (L) segment encodes the viral polymerase. The medium (M) segment encodes the structural glycoproteins, Gn and Gc, as well as nonstructural proteins, including a 78-kDa protein and NSm, a virulence factor suggested to function by inhibiting apoptosis (40). The ambisense small (S) segment encodes, in the viral sense, the nucleoprotein (NP) that is required for RNA synthesis, and the nonstructural NSs protein in the opposite orientation. NSs is the major RVFV virulence factor and functions to inhibit the host immune response (9) by generalized downregulation of host transcription (3, 25), posttranscriptional degradation of protein kinase R (PKR) (16, 18), and repression of the beta interferon (IFN-β) promoter (26). Previous work has indicated the importance of both NSm (6) and NSs (1, 38) in determining virulence in vivo.
The impact of RVFV disease throughout Africa and the Arabian Peninsula, and the potential for viral spread elsewhere, provide strong incentives to develop safe, efficacious, and affordable vaccines. Examples of recently developed candidate vaccines include DNA-vectored (2, 24, 27), virus-like particle (VLP) (11, 29, 31), replicon particle (RRP) (23), and live attenuated (5, 13) vaccines. VLP candidates show promise and remarkable safety but generally require adjuvant and/or multiple immunizations for complete protection. In comparison, live attenuated vaccines are highly immunogenic, presumably due to viral replication in an immunized host. However, early live attenuated vaccines (Smithburn, MP-12) were associated with teratogenesis and abortion in livestock (8, 17). More recently, a naturally occurring RVFV mutant (13, 39) and a reverse genetics-derived candidate developed in our laboratory (7) have been shown to be both safe and efficacious in livestock. To develop an even safer, yet rapidly efficacious vaccine candidate, we combined a characteristic of robust vaccine efficacy with further safety enhancements to produce a replication-competent but nonspreading vaccine candidate. As was the case for our previously described live-attenuated vaccine (7), these RVF virus replicon particles (VRPRVF) contain full-gene deletions of the critical virulence factors NSs and NSm. As an additional safety measure, VRPRVF do not carry the genes for structural glycoproteins and are therefore unable to produce new particles from infected cells, preventing spread within the immunized host and theoretically eliminating the risk of vaccine-induced pathogenicity.
Here, we demonstrate that VRPRVF immunization is both safe and efficacious against virulent RVFV challenge in a mouse model as early as 1 day after vaccination. Interestingly, immunization with nonreplicating VRPRVF (nr-VRPRVF) resulted in significantly lower survival following RVFV challenge at both early and late time points. To explore this further, we compared the early immune responses of immunized mice and found that, relative to results for mock- and nr-VRPRVF-immunized mice, VRPRVF mice developed a stronger systemic antiviral and subsequent adaptive response following immunization, indicating that VRPRVF RNA and protein synthesis, even when confined to the initially infected cells Research Council, are critical for stimulating robust immunity and subsequent protection.
MATERIALS AND METHODS
Ethics statement.
Animal procedures in this study complied with institutional guidelines, the U.S. Department of Agriculture Animal Welfare Act, and the National Research Council guidelines for the humane use of laboratory animals (31a). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Centers for Disease Control and Prevention (CDC; Atlanta, GA).
Cell culture and biosafety.
BSR-T7/5 cells were a generous gift from K. Conzelmann (Max von Pettenkofer-Institut, Munich, Germany). BSR-T7/5 and Vero E6 cells were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and penicillin-streptomycin (Invitrogen). BSR-T7/5 cells were maintained under selection with G-418 every other passage (Geneticin; 1 mg/ml; Invitrogen). All work with infectious RVFV was completed in a biosafety level 4 (BSL4) or BSL3+ laboratory at the CDC. All animals were housed within the BSL4 or BSL3+ laboratories in microisolator pans in HEPA filtration racks, following standard barrier techniques.
Animal immunization and infection.
For the suckling mouse safety test, 2-day-old CD-1 (ICR) mice (Charles River Laboratories) were inoculated intracranially (i.c.) with 1.0 × 104 50% tissue culture infective doses (TCID50) of VRPRVF or with 1.0 ×104 PFU of recombinant RVFV ZH501 (RVFV) in a total volume of 10 μl of DMEM. Mock-immunized mice were inoculated with 10 μl DMEM i.c. Mice were evaluated daily for 28 days postimmunization (dpi), and animals were euthanized if found in distress or moribund.
Four experiments used 10- to 12-week-old female C57BL/6 mice (Jackson Laboratory): (i) VRPRVF dose titration for the minimum effective immunization dose, (ii) vaccine efficacy and immunogenicity of VRPRVF and nr-VRPRVF at 28 dpi, (iii) VRPRVF and nr-VRPRVF efficacy at 1 to 4 dpi, and (iv) systemic immune responses of VRPRVF- and nr-VRPRVF-immunized mice during the first 24 h postimmunization (hpi). In these experiments, mice were immunized subcutaneously (s.c.) with VRPRVF or nr-VRPRVF in doses ranging from 1.0 × 101 to 1.0 × 105 TCID50 prepared in a total volume of 100 μl DMEM. Mock-immunized controls were inoculated with 100 μl DMEM. Mice were challenged s.c. with 1.0 × 105 PFU RVFV in 100 μl DMEM. Animals were evaluated at least once daily for 28 dpi. All animals were euthanized according to a predetermined clinical illness scoring algorithm or if found in acute distress or moribund.
Plasmid construction.
Construction of the full-length RVFL, RVFM, and RVFS plasmids and the RVFM-ΔNSm and RVFS-ΔNSs:GFP plasmids has been described previously (6). The plasmids contain the viral antigenome flanked by the T7 promoter (5′ terminus) and the hepatitis delta virus ribozyme and T7 polymerase terminator motifs (3′ terminus). For this study, the open reading frame (ORF) encoding the RVFV glycoproteins, Gn and Gc, was amplified by PCR (nucleotides [nt] 408 to 3614, as described under GenBank accession number DQ380200) and cloned into the pCAGGS expression plasmid (32) using standard cloning techniques (pC-GnGc). Two silent mutations were introduced into the Gn/Gc ORF used for VRPRVF generation to differentiate this ORF from that of the wild-type virus.
VRPRVF and RVFV production.
VRPRVF, wild-type RVFV, and RVFV-ΔNSm/ΔNSs:GFP viruses were produced using an established three-plasmid RVFV reverse genetics system. As described previously, rescue of recombinant wild-type RVFV ZH501 (RVFV) was accomplished using RVFL, RVFM, and RVFS plasmids (6), and RVFV-ΔNSm/ΔNSs:GFP was rescued using RVFL, RVFM-ΔNSm, and RVFS-ΔNSs:GFP plasmids (5). VRPRVF were rescued similarly, by using RVFL, RVFS-ΔNSm/ΔNSs:GFP, and pC-GnGc plasmids. Briefly, BSR-T7/5 cells were seeded in a 6-well-plate format. Cells were transfected at approximately 75% confluence with 1 μg of each plasmid and LT1 transfection reagent (Mirus) in a ratio of 1 μg of the plasmid to 4 μl of LT1. Rescue of VRPRVF resulted only from cells transfected with all three plasmids. Supernatants were harvested at 4 days posttransfection, subjected to low-speed centrifugation to clear cellular debris, and stored at −80°C. Nonreplicating VRPRVF (nr-VRPRVF) were generated by exposing VRPRVF to gamma irradiation (5 × 106 rads), following a standard CDC protocol for removing antigen preparations from BSL4 containment for diagnostic testing in a BSL2 laboratory. This protocol completely abolishes viral (or VRPRVF) replication while preserving the antigenicity of the sample.
VRPRVF titration and one-step growth curve.
To determine VRPRVF titer and production kinetics, BSR-T7/5 transfection supernatants were harvested from individual wells of a 6-well plate 1, 2, 3, 4, or 5 days posttransfection, clarified by low-speed centrifugation, and frozen at −80°C. VRPRVF titers were determined as TCID50 by using Vero E6 cells. Initial titration of VRPRVF-infected cells was based on enhanced green fluorescent protein (GFP) (eGFP) fluorescence and confirmed by an indirect fluorescent antibody assay (IFA) using an anti-RVF primary antibody.
Total anti-RVFV IgG ELISA.
A total anti-RVFV IgG enzyme-linked immunosorbent assay (ELISA) testing was completed using whole-cell lysates from RVFV-infected Vero E6 cells or uninfected Vero E6 cells at 1:2,000 following standard CDC Viral Special Pathogens Branch diagnostic protocols as described previously (7).
VNT100.
RVFV stock was diluted to 3,000 TCID50 in 50 μl DMEM without FBS. Sera from VRPRVF-, nr-VRPRVF-, and mock-immunized mice were heat inactivated at 56°C for 30 min. In a 96-well plate, 1:10, 1:20, 1:40, 1:80, 1:160, 1:320, and 1:640 serum dilutions were made in 50 μl DMEM. An equal volume of diluted RVFV was added to diluted sera and incubated for 1 h at 37°C. A suspension of approximately 3 × 104 Vero E6 cells was added to each well, and the plates were incubated for 36 h before formalin fixation. Cells were visualized with an IFA using an anti-RVFV primary antibody. Virus neutralization titers (VNT100) were defined as the highest dilution that permitted complete (100%) neutralization of virus input.
Electron microscopy.
VRPRVF and RVFV samples from the supernatants of transfected BSR-T7/5 cells were taken 3 days posttransfection, fixed with 4% paraformaldehyde, and inactivated by gamma irradiation for removal from the containment laboratory. Each sample was incubated overnight on 400 mesh nickel grids at 4°C. Grids were rinsed once and stained with 5% ammonium molybdate and 0.1% trehalose. Specimens were viewed at 120 kV on a Tecnai FEI electron microscope (FEI).
In vivo safety assessment.
A total of 30 2-day-old suckling mice (SM) were inoculated with 1.0 × 104 TCID50 of VRPRVF. Ten SM were inoculated with 1.0 × 104 PFU of RVFV (positive control), and 20 SM were inoculated with 10 μl of DMEM (negative control).
VRPRVF dose titration.
A total of 25 mice were immunized s.c. in groups of 5 with 1.0 × 105, 1.0 × 104, 1.0 × 103, 1.0 × 102, or 1.0 × 101 TCID50 of VRPRVF, and 5 mice were mock immunized with DMEM. Mice were evaluated once daily for clinical signs. At 28 dpi, all mice were challenged with a lethal dose of 1.0 × 105 PFU of RVFV administered s.c.
Efficacy and immunogenicity of VRPRVF and nr-VRPRVF at 28 dpi.
Mice were immunized s.c. in five groups: (i) 1.0 × 105 TCID50 of VRPRVF (n = 10), (ii) 1.0 × 104 TCID50 of VRPRVF (n = 13), (iii) 1.0 × 105 TCID50 of nr-VRPRVF (n = 10), (iv) 1.0 × 104 TCID50 of nr-VRPRVF (n = 13), and (v) sham immunization with DMEM. At 28 dpi, 5 mice from each group were challenged with a lethal dose of 1.0 ×105 PFU of RVFV s.c. and evaluated daily for 28 days. The remaining mice in each group were anesthetized with isoflurane for serum collection for determination of total anti-RVFV IgG and neutralizing antibody titers.
VRPRVF and nr-VRPRVF efficacy at early time points.
A total of 100 mice were immunized s.c. in five groups of 20: (i) 1.0 × 105 TCID50 of VRPRVF, (ii) 1.0 × 104 TCID50 of VRPRVF, (iii) 1.0 × 105 TCID50 of nr-VRPRVF, (iv) 1.0 × 104 TCID50 of nr-VRPRVF, and (v) mock immunization with DMEM. On each of days 1, 2, 3, and 4, a subset of 5 mice from each group (25 total) were challenged s.c. with 1.0 × 105 PFU of virulent RVFV and evaluated twice daily for clinical signs of disease.
Comparison of early immune response of VRPRVF and nr-VRPRVF immunized mice.
A total of 24 mice were immunized s.c. in three groups of 8: (i) 1.0 × 104 TCID50 of VRPRVF, (ii) 1.0 × 104 TCID50 of nr-VRPRVF, or (iii) mock immunization with 100 μl DMEM. At 12 and 24 hpi, 4 mice from each group were anesthetized with isoflurane and perfused with phosphate-buffered saline (PBS). Samples of approximately 100 μg from the perfused liver and brain were placed in RNAlater (Ambion, Inc.) and frozen at −80°C until used for RNA extraction.
RNA extraction.
To extract mRNA from tissues, tissue samples were removed from RNAlater and placed in 1 ml of Tripure isolation reagent (Roche Applied Science). RNA was extracted using a Qiagen RNeasy minikit per the manufacturer's instructions, including the RNase-free DNase step (Qiagen).
Antiviral assays.
Antiviral response quantitative PCR arrays (SABiosciences) were used to determine up- or downregulation of a select panel of 84 antiviral genes in mice immunized with VRPRVF or nr-VRPRVF, relative to mock-immunized mice. Assays were run for liver and brain samples from 3 VRPRVF-immunized mice, 3 nr-VRPRVF-immunized mice, and 3 mock-immunized mice at each time point. For each sample, cDNA was synthesized from 0.8 to 1.0 μg of RNA using an RT2 first-strand kit (SABioscience). Arrays were run on an ABI 7500 PCR system using RT2 SYBR green/ROX PCR master mix according to the manufacturer's instructions (SABioscience).
Statistical analyses.
For efficacy experiments, the Gehan-Breslow-Wilcoxon test was used to determine whether survival curves were significantly different (GraphPad Prism; GraphPad Software, Inc.). Student's t test was used to determine statistical significance of VNT100 and IgG titers.
In the antiviral array analysis, the mean value for each gene was calculated using each set of replicate tissue samples using the threshold cycle (ΔΔCT) method and normalized to the average values for five housekeeping genes (the Gus-β, Hprt, HSP-90AB1, glyceraldehyde-3-phosphate dehydrogenase [GAPDH], and β-actin genes). The P values were calculated based on Student's t test of the replicate 2−ΔCT values (SABioscience) for each gene in the VRPRVF and nr-VRPRVF groups.
RESULTS
VRPRVF high-yield production and growth kinetics.
Efficient VRPRVF production was accomplished with an established RVFV reverse genetics system and simultaneous transfection of three plasmids: RVFL (wild-type L segment), RVFS-ΔNSs:GFP (S segment with GFP replacing NSs), and pC-GnGc (expression vector carrying glycoprotein genes) (Fig. 1A). VRPRVF were produced from cells transfected with all three plasmids and rescued directly from BSR-T7/5 transfection media. Total VRPRVF production increased from 1 day through 4 days after transfection and declined at 5 days (data not shown). In several independent experimental replicates, VRPRVF were rescued with 100% efficiency with titers ranging from 1.0 × 106 to 5.0 × 107 TCID50/ml (average titer, 1.2 × 107 TCID50/ml). Recombinant wild-type RVFV ZH501 and RVFV-ΔNSm/ΔNSs:GFP, a GFP-expressing virus with full-gene deletions of NSs and NSm, were also successfully rescued as described previously (6).
Fig 1.
Reverse genetics-derived VRPRVF are morphologically indistinguishable from wild-type RVFV. (A) Schematic of the Rift Valley fever virus (RVFV) genome (top) and the reverse genetics system as used for virus replicon particle (VRPRVF) production (bottom). vc, viral complementary. (B) Negative-stain electron microscopy images demonstrating the morphological similarity of RVFV particles (left) and VRPRVF particles (right).
VRPRVF are morphologically indistinguishable from RVFV.
To compare the morphology of replicon particles to virus particles, VRPRVF and RVFV particles were harvested from BSR-T7/5 cells at 3 days after transfection for electron microscopy analysis. VRPRVF and classic RVFV particles ranged in size from 80 to 100 nm, were round to slightly pleiomorphic, and were indistinguishable from one another (Fig. 1B).
VRPRVF do not spread beyond initially infected cells.
To illustrate that VRPRVF undergo only one round of infection, Vero E6 cell monolayers were infected with VRPRVF and RVFV-ΔNSm/ΔNSs:GFP and monitored daily for 5 days. While RVFV spreads rapidly throughout the cell monolayer, the VRPRVF do not spread beyond the initially infected cells (Fig. 2A).
Fig 2.
VRPRVF cannot spread beyond initially infected cells and pass a stringent safety test. (A) VRPRVF (top) and RVFV (bottom) were used to infect Vero E6 monolayers. Over the course of 5 days (left to right), VRPRVF remain confined to the initially infected cells, while RVFV gradually spreads throughout the monolayer. Limit of detection, 1 fluorescent focus unit (FFU). (B) Survival curves of newborn suckling mice inoculated intracranially with VRPRVF or RVFV or mock inoculated.
VRPRVF is completely safe in suckling mouse infections.
In a stringent vaccine safety test, newborn (2-day-old) suckling mice were inoculated i.c. with 1.0 × 104 TCID50 of VRPRVF, 1.0 × 104 PFU of RVFV, or 10 μl of DMEM. All RVFV-inoculated mice died 2 days after infection. All mice inoculated with VRPRVF or DMEM survived with no indication of clinical signs (Fig. 2B).
Single-dose immunization with VRPRVF is completely protective from virulent virus challenge.
A dose titration study was conducted to determine the minimum VRPRVF immunization that confers protection from virulent virus challenge. Mice were immunized s.c. in groups of 5 with 10, 1.0 × 102, 1.0 × 103, 1.0 × 104, or 1.0 × 105 TCID50 of VRPRVF, or mock immunized with DMEM, and challenged s.c. with 1.0 × 105 PFU of RVFV. Previous experiments indicated the 50% lethal dose (LD50) of RVFV ZH501 in adult C57BL/6 mice is less than 1 PFU (data not shown); therefore, the virus challenge for all experiments in this study was 100,000-fold higher than the LD50. Results showed a dose-dependent effect on survival. A single-dose immunization with 1.0 × 105 or 1.0 × 104 TCID50 of VRPRVF conferred 100% protection against virus challenge, whereas 1.0 × 103 TCID50 of VRPRVF protected 60% of mice. Although there were no survivors in groups given lower VRPRVF doses, mortality was delayed in mice receiving 1.0 × 102 TCID50 (Fig. 3A).
Fig 3.
Single-dose immunization of replicating VRPRVF confers complete protection. (A) Survival curves of C57BL/6 mice in the VRPRVF dose titration experiment. Mice were immunized with VRPRVF and challenged 28 days later with virulent RVFV. (B) Survival curves of mice immunized with VRPRVF or nr-VRPRVF and challenged 28 days later with virulent RVFV. Survival curves are significantly different (P = 0.005). (C) Total IgG titers of mice immunized with VRPRVF or nr-VRPRVF 28 days prior to serum collection. Limit of detection, 1:100. (D) Virus neutralization titers of mice immunized with VRPRVF or nr-VRPRVF 28 days prior to serum collection. Limit of detection, 1:10.
Active replication of VRPRVF is important for complete protection.
To evaluate the relative importance of VRPRVF replication for vaccine efficacy, mice were immunized with replicating VRPRVF or nonreplicating VRPRVF (nr-VRPRVF) at doses of 1.0 × 105 or 1.0 × 104 TCID50, or mock immunized with DMEM, and were challenged with 1.0 × 105 PFU of virulent RVFV. As seen in the earlier experiment, all mice immunized with a single dose of 1.0 × 105 or 1.0 × 104 TCID50 of VRPRVF survived the lethal virus challenge with no clinical signs. In contrast, only 60% or 20% of the mice immunized with 1.0 × 105 or 1.0 × 104 TCID50 of nr-VRPRVF, respectively, survived the challenge (Fig. 3B).
Replicating VRPRVF induce the IgG response by 28 dpi.
The IgG antibody responses of mice immunized with 1.0 × 105 TCID50 of VRPRVF, 1.0 × 105 TCID50 of nr-VRPRVF, or DMEM were evaluated at 28 dpi. Mice immunized with VRPRVF had total anti-RVF IgG titers ranging from 1,600 (1 of 5 mice) to 6,400 (4 of 5 mice). None of the mice immunized with nr-VRPRVF had detectable IgG response (limit of detection, 1:100) (Fig. 3C).
VRPRVF induce a significantly stronger neutralizing antibody response than nr-VRPRVF.
Also at 28 dpi, the neutralizing antibody response of mice immunized with 1.0 × 104 TCID50 of VRPRVF or nr-VRPRVF was assessed. Seven of the 8 VRPRVF-immunized mice had detectable levels of neutralizing antibodies, compared with 2 of the 8 nr-VRPRVF-immunized mice. VNT100 were significantly higher in VRPRVF-immunized mice than those in nr-VRPRVF-immunized mice (P < 0.05) (Fig. 3D).
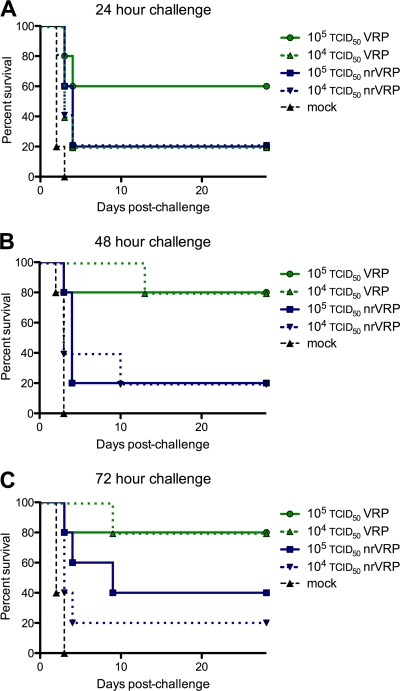
Single-dose VRPRVF immunization provides protection from virulent virus challenge by 24 hpi.
To determine vaccine efficacy at early times postimmunization, mice were immunized with a single dose of 1.0 × 105 or 1.0 × 104 TCID50 of VRPRVF, or 1.0 × 105 or 1.0 × 104 TCID50 of nr-VRPRVF, and challenged with virulent RVFV 1, 2, 3, or 4 dpi. When challenged 24 h after immunization, 60% of the higher-dose VRPRVF-immunized mice survived, and 80% survived the challenge administered on days 2 and 3, irrespective of dose (Fig. 4A to C). All mice immunized with VRPRVF survived the challenge given 4 dpi (data not shown). Mice immunized with nr-VRPRVF displayed lower levels of protection; regardless of the immunization dose, 20% of mice challenged 1 or 2 dpi survived. The highest survivorship of nr-VRP-RVF-immunized mice observed was 40% at 3 dpi (Fig. 4A to C).
Fig 4.
VRPRVF immunization is protective as early as 24 h postimmunization. Survival curves from early protection studies. C57BL/6 mice were immunized with VRPRVF or nr-VRPRVF and challenged with virulent virus (A) 24 h, (B) 48 h, or (C) 72 h later. Survival curves were significantly different at 48 h (P = 0.008) and 72 h (P = 0.025).
VRPRVF-immunized mice show significant upregulation in antiviral gene expression.
Antiviral gene expression levels, including that of IFN-β, was quantified for VRPRVF- and nr-VRPRVF-immunized mice relative to those for mock-immunized mice at 12 and 24 hpi. Genes significantly upregulated are shown in Table 1 (*, P < 0.05; **, P < 0.001). The only gene significantly downregulated relative to mock-immunized mice was the cFOS gene, in the livers of VRPRVF-immunized mice at 12 hpi and in the brains of both VRPRVF- and nr-VRPRVF-immunized mice at 24 hpi. Several genes were significantly upregulated only in VRPRVF-immunized mice, including those encoding CD40, CCL3, CCL5, CXCL9, CXCL10, RIG-I, IFN regulatory factor 3 (IRF3), JUN, MX1, STAT1, TLR9, tumor necrosis factor (TNF), and TNR receptor-associated death domain (TRADD). Both VRPRVF- and nr-VRPRVF-immunized mice had significant upregulation of IRF7, IFN-stimulated gene 15 (ISG15), and LGP2 relative to levels for mock-immunized mice. Significant increases in Nlrp3 and CARD9 were apparent in VRPRVF-immunized mice 12 h earlier than in nr-VRPRVF-immunized mice. All genes upregulated in nr-VRPRVF- relative to mock-immunized mice were also upregulated in VRPRVF-immunized mice.
Table 1.
Fold change in expression levels of selected antiviral genes in VRPRVF-immunized or nr-VRPRVF-immunized mice relative to levels for mock-immunized controlsa
| Gene | Fold change in expression level for indicated sample |
|||||||
|---|---|---|---|---|---|---|---|---|
| Liver, 12 h |
Liver, 24 h |
Brain, 12 h |
Brain, 24 h |
|||||
| VRPRVF | nr-VRPRVF | VRPRVF | nr-VRPRVF | VRPRVF | nr-VRPRVF | VRPRVF | nr-VRPRVF | |
| CARD9 | 0.8 | 0.7 | 1.2 | 1.8* | 2.2** | 1.5 | 1.0 | 0.8 |
| CD40 | 2.2* | 1.6 | 1.5 | 1.0 | 1.2 | 0.9 | 1.0 | 1.1 |
| CCL3 | 0.9 | 1.0 | 3.0* | 1.0 | 1.2 | 1.0 | 1.9 | 0.5 |
| CCL5 | 1.2 | 1.4 | 2.0** | 1.6 | 1.0 | 0.3 | 0.7 | 1.0 |
| CXCL9 | 1.0 | 0.8 | 4.5* | 0.8 | 2.6* | 3.2 | 2.4 | 0.3 |
| CXCL10 | 3.7* | 1.8 | 3.7* | 1.9 | 1.8 | 1.7 | 3.5 | 1.0 |
| cFOS | 0.09* | 0.5 | 0.4 | 0.6 | 1.5* | 1.3 | 0.4* | 0.3* |
| IRF3 | 0.9 | 0.9 | 0.9 | 0.6 | 2.6** | 1.6 | 0.4 | 0.7 |
| IRF7 | 5.0** | 2.1** | 11.2 | 3.6** | 3.2 | 2.2 | 6.0 | 1.3 |
| ISG15 | 16.1* | 3.3* | 14.1* | 2.9* | 1.2 | 0.9 | 6.3 | 0.6 |
| JUN | 0.4 | 0.7 | 0.4 | 1.0 | 2.6* | 1.5 | 0.6 | 0.4 |
| LPG2 | 5.1** | 2.0** | 4.4** | 1.9* | 2.7 | 2.3 | 2.3 | 1.0 |
| MX1 | 1.7 | 0.4 | 7.7** | 2.1 | 0.6 | 0.6 | 1.9* | 0.8 |
| NLRP3 | 0.8 | 0.8 | 1.6 | 2.0 | 2.2* | 1.2 | 0.4 | 0.7 |
| RIG-I | 2.0** | 1.0 | 1.7 | 0.8 | 0.7 | 0.9 | 2.4 | 1.0 |
| STAT1 | 2.7** | 1.3 | 2.6* | 1.2 | 0.7 | 0.8 | 4.0 | 0.8 |
| TLR9 | 3.1 | 1.4 | 1.5 | 0.8 | 2.9* | 1.3 | 0.7 | 0.8 |
| TNF | 2.7* | 1.2 | 4.0 | 1.8 | NA | NA | 2.0 | 0.9 |
| TRADD | 1.1 | 1.0 | 1.0 | 1.3 | 1.6** | 1.2 | 0.8 | 0.9 |
Asterisks indicate significant fold change (*, P < 0.05; **, P < 0.01). NA, not available.
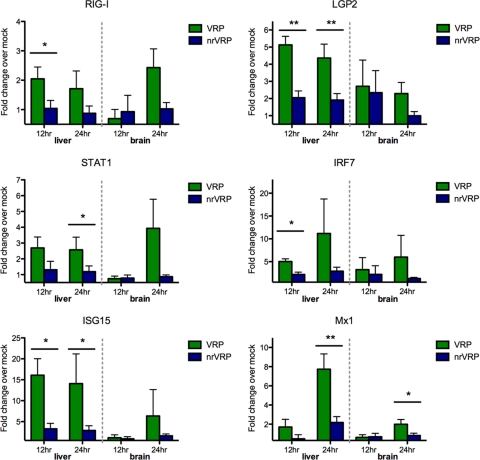
Several genes were expressed at significantly higher levels in VRPRVF-immunized mice than in nr-VRPRVF-immunized mice. In the liver, RIG-I, LGP2, IRF7, and ISG15 expression was elevated in VRPRVF-immunized mice by 12 hpi, and STAT1 and MX1 expression was significantly higher by 24 hpi (Fig. 5). Chemokines CCL3, CCL4, and CXCL9 were significantly elevated in the livers of VRPRVF-immunized mice by 24 hpi (Fig. 5). Also by 24 hpi, MDA5 and CXCL10 were upregulated in the brains of VRPRVF- relative to nr-VRPRVF-immunized mice (Fig. 5 and 6).
Fig 5.
VRPRVF stimulate significantly higher antiviral gene expression levels than those of nonreplicating counterparts. Fold change of relevant antiviral cytokine gene expression levels (over levels for mock-immunized mice) at 12 and 24 h postimmunization with VRPRVF (green) or nr-VRPRVF (blue). Error bars show standard deviations of the fold change. Asterisks indicate significant differences between VRPRVF and nr-VRPRVF results (*, P < 0.05; **, P < 0.01).
Fig 6.
VRPRVF stimulate significantly higher chemokine gene expression than nonreplicating counterparts. Fold change of relevant chemokine gene expression levels (over levels for mock-immunized mice) at 12 and 24 h postimmunization with VRPRVF (green) or nr-VRPRVF (blue). Error bars show standard deviations of the fold change. Asterisks indicates significant differences between VRPRVF and nr-VRPRVF results (*, P < 0.05).
DISCUSSION
Large outbreaks of RVFV can have a devastating impact on human and animal health; however, there are currently no approved vaccines for use outside the areas of endemicity in Africa. In these areas, the widespread use of available livestock vaccines has been limited due to safety concerns or poor immunogenicity. Early live attenuated constructs (i.e., Smithburn and MP12) were associated with abortion or teratogenesis in pregnant animals (8, 17). Inactivated VLP-like vaccines are much safer but require the use of adjuvant or multiple boosters for complete protection. Recently, our laboratory described a rationally designed, reverse genetics-derived vaccine candidate that is safe and efficacious in livestock (7). As an additional countermeasure against RVFV, we paired the robust efficacy of this vaccine with the enhanced safety inherent in nonreplicating constructs. The resulting VRPRVF undergo only one round of infection and are biologically confined to the initially infected cells, but they can actively synthesize viral RNA and express viral nucleoprotein and polymerase.
VRPRVF particles are morphologically indistinguishable from wild-type virus but lack four genes, those encoding virulence factors NSs and NSm and structural proteins Gn and Gc. Deletions of NSm (6) and NSs (38) have been shown to reduce virulence of RVFV in adult rodents. The NSs protein inhibits the host immune response to RVFV infection through multiple mechanisms (3, 16, 18, 25, 26); therefore, its absence or mutation is a common feature of many RVF vaccine candidates (5, 13). Additional full-length deletions of genes encoding the structural proteins Gn and Gc confine VRPRVF replication to the initially infected cells. The resulting inability to spread within the host dramatically reduces the chance of vaccine-induced pathogenicity and likely explains the safety of VRPRVF infections in suckling mice, particularly given the rapid and uniform mortality seen with intracranial inoculation of suckling mice with RVF viruses.
Although extremely attenuated, VRPRVF, like RVFV, contain the polymerase and nucleoprotein, the two factors required for viral replication, allowing for viral RNA expression and de novo viral protein synthesis in the target cells. Intracellular replication of single-stranded RNA viruses (including members of the Bunyaviridae) initiates a strong innate immune response via Toll-like receptors and/or the cytoplasmic RIG-I-like helicases, culminating in the production of important antiviral proteins, including IFN (15, 21). In wild-type RVFV infection, the NSs protein abolishes these host responses. However, immunization with replicating VRPRVF lacking the NSs should allow for unobstructed production of IFN and ISGs, thus preserving critical aspects of the antiviral response. Indeed, in multiple experiments, we demonstrated a significantly stronger immune response and associated protective efficacy in VRPRVF-immunized mice relative to those in nr-VRPRVF- and mock-immunized mice.
Mice immunized with VRPRVF produced significantly higher levels of total IgG and neutralizing antibodies than those in nr-VRPRVF-immunized mice and were completely protected from the virulent virus challenge at 28 dpi, suggesting that replication is critical for robust immunity and subsequent protection. As early as 12 hpi, clear differences in host response were already apparent between the mice immunized with VRPRVF and those immunized with nr-VRPRVF. Relative to both nr-VRPRVF- and mock-immunized mice, VRPRVF immunization resulted in significant systemic induction of IFN-inducible genes, including those encoding STAT1, IRF7, ISG15, RIG-I, LPG2, and MDA5. These genes stimulate the expression of important players in the cellular defense against viruses, including PKR, OAS, IRFs, MX1, and major histocompatibility complex (MHC) classes I and II (20). Activation of ISGs, particularly MHC, provides a mechanism for the improved antibody response and protection seen after immunization with replicating VRPRVF. Additionally, induction of very early cell-mediated and subsequent adaptive immune responses in VRPRVF-immunized mice was evident from the significant upregulation of CCL4 (MIP-1β) and CXCL9 (MIG) expression in the liver and CCL3 (MIP-1α) and CXCL10 (IP-10) expression in the liver and brain. These chemokines play important roles in attracting various immune cells, including monocytes/macrophages, NK cells, and T cells, and in mediating T cell activation, aiding in initiation of cell-mediated and humoral adaptive immunity.
The rapid onset of a systemic antiviral response suggested that VRPRVF immunization could confer early protection. VRPRVF were found to be highly efficacious against virulent virus challenge within days of immunization; a single dose of VRPRVF provided 60% protection by just 1 dpi and complete protection by 4 dpi. This early efficacy suggests that VRPRVF could be a valuable control measure in the field. If RVFV was introduced into an area with large naïve populations, immunization with VRPRVF early in the outbreak could prevent rapid viral spread throughout and beyond the affected region. Furthermore, the low genetic diversity and single serotype of the virus suggests that a VRPRVF vaccine would likely be broadly protective against all known strains of RVFV.
The efficacy of VRPRVF immunization against a virulent virus challenge 100,000-fold higher than the LD50 at early and late time points was remarkable. This protection likely hinges on the ability of the VRPRVF, administered subcutaneously and in a single dose, to elicit a robust immune response in distant tissues within hours of immunization. This systemic response to VRPRVF inoculation is clearly illustrated by the upregulation of antiviral genes in the liver and brain after vaccination. To explain the host-wide effect of localized VRPRVF immunization, we hypothesize that immunization results in VRPRVF infection of resident macrophages or dendritic cells in the skin. Recent work has demonstrated that macrophages and dendritic cells are permissive to replication and are important targets of RVFV infection (28, 30, 34). Given the absence of the NSs protein in the VRPRVF construct, active replication within these cell types should stimulate a strong IFN response, as shown in vitro (30), leading to a systemic antiviral response. At the time points tested in these experiments, we did not detect upregulation of the tested IFN subtypes. However, IFN must clearly have been produced within the host to induce the downstream expression of ISGs that were detected in the liver and brain. The bulk of IFN synthesis may occur at the site of immunization in locally infected macrophages or dendritic cells and then be dispersed systemically. Alternatively, IFN induction might be detectable in the liver and brain only at earlier time points or as subtypes not evaluated here. RVFV is highly sensitive to IFN, and the rapid onset of a strong IFN response associated with VRPRVF immunization provides a plausible explanation for early protection against challenge.
Replication-competent particles are a safe vaccine approach, much like inactivated or VLP-like constructs, yet they stimulate a stronger immune response and therefore provide higher levels of protection from virulent challenge with just a single immunization. Virus-specific VRP vaccine candidates have been described for other diseases, including classical swine fever virus (35) and Venezuelan equine encephalitis virus (22), as well as several vaccines using an alphavirus VRP backbone (1, 12, 19). A similar report of the ability of RVF replicon particles to protect against virulent challenge was very recently published (23). Here, we show that VRPRVF immunization rapidly and systemically initiates a strong cytokine and chemokine response with the resulting protection seen as early as 24 h postimmunization. Further, we demonstrate that the active replication that defines VRPRVF, and distinguishes these particles from classical VLP, is critical for strong innate and adaptive immune responses and, subsequently, complete protection from challenge. Identification of initially infected cells at the site of immunization (presumably resident macrophage and/or dendritic cells of the skin) and determination of their role in the early innate response is ongoing. Further evaluation of VRPRVF efficacy in livestock and nonhuman primates is a critical next step in proving the utility of this method, with the ultimate goal of developing a product for human use.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention.
We thank Christina Spiropoulou and Anita McElroy for critical readings of the manuscript and Tatyana Klimova for thoughtful comments and assistance in editing the manuscript. We also thank Shelley Campbell for assistance with the ELISA. K.A.D. thanks N. J. MacLachlan and P. Pesavento of the University of California, Davis, for their enthusiastic mentorship.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Barnett SW, et al. 2010. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 84:5975–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhardwaj N, Heise MT, Ross TM. 2010. Vaccination with DNA plasmids expressing Gn coupled to C3d or alphavirus replicons expressing gn protects mice against Rift Valley fever virus. PLoS Negl. Trop. Dis. 4:e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billecocq A, et al. 2004. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 78:9798–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bird B, Ksiazek T, Nichol S. 2009. Rift Valley fever virus. J. Am. Vet. Med. Assoc. 234:883–893 [DOI] [PubMed] [Google Scholar]
- 5. Bird BH, et al. 2008. Rift Valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 82:2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bird BH, Albariño CG, Nichol ST. 2007. Rift Valley fever virus lacking NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology 362:10–15 [DOI] [PubMed] [Google Scholar]
- 7. Bird BH, et al. 2011. Rift Valley fever virus vaccine lacking the NSs and NSm genes is safe, non-teratogenic, and confers protection from viremia, pyrexia, and abortion following lethal challenge in adult and pregnant sheep. J. Virol. 85:12901–12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Botros B, et al. 2006. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J. Med. Virol. 78:787–791 [DOI] [PubMed] [Google Scholar]
- 9. Bouloy M, et al. 2001. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J. Virol. 75:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chevalier V, Pépin M, Plée L, Lancelot R. 2010. Rift Valley fever—a threat for Europe? Euro Surveill. 15:19506. [PubMed] [Google Scholar]
- 11. de Boer SM, et al. 2010. Rift Valley fever virus subunit vaccines confer complete protection against a lethal virus challenge. Vaccine 28:2330–2339 [DOI] [PubMed] [Google Scholar]
- 12. Defang GN, Khetawat D, Broder CC, Quinnan GV. 2010. Induction of neutralizing antibodies to Hendra and Nipah glycoproteins using a Venezuelan equine encephalitis virus in vivo expression system. Vaccine 29:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dungu B, et al. 2010. Evaluation of the efficacy and safety of the Rift Valley fever Clone 13 vaccine in sheep. Vaccine 28:4581–4587 [DOI] [PubMed] [Google Scholar]
- 14. Elliott RM. 2009. Bunyaviruses and climate change. Clin. Microbiol. Infect. 15:510–517 [DOI] [PubMed] [Google Scholar]
- 15. Elliott RM, Weber F. 2009. Bunyaviruses and the type I interferon system. Viruses 1:1003–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Habjan M, et al. 2009. NSs protein of Rift Valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 83:4365–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunter P, Erasmus BJ, Vorster JH. 2002. Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J. Vet. Res. 69:95–98 [PubMed] [Google Scholar]
- 18. Ikegami T, et al. 2009. Dual functions of Rift Valley fever virus NSs protein: inhibition of host mRNA transcription and post-transcriptional downregulation of protein kinase PKR. Ann. N. Y. Acad. Sci. 1171(Suppl. 1):E75–E85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston RE, et al. 2005. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine 23:4969–4979 [DOI] [PubMed] [Google Scholar]
- 20. Katze MG, He Y, Gale M. 2002. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2:675–687 [DOI] [PubMed] [Google Scholar]
- 21. Kawai T, Akira S. 2008. Toll-like Receptor and RIG-1-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1–20 [DOI] [PubMed] [Google Scholar]
- 22. Konopka JL, Thompson JM, Whitmore AC, Webb DL, Johnston RE. 2009. Acute infection with Venezuelan equine encephalitis virus replicon particles catalyzes a systemic antiviral state and protects from lethal virus challenge. J. Virol. 83:12432–12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kortekaas J, et al. 2011. Creation of a non-spreading Rift Valley fever virus. J. Virol. 85:12622–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lagerqvist N, et al. 2009. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley fever virus cDNA constructs. Virol. J. 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le May N, et al. 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116:541–550 [DOI] [PubMed] [Google Scholar]
- 26. Le May N, et al. 2008. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lorenzo G, Martín-Folgar R, Hevia E, Boshra H, Brun A. 2010. Protection against lethal Rift Valley fever virus (RVFV) infection in transgenic IFNAR−/− mice induced by different DNA vaccination regimens. Vaccine 28:2937–2944 [DOI] [PubMed] [Google Scholar]
- 28. Lozach P-Y, et al. 2011. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 10:75–88 [DOI] [PubMed] [Google Scholar]
- 29. Mandell RB, et al. 2010. A replication-incompetent Rift Valley fever vaccine: chimeric virus-like particles protect mice and rats against lethal challenge. Virology 397:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McElroy AK, Nichol ST. 2012. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 422:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Näslund J, et al. 2009. Vaccination with virus-like particles protects mice from lethal infection of Rift Valley fever virus. Virology 385:409–415 [DOI] [PubMed] [Google Scholar]
- 31a. National Research Council 2011. Guide to the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC [Google Scholar]
- 32. Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 [DOI] [PubMed] [Google Scholar]
- 33. Schmaljohn CS, Nichol ST. 2007. Bunyaviridae, p 1741–1789 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 34. Smith DR, et al. 2010. The pathogenesis of Rift Valley fever virus in the mouse model. Virology 407:256–267 [DOI] [PubMed] [Google Scholar]
- 35. Suter R, et al. 2011. Immunogenic and replicative properties of classical swine fever virus replicon particles modified to induce IFN-α/β and carry foreign genes. Vaccine 29:1491–1503 [DOI] [PubMed] [Google Scholar]
- 36. Swanepoel R, Coetzer JAW. 1994. Rift Valley fever, p 688–717 In Coetzer JAW, Thomson GR, Tustin RC. (ed), Infectious diseases of livestock with special reference to South Africa. Oxford University Press, Cape Town, South Africa [Google Scholar]
- 37. Turell MJ, Wilson WC, Bennett KE. 2010. Potential for North American mosquitoes (Diptera: Culicidae) to transmit Rift Valley fever virus. J. Med. Entomol. 47:884–889 [DOI] [PubMed] [Google Scholar]
- 38. Vialat P, Billecocq A, Kohl A, Bouloy M. 2000. The S segment of Rift Valley fever phlebovirus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J. Virol. 74:1538–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Teichman B, et al. 2011. Safety and efficacy of Rift Valley fever Smithburn and Clone 13 vaccines in calves. Vaccine 29:5771–5777 [DOI] [PubMed] [Google Scholar]
- 40. Won S, Ikegami T, Peters CJ, Makino S. 2007. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J. Virol. 81:13335–13345 [DOI] [PMC free article] [PubMed] [Google Scholar]