Abstract
Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) primarily infect activated CD4+ T cells but can infect macrophages. Surprisingly, ex vivo tetramer-sorted SIV-specific CD8+ T cells that eliminated and suppressed viral replication in SIV-infected CD4+ T cells failed to do so in SIV-infected macrophages. It is possible, therefore, that while AIDS virus-infected macrophages constitute only a small percentage of all virus-infected cells, they may be relatively resistant to CD8+ T cell-mediated lysis and continue to produce virus over long periods of time.
TEXT
In vivo infection of macrophages is a typical characteristic of lentiviral infections. Neurological complications, such as encephalitis, granulomatous interstitial pneumonia, and progressive dementia, are often associated with progression to AIDS during late-stage human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infection (32). Infected macrophages in the brain appear to be one of several factors that cause these AIDS-associated neuropathies (8, 22). Furthermore, perivascular macrophages are the primary infected cell type in the brains of SIVmac239-infected rhesus macaques (47).
Even though HIV type 1 (HIV-1) and SIVmac239 preferentially infect activated CD4+ T cells (9, 10), several studies have observed infected macrophages in HIV-1-infected patients and SIVmac239-, SIVmac251-, and SIV DeltaB670-infected rhesus macaques (12, 15, 18, 19, 30, 31, 35, 39, 44, 51). Macrophages express the CD4 cell surface receptor, rendering them a potential target for these viruses (7, 15). Using in situ hybridization, infected macrophages were observed in 10 of 21 lymph node biopsy specimens from the acute symptomatic stage and throughout the first year of infection in HIV-1-infected patients (39). Infected macrophages comprised approximately 7% of the entire HIV-1-infected cell population in 10 lymph node samples containing HIV-1-infected macrophages (39). Additionally, approximately 10% of the infected-cell population in endocervix and lymph node samples of acute SIVmac251-infected rhesus macaques expressed macrophage-specific lineage markers (51). Furthermore, HIV-1-, SIVmac239-, SIVmac251-, and SHIVDH12R-infected macrophages were observed as early as 21 days postinfection and persisted for long periods of time (2, 11, 18, 19, 44, 47, 48). Additionally, SHIVDH12R infection of rhesus macaques results in massive and irreversible depletion of CD4+ T cells; however, high viral loads persist in several tissue compartments (18, 19). In this model, macrophages were found to be the principal reservoir for SHIV and responsible for the high viral loads observed. Finally, macrophages are a persistent latent reservoir for HIV-1 (42). Taken together, these studies suggest that macrophages play an important role in maintaining and enhancing HIV/SIV infection in vivo.
Because of the relatively small percentage of infected macrophages, the interaction between antigen-specific CD8+ T cells and infected macrophages in HIV/SIV infection has been poorly studied. We, therefore, sought to determine whether SIV-specific CD8+ T cells could control viral replication in infected macrophages.
Ex vivo tetramer-sorted SIV-specific CD8+ T cells suppressed viral replication in SIV-infected CD4+ T cells.
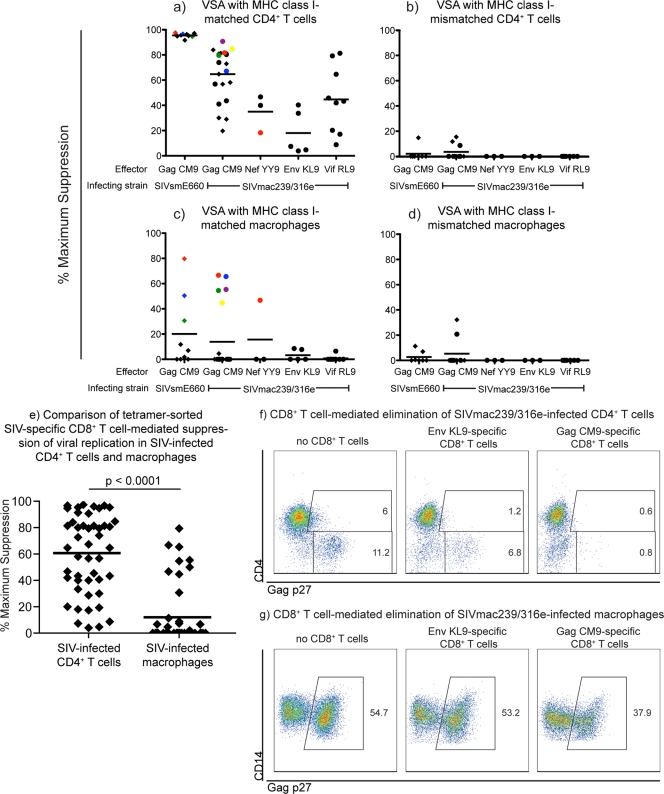
HIV/SIV-specific CD8+ T cells have been shown to suppress viral replication in HIV/SIV-infected CD4+ T cells (26, 27, 36, 43, 45, 49, 50). We confirmed that ex vivo tetramer-sorted SIV-specific CD8+ T cells could reduce viral replication in SIV-infected CD4+ T cells in vitro. Ex vivo tetramer-sorted SIV-specific CD8+ T cells (Table 1) from several progressor and elite controller (EC) animals (Table 2) were incubated with activated SIVmac239/316e- or SIVsmE660-infected CD4+ T cells in viral suppression assays (45). Ex vivo tetramer-sorted SIV-specific CD8+ T cells suppressed viral replication in SIV-infected major histocompatibility complex (MHC) class I-matched CD4+ T cells (Fig. 1a). This suppression was MHC class I dependent because the same ex vivo tetramer-sorted SIV-specific CD8+ T cells did not suppress viral replication in MHC class I-mismatched SIV-infected CD4+ T cell targets (Fig. 1b). Additionally, ex vivo tetramer-sorted SIV-specific CD8+ T cells effectively eliminated SIV-infected CD4+ T cells (Fig. 1f).
Table 1.
Ex vivo SIV-specific CD8+ T cells used in the 48-h virus suppression assay
| Epitope | Protein | Amino acid positions | Sequence | MHC restriction | IC50a (nM) |
|---|---|---|---|---|---|
| CM9 | Gag p27 capsid | 181–189 | CTPYDINQM | A*01 | 22 |
| YY9 | Nef | 159–167 | YTSGPGIRY | A*02 | 2.7 |
| KL9 | Env gp41 | 573–581 | KRQQELLRL | B*08 | 12 |
| RL9 | Vif | 123–131 | RRAIRGEQL | B*08 | 7.5 |
IC50, 50% inhibitory concentration.
Table 2.
MHC class I genotypes and SIV infection details for rhesus macaques used in this study
| Animal | Sexa | MHC class I genotype | Vaccine | Infection strain | Wk 52 chronic-phase viral load (viral RNA copies/ml) |
|---|---|---|---|---|---|
| r95061 | F | A*01, A*02, B*17, B*29 | HBcAg/MVA | nef open/239 | 30 |
| r96141 | F | A*01, A*11, B*06, B*22, B*30 | None | SIVmac239-b08-8x | 30 |
| r98016 | M | A*02, A*07, B*06, B*08, B*17, B*29 | None | SIVmac239 | 30.4 |
| r01056 | M | A*01, B*17, B*29, B*52, B*55, B*5802 | BCG; rYF-17D/SIVGag45–269 | SIVsmE660 | 1.94 × 106 |
| r03130 | M | A*01, B*29, B*46, B*47 | rYF-17D/SIVGag45–269 | SIVsmE660 | 2.14 × 104 |
| r03047 | F | A*08, B*06, B*08, B*30, B*46 | SIVmac239 Delta nef | SIVmac239 | 30 |
| r04091 | M | A*01, A*08, B*22, B*30, B*46 | rYF-17D/SIVGag45–269 | SIVsmE660 | 2.70 × 105 |
M, male; F, female.
Fig 1.
Ex vivo tetramer-sorted SIV-specific CD8+ T cells suppressed viral replication in SIV-infected CD4+ T cells but were ineffective at suppressing viral replication in SIV-infected macrophages. We calculated the maximum percentage of viral suppression for each ex vivo tetramer-sorted SIV-specific CD8+ T cell population using the number of viral RNA (vRNA) copies per milliliter of culture supernatant at 48 h with and without effector cells: (vRNA copies/ml without CD8+ T cells − vRNA copies/ml with CD8+ T cells)/vRNA copies/ml without CD8+ T cells × 100. We used only tetramer-sorted SIV-specific CD8+ T cells that were greater than 50% specific as measured by postsort tetramer stains. The purity of the tetramer-sorted SIV-specific CD8+ T cells did not correlate with their ability to suppress viral replication in SIV-infected CD4+ T cells or macrophages. Experiments in panels a and c as well as panels b and d were directly matched: targets were derived from the same animals on the same day, infected simultaneously the same way 4 days after harvesting, and incubated with the same tetramer-sorted effectors for 48 h. We used nonautologous targets because effector cells were harvested from SIV-infected animals and using autologous targets would not allow for an MHC class I-mismatch control. (a) Ex vivo tetramer-sorted SIV-specific CD8+ T cells effectively suppressed viral replication in SIVmac239-, SIVmac239/316e-, and SIVsmE660-infected MHC class I-matched CD4+ T cell targets at an effector-to-target ratio of 1:1 after 48 h of coincubation. (b) Percent maximum suppression of SIV-specific CD8+ T cells incubated with MHC class I-mismatched SIV-infected CD4+ T cells. The range of viral replication in the SIV-infected CD4+ T cells without CD8+ T cells was 1 × 106 to 1 × 107/ml of viral RNA copies/ml of supernatant. (c) Ex vivo tetramer-sorted SIV-specific CD8+ T cells poorly suppressed both SIVmac239/316e- and SIVsmE660-infected MHC class I-matched macrophages. (d) Percent maximum suppression of SIV-specific CD8+ T cells incubated with MHC class I-mismatched SIV-infected macrophages. The range of viral replication in the SIV-infected macrophages without CD8+ T cells was 1 × 105 to 1 × 106 viral RNA copies/ml of supernatant. The average percent maximum suppression capacity is indicated for each animal with black bars. SIV-specific CD8+ T cell populations isolated from elite controllers are indicated with circles, while SIV-specific CD8+ T cell populations isolated from progressors are indicated with diamonds. The colored symbols in panel a correspond to the tetramer-sorted SIV-specific CD8+ T cell populations that suppressed viral replication in SIV-infected macrophages in panel c. Each data point represents the average of one experiment performed in duplicate or triplicate. Ex vivo tetramer-sorted SIV-specific CD8+ T cells were harvested from several time points throughout the chronic phase of infection of SIV-infected rhesus macaques. (e) Statistical comparison of all tetramer-sorted SIV-specific CD8+ T cell-mediated suppression of viral replication in SIV-infected CD4+ T cells and macrophages. The difference in suppression of viral replication observed between CD4+ T cells and macrophages was statistically significant (P < 0.0001). (f) Intracellular Gag p27 staining of a representative experiment of MHC class I-matched SIVmac239/316e-infected CD4+ T cells incubated for 48 h alone (left panel), with Mamu-B*08+ EnvKL9-specific CD8+ T cells (middle panel), or with Mamu-A*01+ GagCM9-specific CD8+ T cells (right panel). Dot plots were generated by gating on live, CD8− cells. (g) Intracellular Gag p27 staining of a representative experiment of MHC class I-matched SIVmac239/316e-infected macrophages incubated for 48 h alone (left panel), with Mamu-B*08+ EnvKL9-specific CD8+ T cells (middle panel), or with Mamu-A*01+ GagCM9-specific CD8+ T cells (right panel). Dot plots were generated by gating on live, HLA-DR+ CD14+ macrophages.
Most ex vivo tetramer-sorted SIV-specific CD8+ T cells cannot eliminate or suppress viral replication in SIV-infected macrophages.
HIV/SIV-specific CD8+ T cell lines and clones have been shown to eliminate HIV/SIV-infected macrophages (14, 38). Indeed, HIV-specific CD8+ T cell clones killed HIV-infected macrophages more efficiently than they killed HIV-infected CD4+ T cells (14). Additionally, GagCM9-specific CD8+ T cells clones effectively eliminated SIVmac239/316e-infected macrophages in vitro (38). Though CD8+ T cell lines and clones can suppress viral replication in HIV- and SIV-infected macrophages, the suppressive properties of these cell lines and clones may not reflect the abilities of CD8+ T cells in vivo. Cell lines and clones are maintained in tissue culture media containing interleukin-2 (IL-2) and are regularly restimulated, and selection for particular clonotypes can occur in vitro. We, therefore, sought to determine whether ex vivo tetramer-sorted SIV-specific CD8+ T cells could suppress viral replication in SIVmac239/316e- and SIVsmE660-infected macrophages. We reasoned that freshly sorted CD8+ T cells might be more representative of the in vivo properties of CD8+ T cells than in vitro cultured cell lines and clones. SIVmac239/316e encodes amino acid replacements in Env that facilitate macrophage infection in vitro. We also infected macrophages with SIVsmE660 because some of the animals were initially infected with SIVsmE660. We, therefore, infected monocyte-derived macrophages from naïve animals with either SIVmac239/316e or SIVsmE660. Most ex vivo tetramer-sorted SIV-specific CD8+ T cells that suppressed viral replication in SIVmac239/316e-infected CD4+ T cells (Fig. 1a) failed to reduce viral replication in SIVmac239/316e-infected macrophages (Fig. 1c). In fact, the average percent maximum suppression of viral replication in SIV-infected CD4+ T cells was 60%, compared to 12% maximum suppression of viral replication in SIV-infected macrophages; the difference in the level of suppression observed between CD4+ T cells and macrophages was statistically significant (P < 0.0001; Fig. 1e). Some tetramer-sorted GagCM9-specific CD8+ T cells suppressed viral replication in SIVmac239/316e- and SIVsmE660-infected macrophages (Fig. 1c); however, there was no correlation between suppression of viral replication in SIV-infected macrophages and the disease status or viral load of the animals (Table 2 and Fig. 1c) or the purity to which the SIV-specific CD8+ T cells were sorted (data not shown). There was no common distinguishing feature shared among the tetramer-sorted SIV-specific CD8+ T cells that suppressed viral replication in SIV-infected macrophages nor among the animals from which these cells were derived. Additionally, tetramer-sorted CD8+ T cells that suppressed viral replication in CD4+ T cells most effectively were not always the tetramer-sorted SIV-specific CD8+ T cells that suppressed viral replication in SIV-infected macrophages (Fig. 1a and c). Suppression of viral replication that was observed in the few cases was MHC class I dependent because the same ex vivo tetramer-sorted SIV-specific CD8+ T cells did not suppress viral replication in MHC class I-mismatched SIVmac239/316e-infected macrophages (Fig. 1d). Finally, ex vivo tetramer-sorted CD8+ T cells restricted by both Mamu-A*01 and Mamu-B*08 failed to eliminate SIVmac239/316-infected macrophages (Fig. 1g).
Our data suggest that macrophages may be an important reservoir for SIV because it may be difficult for SIV-specific CD8+ T cells to suppress viral replication in this particular cell type.
Bulk CD8+ T cells that suppress viral replication in SIV-infected CD4+ T cells poorly suppressed viral replication in SIV-infected macrophages.
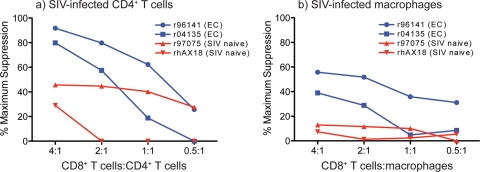
To extend our findings that freshly sorted SIV-specific CD8+ T cells cannot efficiently suppress viral replication in SIV-infected macrophages, we next tested bulk CD8+ T cells in the viral suppression assay as previously described (17, 28). We isolated bulk CD8+ T cells from ECs and naïve animals using an anti-CD8 antibody that recognizes a conformational epitope of the CD8αβ heterodimer, thereby excluding natural killer cells, which express only CD8α (40, 46). Autologous CD4+ T cells and macrophages were isolated, grown, and infected as described above. CD8+ T cells were added to the infected targets at various concentrations and incubated for 3 days. CD8+ T cells from ECs suppressed viral replication in autologous SIVmac239/316e-infected CD4+ T cells (Fig. 2a). However, at similar effector-to-target ratios, the same CD8+ T cells were inefficient at suppressing viral replication in autologous SIVmac239/316e-infected macrophages (Fig. 2b). CD8+ T cells from SIV-naïve animals exerted some level of nonspecific suppression of viral replication in SIV-infected CD4+ T cell targets only at the highest effector-to-target ratios; however, these levels rapidly decreased as the number of effectors was diluted. CD8+ T cells from SIV-naïve animals could not suppress viral replication in SIV-infected macrophages at any effector-to-target ratio.
Fig 2.
Bulk CD8+ T cells suppressed viral replication in autologous SIV-infected CD4+ T cells but were ineffective at suppressing viral replication in autologous SIV-infected macrophages. (a) Freshly harvested bulk CD8+ T cells from SIVmac239-infected ECs and SIV-naïve animals were incubated with SIVmac239/316e-infected autologous CD4+ T cells for 48 h at various concentrations. Dot plots were generated by gating on live, CD8− cells. (b) Freshly harvested bulk CD8+ T cells from SIVmac239-infected ECs and SIV-naïve animals were incubated with SIVmac239/316e-infected autologous macrophages for 48 h at various concentrations. Dot plots were generated by gating on live, HLA-DR+ CD14+ macrophages. We infected the CD4+ T cells and macrophages to achieve similar intracellular Gag p27 levels at the end of the assay. CD4+ T cells and macrophages from r96141 were approximately 25% infected, and CD4+ T cells and macrophages from r04135 were approximately 16% infected. These data were representative of two independent experiments.
HIV/SIV-specific CD8+ T cells play an essential role in reducing peak and chronic-phase viral replication (3, 13, 20, 21, 23, 24, 29, 34, 41). However, the SIV-specific CD8+ T cells that we tested in this study did not appear to eliminate and suppress viral replication in SIV-infected macrophages. This does not mean that all CD8+ T cells are incapable of suppressing viral replication in SIV-infected macrophages. For example, vaccine-induced CD8+ T cells generated by certain vectors may be better than those generated by other vectors at suppressing viral replication in SIV-infected macrophages. Additionally, CD8+ T cells from different stages of infection may have different abilities to suppress viral replication in macrophages. Unfortunately we did not have sufficient cell numbers to measure levels of expression markers, perforin, and granzyme to assess the “quality” of the CD8+ T cells in our studies.
We previously observed differential abilities of SIV-specific CD8+ T cells to suppress viral replication in SIV-infected CD4+ T cells depending on the culturing method (5, 26, 27, 36, 45). The culture conditions of CD8+ T cell lines and clones may result in activated cell populations that have unusually high antiviral efficacy in vitro. Thus, these cultured cell populations may not reflect how CD8+ T cells function in vivo.
Though HIV and SIV preferentially infect activated CD4+ T cells (9), several studies have suggested that HIV and SIV can also infect macrophages in vivo (18, 31, 39, 51). The importance of infected macrophages in vivo may, therefore, be underappreciated. Even with low numbers of infected macrophages in the total HIV/SIV-producing cellular compartment, macrophages may continually produce infectious virions and/or infect CD4+ T cells in trans (4, 16, 42). It is also possible that macrophages are relatively resistant to CD8+ T cell-mediated lysis. Activated CD4+ T cells produce virus 24 h after infection (45) when cell lysis begins (25, 33). These infected cells are most susceptible to CD8+ T cell-mediated lysis during the first 12 h of this replicative cycle, before Nef downregulates MHC class I on the cell surface (1, 37). For macrophages, which can be long lived after infection (6, 42), this CD8+ T cell-mediated lytic window is likely also to be 12 h. However, if an infected macrophage is not lysed by CD8+ T cells during this short window, the infected macrophage might continue producing virus for several months (42). Thus, macrophages could actually be contributing significantly to viral production. Induction of HIV/SIV-specific CD8+ T cells capable of killing infected macrophages or preventing establishment of the macrophage reservoir for HIV might be critical for controlling viral replication.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Disease grants R01 AI076114, R01 AI049120, R24 RR015371, and R24 RR016038 and in part by grant R51 RR000167 from the National Center for Research Resources (NCRR) awarded to the WNPRC, University of Wisconsin—Madison, and by grant RR000168 awarded to the New England Primate Research Center. The following reagents were obtained through the NIH AIDS Reagent and Reference Reagent Program, Division of AIDS, NIAID, NIH: IL-2, human (item no. 136), from Hoffman-La Roche; SIVmac p27 hybridoma (55-2F12, item no. 1547) from Niels Pedersen.
We gratefully acknowledge Ronald Desrosiers for providing SIVmac239/316e and Justin Greene for providing insight on the bulk CD8+ T cell viral suppression assay. We also acknowledge Caitlin McNair, Jennifer Nelson, and Thomas Friedrich for production of high-titer SIV and viral load analysis and Chrystal Glidden, Gretta Borchardt, and Debra Fisk for MHC typing of animals.
Footnotes
Published ahead of print 8 February 2012
REFERENCES
- 1. Adnan S, et al. 2006. Nef interference with HIV-1-specific CTL antiviral activity is epitope specific. Blood 108:3414–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borda JT, et al. 2004. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am. J. Pathol. 165:2111–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cameron PU, et al. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383–387 [DOI] [PubMed] [Google Scholar]
- 5. Chung C, et al. 2007. Not all cytokine-producing CD8+ T cells suppress simian immunodeficiency virus replication. J. Virol. 81:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collman RG, Perno CF, Crowe SM, Stevenson M, Montaner LJ. 2003. HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: new answers yield new questions. J. Leukoc. Biol. 74:631–634 [DOI] [PubMed] [Google Scholar]
- 7. Crowe SM, et al. 1994. HIV infection of monocyte-derived macrophages in vitro reduces phagocytosis of Candida albicans. J. Leukoc. Biol. 56:318–327 [DOI] [PubMed] [Google Scholar]
- 8. Desrosiers RC, et al. 1991. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am. J. Pathol. 139:29–35 [PMC free article] [PubMed] [Google Scholar]
- 9. Douek DC, et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95–98 [DOI] [PubMed] [Google Scholar]
- 10. Douek DC, Picker LJ, Koup RA. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265–304 [DOI] [PubMed] [Google Scholar]
- 11. Eckstein DA, et al. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med. 194:1407–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Embretson J, et al. 1993. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc. Natl. Acad. Sci. U. S. A. 90:357–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedrich TC, et al. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81:3465–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujiwara M, Takiguchi M. 2007. HIV-1-specific CTLs effectively suppress replication of HIV-1 in HIV-1-infected macrophages. Blood 109:4832–4838 [DOI] [PubMed] [Google Scholar]
- 15. Gartner S, et al. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215–219 [DOI] [PubMed] [Google Scholar]
- 16. Geijtenbeek TB, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597 [DOI] [PubMed] [Google Scholar]
- 17. Greene J, et al. 2010. Extralymphoid CD8+ T Cells resident in tissue from simian immunodeficiency virus SIVmac239 nef-vaccinated macaques suppress SIVmac239 replication ex vivo. J. Virol. 84:3362–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Igarashi T, et al. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. U. S. A. 98:658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Igarashi T, Imamichi H, Brown CR, Hirsch VM, Martin MA. 2003. The emergence and characterization of macrophage-tropic SIV/HIV chimeric viruses (SHIVs) present in CD4+ T cell-depleted rhesus monkeys. J. Leukoc. Biol. 74:772–780 [DOI] [PubMed] [Google Scholar]
- 20. Jin X, et al. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 22. Koenig S, et al. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089–1093 [DOI] [PubMed] [Google Scholar]
- 23. Koup RA, et al. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuroda MJ, et al. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127–5133 [PubMed] [Google Scholar]
- 25. Levy JA. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 57:183–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loffredo JT, et al. 2007. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T cells is unrelated to epitope specificity and is abrogated by viral escape. J. Virol. 81:2624–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loffredo JT, et al. 2005. Tat(28–35)SL8-specific CD8+ T lymphocytes are more effective than Gag(181–189)CM9-specific CD8+ T lymphocytes at suppressing simian immunodeficiency virus replication in a functional in vitro assay. J. Virol. 79:14986–14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martins MA, et al. 2010. T-cell correlates of vaccine efficacy after a heterologous simian immunodeficiency virus challenge. J. Virol. 84:4352–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matano T, et al. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meltzer MS, et al. 1990. Macrophages as susceptible targets for HIV infection, persistent viral reservoirs in tissue, and key immunoregulatory cells that control levels of virus replication and extent of disease. AIDS Res. Hum. Retroviruses 6:967–971 [DOI] [PubMed] [Google Scholar]
- 31. Ortiz AM, et al. 2011. Depletion of CD4+ T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J. Clin. Invest. 121:4433–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Price RW. 1996. Neurological complications of HIV infection. Lancet 348:445–452 [DOI] [PubMed] [Google Scholar]
- 33. Rasheed S, Gottlieb AA, Garry RF. 1986. Cell killing by ultraviolet-inactivated human immunodeficiency virus. Virology 154:395–400 [DOI] [PubMed] [Google Scholar]
- 34. Reimann KA, et al. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 68:2362–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reinhart TA, et al. 1997. Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J. Infect. Dis. 176:1198–1208 [DOI] [PubMed] [Google Scholar]
- 36. Sacha JB, et al. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 178:2746–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sacha JB, et al. 2007. Pol-specific CD8+ T cells recognize simian immunodeficiency virus-infected cells prior to Nef-mediated major histocompatibility complex class I downregulation. J. Virol. 81:11703–11712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sacha JB, et al. 2009. Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection. Proc. Natl. Acad. Sci. U. S. A. 106:9791–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schacker T, et al. 2001. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J. Infect. Dis. 183:555–562 [DOI] [PubMed] [Google Scholar]
- 40. Schmitz JE, et al. 1998. Expression of the CD8alpha beta-heterodimer on CD8(+) T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus- and human immunodeficiency virus+ individuals. Blood 92:198–206 [PubMed] [Google Scholar]
- 41. Schmitz JE, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860 [DOI] [PubMed] [Google Scholar]
- 42. Sharova N, Swingler C, Sharkey M, Stevenson M. 2005. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 24:2481–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsubota H, Lord CI, Watkins DI, Morimoto C, Letvin NL. 1989. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J. Exp. Med. 169:1421–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veazey RS, et al. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- 45. Vojnov L, et al. 2010. Effective simian immunodeficiency virus-specific CD8+ T cells lack an easily detectable, shared characteristic. J. Virol. 84:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Webster RL, Johnson RP. 2005. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology 115:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams KC, et al. 2001. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J. Exp. Med. 193:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wykrzykowska JJ, et al. 1998. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J. Exp. Med. 187:1767–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang OO, et al. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang OO, et al. 2003. Impacts of avidity and specificity on the antiviral efficiency of HIV-1-specific CTL. J. Immunol. 171:3718–3724 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Z, et al. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353–1357 [DOI] [PubMed] [Google Scholar]