Abstract
Respiratory syncytial virus (RSV) is the most important cause of lower respiratory tract disease in young children. In the 1960s, infants vaccinated with formalin-inactivated RSV developed a more severe disease characterized by excessive inflammatory immunopathology in lungs upon natural RSV infection. The fear of causing the vaccine-enhanced disease (VED) is an important obstacle for development of safe and effective RSV vaccines. The recombinant vaccine candidate G1F/M2 immunization also led to VED. It has been proved that cellular memory induced by RSV vaccines contributed to VED. Interleukin-27 (IL-27) and IL-23 regulate Th1, Th17, and/or Th2 cellular immune responses. In this study, mice coimmunized with pcDNA3–IL-27 and G1F/M2 were fully protected and, importantly, did not develop vaccine-enhanced inflammatory responses and immunopathology in lungs after RSV challenge, which was correlated with moderate Th1-, suppressed Th2-, and Th17-like memory responses activated by RSV. In contrast, G1F/M2- or pcDNA3–IL-23+G1F/M2-immunized mice, in which robust Th2- and Th17-like memory responses were induced, developed enhanced pulmonary inflammation and severe immunopathology. Mice coimmunized with G1F/M2 and the two cytokine plasmids exhibited mild inflammatory responses as well as remarkable Th1-, suppressed Th2-, and Th17-like memory responses. These results suggested that Th1-, Th2-, and Th17-like memory responses and, in particular, excessive Th2- and Th17-like memory responses were closely associated with VED; IL-27 may inhibit VED following respiratory syncytial virus infection by regulating cellular memory responses.
INTRODUCTION
Respiratory syncytial virus (RSV) is the most important cause of lower respiratory tract disease in infants and young children, accounting for approximately 5% of hospitalizations due to lower respiratory tract infections (15). Premature infants, immunocompromised individuals, and the elderly are the populations at the greatest risk to develop life-threatening RSV infections (10, 29). Moreover, reinfections occur frequently during life due to incomplete immunity after RSV infection. A number of clinical epidemiology studies have identified that there is a strong association between RSV bronchiolitis in infancy and the development of wheezing and asthma in later childhood (31, 36, 37). The World Health Organization has established a high priority for the development of an RSV vaccine. However, efforts to develop a safe and effective vaccine have been unsuccessful. In the 1960s, a formalin-inactivated RSV (FI-RSV) vaccine was used to immunize infants; these infants developed a more severe disease upon natural RSV infection; 80% of them but only 5% of infants that received a control vaccine required hospitalization, and two died (21). Histology performed on the lungs of the deceased revealed extensive neutrophils, mononuclear cells, and lymphocytes and increased numbers of eosinophils infiltrating the lungs (33).
Although the vaccine-enhanced disease (VED) is a major concern in the development of a safe and effective RSV vaccine, its immunological mechanisms are unclear. Many pieces of evidence showed that VED was associated with an exaggerated Th2-type response. Depletion of Th2 cytokine interlukin-4 (IL-4) or IL-13 of mice immunized with FI-RSV inhibited the development of pulmonary disease after RSV challenge (18). The Th2 polarization may have been related to the absence of a Th1-promoting RSV-specific cytotoxic T lymphocyte (CTL) response after immunization with FI-RSV (29). RSV challenge of FI-RSV-immunized mice elicited a robust memory CD4+ T cell response in the absence of a detectable memory CD8+ T cell response, pulmonary eosinophilia, and VED (29). Immunization of BALB/c mice with a recombinant vaccinia virus (vacv) that expresses the RSV M2 protein (vacvM2) elicited an RSV-specific memory CD8+ T cell response. Mice coimmunized with FI-RSV and vacvM2 after RSV challenge did not develop pulmonary eosinophilia, along with downregulation of pulmonary Th2 cytokines and upregulation of Th1 cytokines (41). Despite the beneficial role, Th1-biased or CD8+ T-biased cellular memory may also be detrimental to the host. RSV challenge of mice previously immunized with vacv expressing the RSV F protein (vacvF) exhibited a memory Th1 response in the absence of a detectable memory Th2 response and developed lung inflammation characterized by mononuclear cell infiltration (5, 17, 28). In the mice immunized with vacvM2 or vacvF, systemic disease, including weight loss, occurs after RSV challenge, although no eosinophilia was detectable (5, 17, 28). These data clearly suggested that both a Th2 memory response and a Th1 or CD8+ T memory response contribute to VED. Induction of a carefully balanced and controlled cellular memory would be critical for a safe RSV vaccine.
We have engineered a recombinant vaccine candidate, G1F/M2 (46), consisting of a domain of RSV G protein (amino acids [aa] 125 to 225; G1), linker residues, and a chimera F/M2 composed of a CTL epitope (aa 81 to 95; M2) of RSV M2 protein and a fusion peptide from F1 protein of measles virus (aa 113 to 131), which mediate binding and internalization of the chimeric polypeptide in cells, aiding presentation via the class I pathway (16, 11). It has been shown that F/M2 is effective in induction of M2 (aa 81 to 95 peptide)- and RSV-specific CTL responses as well as protective immunity in mice (16, 11). G1F/M2 immunization of mice with Al(OH)3 as an adjuvant induced a mixed Th1/Th2 response, CD8+ CTLs, and neutralizing antibodies (46). However, the immunized mice developed vaccine-enhanced pulmonary inflammation and immunopathology after RSV challenge (unpublished data). A regulatory adjuvant may be required to regulate cellular memory for attenuation of the excessive memory responses following RSV challenge.
The incorporation of cytokines as vaccine adjuvants in order to regulate the activation, expansion, and selection of vaccine-specific effector and memory T cells has received great attention. IL-12 has been recognized to be the signature cytokine that links innate and adaptive immunity. Recently, IL-23 and IL-27, two novel IL-12 cytokine family members, were discovered, and these are similar to yet clearly distinct from IL-12 in their structures and T cell stimulatory mechanisms (20, 30). IL-23 induces the production of gamma interferon (IFN-γ) from Th1 and IL-17 from Th17 (22, 30). It possesses unique immunological roles in the proliferation and development of memory T cells (30). IL-27 is composed of the Epstein-Barr virus-induced gene 3 (EBI3) and p28 subunits that are structurally related to the p40 and p35 subunits of IL-12, respectively. Early work showed the proinflammatory activities of IL-27 (43). However, based on recent findings, IL-27 has emerged as a rather immunoregulatory cytokine. Accumulating evidence from IL-27 receptor (IL-27R)-deficient mice indicated a dominant role in the suppression of immune hyperactivity. For example, IL-27ra−/− mice infected with Toxoplasma gondii developed a lethal CD4+ Th cell-mediated inflammatory disease (40). Similarly, in a model of asthma, the absence of IL-27 signaling led to an exacerbated Th2 response (27). IL-27 appears to have an inhibitory effect on Th1, Th2, and Th17 responses (2, 27, 45). Could IL-27 serve as an adjuvant of G1F/M2 to suppress vaccine-enhanced inappropriate immune responses?
In the study, we examined the immunoregulatory effects of IL-27 and/or IL-23 delivered as plasmid vectors pcDNA3–IL-27 (pIL-27) and pcDNA3–IL-23 (pIL-23), respectively, on G1F/M2-induced immune memory and cellular memory responses and pulmonary inflammatory immunopathology after RSV challenge in BALB/c mice. The results indicated that pIL-27 suppressed G1F/M2-enhanced pulmonary disease after RSV challenge and Th1-, Th2-, and Th17-like cellular memory responses. In particular, excessive Th2- and Th17-like memory responses were closely associated with VED.
MATERIALS AND METHODS
Virus and mice.
RSV A (Long strain, from ATCC) was propagated in HEp-2 cells and purified by a discontinuous sucrose gradient as previously described (47). RSV was stored at −70°C until use. Female BALB/c mice aged 6 to 9 weeks were purchased from Hebei Laboratory Animal Center (Shijiazhuang, China), housed and manipulated according to the Care and Use of Laboratory Animals (China), and kept under specific-pathogen-free conditions. They were confirmed to be seronegative for RSV before inclusion in the studies.
Preparation of G1F/M2 protein and plasmids pIL-27 and pIL-23.
Gene assembly, vector constructions, protein expression in Escherichia coli, purification by affinity chromatography, renaturation by dialysis, and analysis of G1F/M2 were undertaken as previously described (46). pIL-23 and pIL-27 were constructed as previously described (6, 35).
Immunizations and RSV challenge procedures.
Female BALB/c mice were randomly divided into 6 groups, the pIL-23+G1F/M2, pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, pcDNA3+G1F/M2, G1F/M2, and phosphate-buffered saline (PBS) groups, and each group included 12 mice. For the pIL-23+G1F/M2, pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, and pcDNA3+G1F/M2 groups, mice were vaccinated intraperitoneally (i.p.) with 20 μg G1F/M2 in 200 μl PBS 2 days after injection intramuscularly (i.m.) of 100 μg pIL-23 or/and pIL-27 plasmid adjuvant with 100 μl 25% sucrose. For the G1F/M2 and PBS groups, mice were vaccinated i.p. with 20 μg G1F/M2 or PBS with a total 200-μl volume. An interval of 2 weeks was invariably employed for the second and third immunizations. Except where indicated, half of the mice were bled 3 weeks after the last immunization for immune memory studies. The other half of the mice were challenged by intranasal (i.n.) instillation of 105 50% tissue culture infective doses (TCID50s)/100 μl RSV 3 weeks after the last immunization and sacrificed 5 days later for protection and immune pathology studies.
Standard ELISPOT assay.
IFN-γ- and IL-4-secreting cells were quantified using an enzyme-linked immunosorbent spot (ELISPOT) assay kit (Cayman) according to the manufacturer's instructions. Briefly, 96-well filtration plates were coated with anti-IFN-γ or anti-IL-4 antibody. Splenocytes (1 × 106/well) were cocultured with 20 μg/ml G1F/M2 for 48 h at 37°C in triplicate wells. After the cells were removed, plates were incubated with biotinylated anti-IFN-γ or anti-IL-4 antibodies for 2 h, streptavidin-horseradish peroxidase for 45 min, and then fresh substrate solution. Spots were then counted using an immunospot image analyzer. Results are shown as the mean value obtained for triplicate wells.
Flow cytometric assay.
Percentages of CD4+ T and CD8+ T cells in the spleen cells of mice were analyzed with a flow cytometer. Ten microliters of anti-CD4–fluorescein isothiocyanate (FITC) or anti-CD8–FITC, anti-CD44–phycoerythrin (PE), and anti-CD62L-Pecy5 was incubated with 1.0 × 106 spleen cells in a 100-μl staining volume for 20 min at room temperature. The cells were run on a flow cytometer (Beckman Coulter), and fluorescence-activated cell sorter data were analyzed using CellQuest software.
Specific CTL activity.
Specific CTL activity was assessed using a released lactate dehydrogenase (LDH) cytotoxicity assay kit (Cayman). Spleens from immunized BALB/c mice were removed 3 weeks after the last immunization. Splenocytes were suspended in complete RPMI 1640 with 10% fetal calf serum and stimulated in vitro for 5 days with 0.5 μM G1F/M2 to produce effectors. Target cells were SP2/0 cells pulsed with UV-inactivated RSV. SP2/0 cells were used as a control. Effectors and targets (1 × 104 cells) were cocultured for 6 h at 37°C in 5% CO2 at a ratio of 50.0:1. LDH was measured according to the manufacturer's protocol. The percentage of specific killing was calculated as (experimental release − spontaneous release)/(total release − spontaneous release). The data are represented as the mean percentages of the specific lysis values from six mice.
ELISAs.
IgG, IgG1, or IgG2a antibody titers in sera from vaccinated mice were assessed by a standard enzyme-linked immunosorbent assay (ELISA) protocol (46, 47). Antibody titers were expressed as the reciprocal of the last sample dilution giving an absorbance of at least 2-fold that of the preimmune sample and with an optical density of ≥0.15.
Neutralization assays.
Virus neutralization assays were undertaken in triplicate as previously described (32). Briefly, a viral suspension that was standardized to yield 50 plaques per well in HEp-2 cell monolayer cultures was used. Mixtures of 30 μl each of the RSV suspension and 2-fold serial dilutions of antiserum were incubated for 1 h at 37°C. The suspension was absorbed onto HEp-2 cells and then overlaid 2 h later with a 0.8% (wt/vol) semisolid methylcellulose overlay. After 3 to 4 days at 37°C in a humidified atmosphere containing 5% CO2, the cells were fixed and stained with 0.1% (wt/vol) crystal violet. Neutralization titers were expressed as the geometric mean titers of sera that neutralized 60% of plaques on RSV-infected HEp-2 cells.
Real-time RT-PCR.
RNA was isolated from the lungs using the TRIzol reagent (Invitrogen, Carlsbad, CA). Moloney murine leukemia virus reverse transcriptase and oligo(dT) (MBI Fermentas) were used for synthesis of the first strand of cDNA. The expression of the RSV gene in lung tissue was assessed by amplification of the RSV N gene by semiquantitative reverse transcription-PCR (RT-PCR) and real-time quantitative RT-PCR (qRT-PCR) with SYBR green to detect amplified products, using the following primers: sense primer 5′ GCG ATG TCT AGG TTA GGA AGA A 3′ and antisense primer 5′ GCT ATG TCC TTG GGT AGT AAG CCT 3′. The mouse housekeeping gene (β-actin) was used as a control. The semiquantitative PCR products were electrophoresed in a 1.2% agarose gel. Gene expression by qRT-PCR was normalized to β-actin expression before the fold change was calculated. Following RSV challenge, the fold reduction of RSV N-gene expression in each experimental group was calculated by comparison to that in PBS-treated mice. The levels of cytokines, chemokines, and mucus-associated gene gob5 in lung were similarly assessed. The fold change of the levels in each group was calculated by comparison to the level in naïve mice without any treatment.
Histology.
The lungs were isolated and immediately fixed in 4% paraformaldehyde. Lung tissue samples were subsequently processed, embedded in paraffin, thin sectioned, and placed on l-lysine-coated slides. Sections were stained with hematoxylin and eosin (H&E). Five sections per mouse were obtained. Sections from each mouse were scored blindly for the degree of inflammation in the peribronchial and perivascular spaces on a scale of 0 to 3 and in the alveolar tissue on a scale of 0 to 4, as previously described (26). Mean scores were calculated for each mouse.
Statistical analyses.
Statistical analyses were performed using the software SPSS (version 12.0). P values of <0.05 were considered statistically significant.
RESULTS
Cellular memory induced by pIL-27+G1F/M2.
On the basis of the homing characteristics, memory cells are classified into at least two functionally distinct subsets, effector memory T cells (TEM) and central memory T cells (TCM) (34). TEM that do not express the lymph node homing molecules CD62L and chemokine (C-C motif) receptor 7 (CCR7) display immediate effector function, and TCM, which are CD62L+, CCR7+, and CD44+, lack immediate effector function but efficiently stimulate dendritic cells (DCs) and differentiate into CCR7-negative effector cells upon secondary stimulation (34). To characterize the immune memory induced by adjuvanted G1F/M2, central memory and effector memory T cells were investigated by flow cytometric assay and standard ELISPOT assay, respectively.
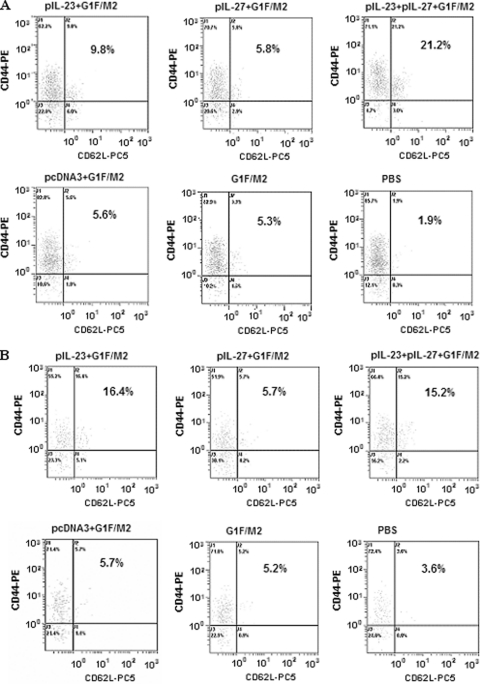
CD44+ CD62L+ is a phenotype that generally typifies central memory cells (25). Both pIL-23+G1F/M2 and pIL-23+pIL-27+G1F/M2 induced higher levels of CD4+/CD8+ CD44+ CD62L+ central memory cells than G1F/M2 without any adjuvants (Fig. 1). In contrast, mice immunized with pIL-27+G1F/M2 exhibited a frequency of CD4+/CD8+ CD44+ CD62L+ cells similar to the frequency in those immunized with G1F/M2. pcDNA3+G1F/M2 induced central memory cells at a frequency similar to that for G1F/M2. No or a few CD44+ CD62L+ cells were detected in the PBS group. Besides, the number of CD4+ CD44+ CD62L+ cells in the pIL-23+pIL-27+G1F/M2 group was markedly greater than that in the pIL-23+G1F/M2 or pIL-27+G1F/M2 group, suggesting a synergistic effect of IL-23 and IL-27 on the development of CD4+ central memory cells. These data suggest that pIL-27 did not affect the number of central memory T cells induced by G1F/M2, while pIL-23 alone or pIL-23+pIL-27 could promote the development of these cells, consistent with the ability of IL-23 to stimulate the proliferation and development of memory T cells (22, 30).
Fig 1.
Percentage of CD44+ CD62L+ cells of CD4+ or CD8+ spleen cells in immunized mice. Mice were immunized i.p. three times as described in Materials and Methods. Spleens from immunized mice were removed 3 weeks after the last immunization. Splenocytes were incubated with anti-CD4-FITC/anti-CD8-FITC, anti-CD44-PE, and anti-CD62L-Pecy5 (PC5) and then detected by flow cytometry. Percentages of CD4+ CD44+ CD62L+ T cells (A) and CD8+ CD44+ CD62L+ T cells (B) in the splenocytes of mice immunized with pIL-23+G1F/M2, pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, pcDNA3+G1F/M2, G1F/M2, or PBS are shown.
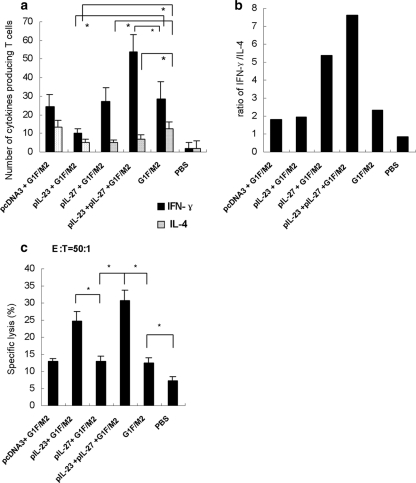
It has been proved that IL-27 is characterized by the capability of both proinflammation and inhibition of excessive Th1, Th2, and CD8+ T cell responses, while IL-23 promotes a Th1 memory response (22, 30, 40, 45). The cellular memory responses elicited by G1F/M2 are likely regulated by IL-27 and/or IL-23. To confirm the hypothesis, mice were immunized with pIL-27+G1F/M2, pIL-23+G1F/M2, or pIL-23+pIL-27+G1F/M2; and as controls, one group was immunized with pcDNA3+G1F/M2, one group was immunized with G1F/M2, and one group received PBS alone. The numbers of specific cytokine-secreting effector memory T cells were tested 3 weeks after the third immunization using a standard ELISPOT assay (4). Th1 responses are characterized by the production of IFN-γ, while Th2 responses are characterized by the production of IL-4, IL-5, and IL-13. CD8+ T cells can also produce significant amounts of IFN-γ. The splenocytes from immunized mice were restimulated in vitro by G1F/M2 protein, and specific Th1, CD8+ T, or Th2 effector memory cells could be activated to secrete IFN-γ or IL-4. As shown in Fig. 2a, no difference in the frequency of IFN-γ-secreting cells in pIL-27+G1F/M2-immunized mice compared with that in G1F/M2-immunized mice was observed, while the frequency of IL-4-secreting cells in pIL-27+G1F/M2-immunized mice was significantly lower than that in G1F/M2-immunized mice (P < 0.05), suggesting an inhibiting effect of pIL-27 on development of Th2 effector memory cells. The ratio of IFN-γ-secreting/IL-4-secreting cells of pIL-27+G1F/M2-immunized mice was higher than that of G1F/M2-immunized mice (Fig. 2b). Unexpectedly, the number of IFN-γ- or IL-4-secreting cells of pIL-23+G1F/M2-immunized mice was less than that of G1F/M2-immunized mice, suggesting an inhibiting effect of pIL-23 on effector memory cells. The frequency of IFN-γ-secreting cells of pIL-23+pIL-27+G1F/M2-immunized mice was significantly higher than that of the other groups, indicating a strong IFN-γ-secreting T effector memory response. These data showed that pIL-27 alone inhibited the development of Th2 effector memory cells and neither suppressed nor increased IFN-γ-secreting effector memory T cell differentiation, while pIL-23+pIL-27 promoted IFN-γ-secreting effector memory cell production.
Fig 2.
Specific cellular memory induced by the vaccine candidate in BALB/c mice. Mice were immunized i.p. three times as described in Materials and Methods. Spleens from immunized mice were removed 3 weeks after the last immunization. (a) Frequency of G1F/M2-specific IFN-γ- or IL-4-secreting T cells in 106 splenocytes of immunized mice. Splenocytes were restimulated for 48 h with 20 μg G1F/M2. The numbers of specific IFN-γ- and IL-4-secreting T cells were evaluated using an ELISPOT assay. Results are shown as the mean value ± SD of the number of spots observed for 106 spleen cells of 6 mice per group, obtained from triplicate wells. *, P < 0.05. (b) Ratio of IFN-γ- and IL-4-secreting T cells. (c) Induction of G1F/M2-specific CTL responses in immunized mice. Splenocytes were restimulated in vitro for 5 days with 0.5 μM G1F/M2 to produce effectors. Target cells were SP2/0 cells pulsed with UV-unactivated RSV. Data represent the percentage of specific lysis at an effector-to-target cell ratio of 50:1. Results are the means ± SDs of 5 or 6 mice per group. *, P < 0.05.
To further examine the effect of the adjuvants on the function of specific CD8+ memory T cells induced by G1F/M2, the splenocytes from immunized mice were restimulated with G1F/M2 in vitro for 5 days. The specific CD8+ memory T cells were activated and differentiated into effector CTLs, which could specifically kill RSV-treated target cells. CTL activity was tested using an LDH cytotoxicity assay kit. The data illustrated that the level of CTL activity elicited by pIL-27+G1F/M2, G1F/M2, or pcDNA3+G1F/M2 significantly increased compared with that elicited by PBS (P < 0.05; Fig. 2c). No difference in CTL activity was observed among mice immunized with pIL-27+G1F/M2, G1F/M2, and pcDNA3+G1F/M2. In contrast, pIL-23+G1F/M2 or pIL-23+pIL-27+G1F/M2 induced significantly higher levels of specific CTL activity than G1F/M2 alone. The results indicated that pIL-27 did not promote CD8+ memory T cell development induced by G1F/M2, while pIL-23 or pIL-23+pIL-27 enhanced the CD8+ memory response, which is consistent with the results for CD8+ CD44+ CD62L+ central memory T cells detected by flow cytometry (Fig. 1). The low CTL responses may be due to the method chosen for in vitro stimulation.
Humoral immune response induced by pIL-27+G1F/M2.
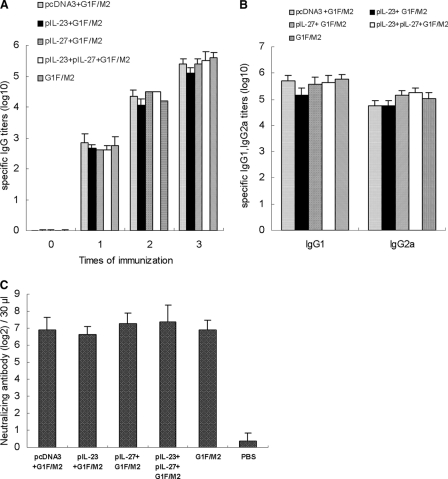
Many studies indicate that protection against RSV infection is mainly conferred by neutralizing antibodies. The G and F glycoproteins are the only viral antigens able to induce neutralizing antibodies (reviewed in reference 8). In this study, we detected the titers of IgG, IgG1, and IgG2a antibodies and neutralizing antibody induced by the vaccine candidate. Serum samples were 2-fold serially diluted, and specific IgG, IgG1, and IgG2a antibodies were tested with ELISA. Each group exhibited a high titer of specific IgG antibody just after the first immunization (Fig. 3A). Levels of IgG continued to rise after the second and third immunizations. In addition, G1F/M2 with or without adjuvants induced high titers of IgG1 and IgG2a antibodies after the third immunization (Fig. 3B). Neutralizing antibody was tested by neutralization assays. As shown in Fig. 3C, mice immunized with adjuvanted G1F/M2 or G1F/M2 alone exhibited high levels of RSV-specific neutralizing antibody compared with the level in mice immunized with PBS (P < 0.05). No difference was observed among these experimental groups (P > 0.05). The results indicated that the cytokine adjuvants had no effect on induction of neutralizing antibody by G1F/M2 antigen.
Fig 3.
Humoral immunity induced by the vaccine candidate in BALB/c mice. Mice were immunized i.p. three times with pIL-23+G1F/M2, pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, pcDNA3+G1F/M2, G1F/M2, or PBS. Serum samples were collected at 14 days after each immunization. G1F/M2-specific IgG (A) and IgG1 and IgG2a (B) antibodies 14 days after the third immunization were determined by ELISA. Results are expressed as geometric means of serum antibody titers and the means ± SDs from triplicate wells. *, P < 0.05. (C) Serum neutralizing antibody titers against RSV obtained in a neutralization assay. Results are expressed as the geometric mean titer of sera that neutralized 60% of plaques on RSV-infected HEp-2 cells.
Immunization with adjuvanted G1F/M2 protected mice against RSV infection.
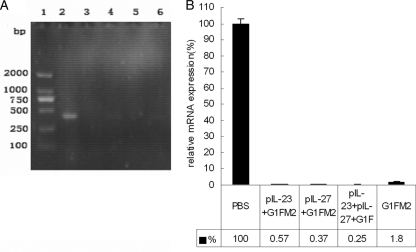
To determine the protective effect, mice were challenged with RSV i.n. 3 weeks after the final immunization. Five days after challenge, when the viral load peaked in the lungs, mice were sacrificed, total RNA was extracted from lung tissue, and the RSV load in the lungs was measured with semiquantitative PCR and qPCR of the RSV N gene. The N-gene expression elicited by RSV challenge of PBS-treated mice served as the control. As shown in Fig. 4A, obvious RSV gene was observed in mice treated with PBS following RSV challenge, while little or no RSV gene was observed in other mice immunized with adjuvanted G1F/M2 or G1F/M2 alone. The results from qPCR are shown in Fig. 4B. The relative levels of RSV N mRNA expression of mice immunized with pIL-23+G1F/M2, pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, or G1F/M2 after RSV challenge were 0.57%, 0.37%, 0.25%, and 1.8%, respectively, while the level for mice treated with PBS after RSV challenge was 100%. No statistically significant difference in expression of the RSV N gene was observed between the G1F/M2 group and the pcDNA3+G1F/M2 group (data no shown). These data suggested that immunization of mice using G1F/M2 with or without the adjuvants dramatically decreased the viral load in lungs compared with that obtained using PBS.
Fig 4.
Immunization with the vaccine candidate protects BALB/c mice against RSV challenge. Mice were immunized i.p. three times as described in Materials and Methods. Three weeks after the final immunization, mice were challenged i.n. with 105 TCID50s/100 μl RSV A. At 5 days postchallenge, lungs were removed. RSV replication in lungs was measured using RT-PCR. (A) Semiquantitative RT-PCR products. Lane 1, DL2000 DNA maker; lane 2, PBS group; lane 3, pIL-23+G1F/M2 group; lane 4, pIL-27+G1F/M2 group; lane 5, pIL-23+ pIL-27+G1F/M2 group; lane 6, G1F/M2 group. (B) Relative expression of RSV N mRNA by qRT-PCR. The fold reduction of RSV N-gene expression in each experimental group was calculated by comparison to that in PBS-treated mice (100%). Results are means ± SDs of 5 or 6 mice per group and are representative of two experiments.
RSV challenge of pIL-27+G1F/M2-immunized mice activated moderate Th1-like responses, low Th2-like responses, and no Th17-like responses.
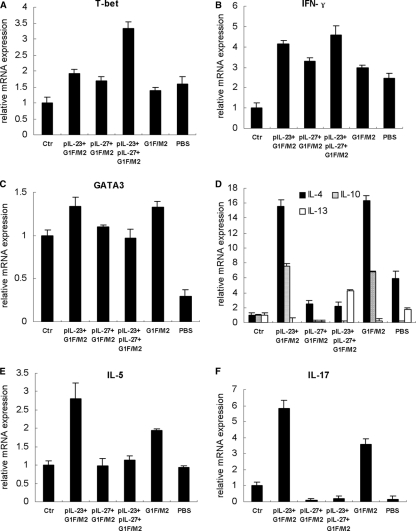
VED is characterized by a Th2-biased response (29). In the current study, we focused on the regulating effects of pIL-27 and/or pIL-23 on Th1-, Th2-, and Th17-like responses stimulated by RSV challenge of preimmunized mice. Since IL-23 promotes the development of Th1 and Th17 cells and IL-27 inhibits the development of Th1, Th2, and Th17 cells (reviewed in reference 20), the CD4+ memory T cells induced by G1F/M2 with or without adjuvants should be activated by RSV infection and produce Th1-, Th2-, and Th17-type cytokines. To understand whether the cytokines were regulated at the transcriptional level, we employed qRT-PCR to determine the mRNA. Naïve mice neither immunized nor challenged were used as controls. T-bet is a Th1-specific transcription factor that plays a central role in Th1 development (39). NK cells also express T-bet. T-bet correlates with IFN-γ expression in Th1 and NK cells (39). As Fig. 5A and B show, RSV challenge of mice preimmunized with pIL-23+G1F/M2, pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, G1F/M2, or PBS induced higher relative levels of mRNA expression of T-bet or IFN-γ than those in control naïve mice (P < 0.05), implicating that Th1, CD8+ T, and/or NK cell responses were activated in these experimental mice. The level of IFN-γ in the pIL-27+G1F/M2 group was significantly lower than that in the pIL-23+G1F/M2 or pIL-23+pIL-27+G1F/M2 group (P < 0.05), which may suggest that moderate Th1, CD8+ T, and/or NK cell responses in the pIL-27+G1F/M2 group were activated by RSV challenge.
Fig 5.
RSV challenge of pIL-27+G1F/M2-immunized mice activated moderate Th1, few Th2, and no Th17 memory responses in lungs. Mice were immunized i.p. three times as described in Materials and Methods. Three weeks after the final immunization, mice were challenged i.n. with 105 TCID50s/100 μl RSV A. At 5 days postchallenge, lungs were removed. The relative expression of the transcription factors and cytokines in lungs was measured using qRT-PCR. Naïve mice neither immunized nor challenged were used as controls (Ctr). The results are represented as the fold expression of each gene in experimental mice relative to control mice. (A and B) Th1-type transcription factors and cytokines; (C to E) Th2-type transcription factors and cytokines; (F) Th17 typical cytokine. Results are means ± SDs of 5 or 6 mice per group and are representative of two experiments.
As a Th2 cell lineage-specific transcription factor, the relative level of mRNA expression of GATA3 in the G1F/M2 or pIL-23+G1F/M2 group was higher than that in control mice (P < 0.05), while the levels in the other groups were similar to those in control mice, which suggests that RSV challenge of mice preimmunized with pIL-23+G1F/M2 or G1F/M2 activated Th2-like responses (Fig. 5C). Consistent with the findings for GATA3, mRNA expression of IL-4 and IL-10, Th2-type cytokines, in the G1F/M2 or pIL-23+G1F/M2 group was significantly higher than that in the control or the other groups (P < 0.05; Fig. 5D). IL-4 and IL-10 mRNA levels in the pIL-27+G1F/M2 or pIL-23+pIL-27+G1F/M2 group were markedly lower than those in the pIL-23+G1F/M2 or G1F/M2 group (P < 0.05).
IL-13 plays important roles in RSV disease. Neutralization of IL-13 blocks RSV-induced airway hyperreactivity during allergen challenge (24). Unexpectedly, the levels of IL-13 mRNA in the pIL-23+pIL-27+G1F/M2 and PBS groups were significantly higher than those in the control and the other groups (P < 0.05), suggesting GATA3-independent IL-13 secretion (Fig. 5D). IL-5 is known to be a Th2 cell product and also a potent autocrine growth, survival, and function cytokine produced by eosinophils themselves (7). Relative mRNA expression of IL-5 in the G1F/M2 group was significantly higher than that in control mice (P < 0.05). Mice in the pIL-23+G1F/M2 group exhibited markedly higher levels of IL-5 mRNA than mice in the control or the other groups (P < 0.05; Fig. 5E). In contrast, no significant alteration in the level of IL-5 mRNA in mice in the pIL-27+G1F/M2 or pIL-23+pIL-27+G1F/M2 group compared with that in control mice was observed (P > 0.05; Fig. 5E).
Since IL-23 was proved to promote CD4+ Th cells to differentiate into Th17 cells, which produce IL-17, a proinflammatory cytokine (12, 20), the expression of IL-17 mRNA was tested. As shown in Fig. 4F, RSV challenge of mice preimmunized with G1F/M2 induced a higher level of IL-17 mRNA expression than challenge of control mice (P < 0.05), suggesting that a Th17-like memory response was induced in the G1F/M2 group. The relative level of IL-17 mRNA in mice from the pIL-23+G1F/M2 group was significantly higher than that in mice from the G1F/M2 group (P < 0.05), suggesting that IL-23 increased the development of G1F/M2-specific Th17 memory cells, which may be a part of the increased CD4+ central memory T cells (Fig. 1). IL-17 mRNA expression in the pIL-27+G1F/M2, pIL-23+pIL-27+G1F/M2, or PBS group was significantly suppressed compared to that in control mice (P < 0.05; Fig. 4F). No differences in expression of these molecules associated with Th cells were observed between the G1F/M2 group and the pcDNA3+G1F/M2 group (data no shown). These data indicated that RSV challenge of mice preimmunized with G1F/M2 activated Th2-like and Th17-like memory responses, while RSV challenge of mice preimmunized with pIL-27+G1F/M2 activated moderate Th1, CD8+ T, and/or NK cell responses, few Th2-like memory responses, and no Th17-like memory response, which were probably beneficial to avoiding VED after RSV infection.
pIL-27+G1F/M2-immunized mice showed few inflammatory cytokines following RSV challenge.
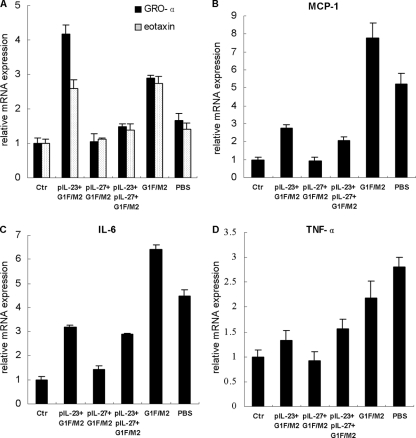
The bronchi and lungs of dead infants in clinical trials in the 1960s were filled with large numbers of neutrophils, macrophages, lymphocytes, and eosinophils (33). In this study, we characterized the proinflammatory cytokines and chemokines associated with these inflammatory cells. Growth-regulated oncogene alpha (GRO-α) and eotaxin are the chemokines recruiting neutrophils and eosinophils, respectively. RSV challenge of mice immunized with G1F/M2 or pIL-23+G1F/M2 elicited significantly increased relative mRNA expression of both GRO-α and eotaxin compared with RSV challenge of mice treated with PBS (P < 0.05; Fig. 6A), which implicated vaccine-enhanced pulmonary inflammation in pIL-23+G1F/M2- or G1F/M2-immunized mice following RSV challenge. In contrast, relative mRNA expression of both GRO-α and eotaxin in mice immunized with pIL-27+G1F/M2 following RSV challenge was less than that in mice treated with PBS (P < 0.05). Moreover, no difference in the relative mRNA expression of GRO-α or eotaxin was observed between the pIL-27+G1F/M2 group and the control group (P > 0.05). pIL-23+pIL-27+G1F/M2 immunization following RSV challenge did not increase or suppress the expression of GRO-α or eotaxin mRNA (Fig. 6A).
Fig 6.
pIL-27+G1F/M2-immunized mice showed infiltration of few inflammatory cytokines in lungs following RSV challenge. Mice were immunized i.p. three times as described in Materials and Methods and then challenged i.n. with 105 TCID50s/100 μl RSV A 3 weeks after the final immunization. Lungs were removed at 5 days postchallenge. The relative expression of the inflammatory cytokines GRO-α and eotaxin (A), MCP-1 (B), IL-6 (C), and TNF-α (D) in lungs was measured using qRT-PCR. Naïve mice neither immunized nor challenged were used as controls (Ctr). Results are means ± SDs of 5 or 6 mice per group and are representative of two different experiments.
Monocyte chemoattractant protein 1 (MCP-1), a chemokine of mononuclear cells and T lymphocytes, plays an important role in severe RSV infection and VED (3, 31). RSV challenge of G1F/M2-immunized mice induced dramatically increased levels of MCP-1 mRNA compared with RSV challenge of mice treated with PBS (P < 0.05), suggesting a G1F/M2-enhanced MCP-1 expression after RSV challenge. In contrast, relative mRNA expression of MCP-1 in mice immunized with pIL-27+G1F/M2 following RSV challenge was the same as that in control mice (Fig. 6B). Mice immunized with pIL-23+G1F/M2 or pIL-23+pIL-27+G1F/M2 exhibited reduced MCP-1 mRNA levels after RSV challenge compared with mice immunized with G1F/M2 without any adjuvants (P < 0.05).
IL-6 is a proinflammatory cytokine. Infection of DCs, macrophages, and peripheral blood mononuclear cells with RSV induced secretion of IL-6 (19). High IL-6 responsiveness predicted a severe RSV infection in children (19). In the current study, although the RSV load in lungs was dramatically suppressed, the levels of IL-6 mRNA were still high in all experimental groups except the pIL-27+G1F/M2 group. RSV challenge of G1F/M2-immunized mice elicited a level of IL-6 mRNA significantly higher than that in PBS-treated mice (P < 0.05), which suggested a vaccine-enhanced expression of IL-6 in G1F/M2-immunized mice after RSV challenge (Fig. 6C). In contrast, pIL-23+G1F/M2-, pIL-27+G1F/M2-, or pIL-23+pIL-27+G1F/M2-immunized mice produced significantly lower levels of IL-6 mRNA than G1F/M2-immunized mice (P < 0.05). The level of IL-6 mRNA of mice immunized with pIL-27+G1F/M2 was weakly higher than that of control mice and markedly lower than that of mice immunized with pIL-23+G1F/M2 or pIL-23+pIL-27+G1F/M2 (P < 0.05).
Tumor necrosis factor alpha (TNF-α) is also a proinflammatory cytokine that could provoke a pronounced inflammation of the airways and increase pathology after RSV infection (19). The data in Fig. 6D show that mice immunized with pIL-27+G1F/M2 after RSV challenge produced the same level of relative TNF-α mRNA as control mice (P > 0.05), while RSV challenge of the other mice stimulated a significantly higher level of TNF-α mRNA to various degrees (P < 0.05). The level of TNF-α mRNA of mice without immunization (in the PBS group) after RSV challenge was the highest among all these groups. Moreover, the level of TNF-α mRNA of pIL-23+G1F/M2- or pIL-23+pIL-27+G1F/M2-immunized mice remarkably decreased compared with that of G1F/M2-immunized mice (P < 0.05). No differences in expression of these inflammatory cytokines were observed between the G1F/M2 group and the pcDNA3+G1F/M2 group (data no shown). These data suggested that mice immunized with G1F/M2 without adjuvants showed vaccine-enhanced pulmonary inflammatory responses following RSV infection, while RSV challenge of mice immunized with pIL-27+G1F/M2 stimulated no or little inflammation in lungs.
pIL-27+G1F/M2 immunization prevented pulmonary pathology following RSV infection.
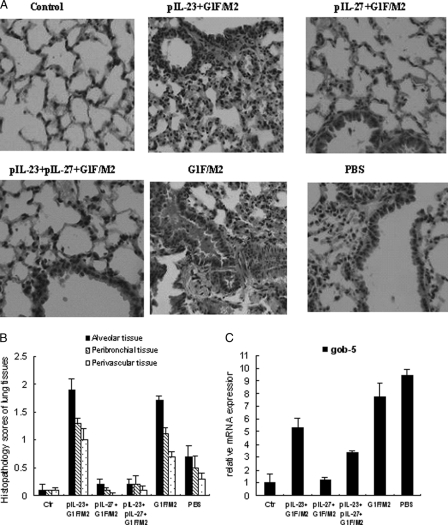
To further characterize the pulmonary immunopathology, lungs of mice immunized and challenged were stained with H&E (Fig. 7A). Naïve mice without any treatment served as controls. We observed multifocal acute alveolitis with intra-alveolar edema in the parenchymal lung tissue of mice in the G1F/M2 group (and the pcDNA3+G1F/M2 group; image no shown), with hemorrhage and mononuclear and lymphocyte cell infiltrates detected throughout. This was accompanied by bronchiolitis with shed epithelium and mucus in the bronchus. The postchallenge lungs of mice in the pIL-23+G1F/M2 group had inflammatory cell infiltration in alveolar walls, intra-alveolar edema, and hemorrhage similar to that in mice immunized with G1F/M2. In contrast, pIL-23+pIL-27+G1F/M2 immunization dramatically suppressed the above-described immunopathology. As expected, lungs of pIL-27+G1F/M2-immunized mice contained few inflammatory cells after RSV challenge, and no mucus or alveolitis was observed, which was similar to the findings for the control mice. Mice from the PBS group showed mild inflammatory cell infiltration and alveolitis. The mice in the G1F/M2 or pIL-23+G1F/M2 group also scored significantly higher for histopathology than their counterparts in the other groups (P < 0.05; Fig. 7B). The inflammation scores for the pIL-27+G1F/M2- or pIL-23+pIL-27+G1F/M2-immunized mice showed no statistical differences from scores for the control mice (Fig. 7B).
Fig 7.
pIL-27+G1F/M2 immunization prevented pulmonary pathology following RSV infection. (A) Lung histology was determined by H&E staining at 5 days postchallenge. An image for a representative animal is shown for each experimental group or for naïve mice (control). (B) Scores for pulmonary inflammation. Tissue sections obtained from each mouse were scored for inflammation on a scale of from 0 to 3 or 4 as described in Materials and Methods. (C) Relative expression of gob5 gene in lungs at 5 days postchallenge. Preimmunized mice were challenged with RSV A 3 weeks after the final immunization. Total RNAs were extracted from the lungs. The relative expression of gob5 mRNA was measured by qRT-PCR. Naïve mice neither immunized nor challenged were used as controls. Results are means ± SDs of 5 to 6 mice/group and are representative of two experiments.
Mucus overproduction is one of the hallmarks of severe RSV-induced disease in infants. After RSV challenge, the lungs of FI-RSV-immunized mice also showed increased intracellular mucus production (29). Mucus-associated gene gob5 in lungs was examined (Fig. 7C). Following RSV challenge, mice in the G1F/M2 or PBS group showed a substantial increase in expression of gob5 mRNA compared with control mice (P < 0.05). The relative level of gob5 mRNA in the pIL-23+G1F/M2 or pIL-23+pIL-27+G1F/M2 group was higher than that in control mice but lower than that in the G1F/M2 or PBS group (P < 0.05). No significant difference between the pIL-27+G1F/M2 and control groups was observed (P > 0.05). These results indicated that RSV challenge of pIL-27+G1F/M2-immunized mice did not elicit immunopathology and mucus compared with RSV challenge of G1F/M2-immunized mice.
DISCUSSION
Although many governmental bodies and industrial organizations have identified RSV to be a major target for vaccine development, 4 decades of effort have yet to produce an effective and safe vaccine against RSV for human use. The fear of causing VED is an important obstacle for RSV vaccine development. Immune memory is the conceptual basis for how vaccines work. Many studies suggest that cellular immune responses induced by RSV infection of infants immunized with FI-RSV or mice immunized with FI-RSV or subunit vaccine candidates contribute to VED (28, 29, 41). In this study, we focused on the inflammatory immunopathology associated with excessive or unbalanced specific cellular memory responses that must be overcome for a safe and effective RSV vaccine. We evaluated the immune regulation effect of IL-27 as a molecular adjuvant for the RSV vaccine candidate G1F/M2 on immune memory, protection, and vaccine-enhanced inflammatory response and immunopathology.
This study showed that pIL-27+G1F/M2 immunization neither increased nor suppressed CD4+/CD8+ central memory T cells (Fig. 1), IFN-γ-secreting Th1 and/or CD8+ T effector memory cells, and CTL activity compared to G1F/M2 immunization (Fig. 2). These results were consistent with the regulation effect of IL-27 on T cell differentiation, since IL-27 suppressed excessive T cell responses (40), and the Th1 response induced by G1F/M2 was not hyperactive but might be moderate. In addition, mice immunized with pIL-27+G1F/M2 exhibited decreased Th2 effector memory cells (Fig. 2a), which was consistent with previous studies (27, 44). IL-27ra−/− mice challenged with Trichuris muris produced increased Th2-associated cytokines and developed Th2-associated exaggerated goblet cell hyperplasia and mastocytosis in the gut (27). Intranasal administration of IL-27 inhibited ovalbumin (OVA)-induced Th2 cytokines and airway hyperresponsiveness in OVA-sensitized animals (44). These studies and our result support the negative regulation effect of IL-27 on the development of Th2 memory cells. Villarino et al. (40) demonstrated that increased production of Th2 cytokines in the T. muris-infected mice was not the consequence of an inability to generate an effective IFN-γ response in the absence of IL-27R signaling. Consistent with that result, we found that the inhibition of pIL-27+G1F/M2 on specific IL-4-production was not parallel with an increase of IFN-γ (Fig. 2a).
In contrast to IL-27, IL-23 preferentially acts on memory Th1 cells for their proliferation and IFN-γ production (22). pIL-23 as an adjuvant of G1F/M2 markedly increased the frequency of CD4+ central memory T cells and Th1-promoting CD8+ central memory T cell (Fig. 1) and CD8+ CTL activity (Fig. 2c), which suggested the promotion effect of IL-23 on Th1 memory cells (22). However, we found that pIL-23 suppressed the specific CD4+ effector memory T cells induced by G1F/M2 (Fig. 2a). No similar study about the effect of IL-23 on effector memory cells was reported. Further understanding of the role of IL-23 in T cell differentiation is required.
pIL-23+pIL27+G1F/M2 immunization of mice induced high levels of IFN-γ-secreting effector memory cells, CD4+ central memory cells, and specific CTL activity, while the Th2 memory response was suppressed by pIL-23+pIL27+G1F/M2 (Fig. 1 and 2). These data suggested the property of IL-27 inhibiting Th2 cell differentiation (27) and a synergy effect of IL-27 and IL-23 on promoting IFN-γ-secreting T cell differentiation. It has been demonstrated that IL-27 has a double-sided role in the regulation of the immune response: one as an initiator of Th1 responses and the other as an attenuator of inflammatory responses (40, 43, 44). Stimulated under weakly polarizing conditions in vitro, IL-27R-deficient T cells are deficient in IFN-γ production, but in a strongly polarizing environment, they produce elevated levels of IFN-γ (2, 40, 43, 45), indicating that IL-27 is capable of inhibiting IFN-γ production only when CD4+ T cells are in an uncommitted state after activation and before differentiation, while it promotes IFN-γ production after polarization of CD4+ T cells. It is possible that the Th1 memory response activated by pIL-23 provided conditions for pIL-27 promotion of Th1 differentiation.
In the challenge phase, the viral load, inflammatory response, and pathology lesions in lungs of vaccinees are controlled by both the innate immune responses and adaptive memory responses activated by RSV infection. Following RSV challenge, mice immunized with pIL-27+G1F/M2 were protected. Importantly, few inflammatory mediators, no immunopathology, and no mucus were observed in the lungs of these mice, while mice immunized with G1F/M2 exhibited large numbers of proinflammatory cytokines and chemokines, serious immunopathology, and mucus in the lungs.
The specific neutralizing antibody as well as CTL may contribute to protection against RSV challenge (8). Interestingly, vaccine-enhanced inflammatory immunopathology, a tough problem for a safe RSV vaccine, may be inhibited by IL-27, serving as the adjuvant in this mouse model. RSV challenge of pIL-27+G1F/M2-immunized mice elicited moderate Th1 and/or CD8+ T cell memory responses characterized by expression of IFN-γ and T-bet and few Th2-like memory responses characterized by expression of IL-4, IL-5, IL-10, IL-13, and GATA3 (Fig. 5), which were correlated with the Th1, CD8+ T, and Th2 immune memory induced by pIL-27+G1F/M2 immunization (Fig. 1 and 2). In addition, the expression of IL-17, produced by activated memory Th17 cells (1), was suppressed in pIL-27+G1F/M2-immunized mice following RSV challenge (Fig. 5). In contrast, RSV challenge of G1F/M2-immunized mice elicited low levels of Th1-like responses but robust Th2-like responses and high levels of expression of IL-17 (Fig. 5). The Th1-, Th2-, and Th17-like memory response profiles activated by RSV challenge of preimmunized mice may be largely associated with the pulmonary inflammatory responses and immunopathology.
While the Th1 immune response is pivotal for host defense against pathogens, dysregulated Th1 responses may cause immunopathology. For instance, strong Th1 memory induced by vacvF led to infiltration of a number of inflammatory mononuclear cells in lungs following RSV challenge (5). Thus, initiation, reinforcement, and maintenance of the Th1 memory induced by RSV vaccines should be tightly controlled. IL-27 may be a key checkpoint for development of Th1 cells which can regulate Th1 differentiation and suppress an overmuch Th1 response (40, 43). In contrast, IL-23 promotes the Th1 memory response (30). RSV challenge of mice induced more Th1-like memory responses in mice immunized with pIL-23+G1F/M2 than mice immunized with pIL-27+G1F/M2 (Fig. 5), which might be associated with the inflammatory cell infiltration in lungs of these mice (Fig. 7A). However, only strong Th1-like responses could not fully explain the enhanced inflammation and immunopathology in the pIL-23+G1F/M2 group, since mice from the pIL-23+pIL-27+G1F/M2 group, which showed stronger Th1-like responses than mice from the pIL-23+G1F/M2 group, exhibited mild inflammation and pathology (Fig. 6 and 7).
Evidence suggested that Th2 responses contribute to VED (17, 29). In animals preimmunized with FI-RSV vaccine, high levels of IL-4 were detected (17). In mice, depletion of Th2 cells or IL-4 and IL-10 reduced the severity of VED (18, 29). The current study showed that in mice preimmunized with G1F/M2 or pIL-23+G1F/M2, high levels of mRNA of Th2-type cytokines (IL-4, IL-5, IL-10) and transcription factor GATA3 were detected (Fig. 5). At the same time, enhanced inflammatory cytokines and chemokines (GRO-α, eotaxin, MCP-1, and IL-6) and pulmonary pathology existed in lungs (Fig. 6 and 7). In contrast, RSV challenge of mice immunized with pIL-27+G1F/M2 or pIL-23+pIL-27+G1F/M2 induced markedly weaker Th2-like memory responses along with fewer inflammatory cytokines and chemokines and less immunopathology. These results supported the involvement of Th2 memory responses in VED and are consistent with the inhibitory effect of IL-27 on Th2 responses (27, 43, 45).
Recently, a subset of IL-17-producing CD4+ T cells, Th17, which is distinct from either IFN-γ-producing Th1 or IL-4-producing Th2 and able to cause inflammation and autoimmune disease was identified (12). IL-17, a proinflammatory cytokine, enhances T cell priming and stimulates production of multiple proinflammatory cytokines and chemokines, resulting in the induction of inflammation, such as recruitment of neutrophils (42, 45). It was reported that IL-17 expression is associated with RSV diseases (27, 23). IL-17 may play a pathogenic role during RSV-associated allergic airway responses (27, 13). VED is known to be an allergy-like disease characterized by a Th2-biased response and granulocyte infiltration. We found that RSV challenge of mice immunized with G1F/M2 induced significant expression of IL-17 mRNA, while PBS-treated mice with primary RSV infection exhibited suppressed expression of IL-17 mRNA (Fig. 5F), which implied that IL-17 was involved in the VED. It was demonstrated that IL-23 maintains Th17 effector function and promotes IL-17 production (38), while IL-27 acts as a negative regulator of Th17 to produce IL-17, similar to its ability to differentiate Th2 (9). We found that the relative expression of IL-17 mRNA was significantly increased upon RSV challenge of pIL-23+G1F/M2-immunized mice with severe enhanced immunopathology, while it was suppressed upon RSV challenge of pIL-27+G1F/M2-immunized mice with no enhanced immunopathology (Fig. 5 and 7), which supported the suggestion that Th-17/IL-17 may contribute to G1F/M2-enhanced disease. This is consistent with the result in allergenically sensitized RSV-infected mice. OVA-sensitized, RSV-infected (OVA/RSV) mice expressed increased levels of IL-17 and mucus (14). Airway hypersensitivity was suppressed in IL-17-deficient mice (14). Moreover, RSV challenge of pIL-23+pIL-27+G1F/M2-immunized mice induced strong Th1-like, weak Th2-like, and suppressed Th17-like responses; these mice showed a few inflammatory responses in lungs.
All the results indicated that Th1, Th2, and Th17 polarization of Th cells is associated with G1F/M2-enhanced disease and, in particular, that excessive Th2- and Th17-like memory responses activated by RSV challenge may be closely associated with the development of VED. In pIL-27+G1F/M2-immunized mice, the regulation effect of IL-27 on Th1-, Th2-, and Th17-like responses promoted G1F/M2 induction of appropriate cellular memory, which may lead to appropriate innate immune and adaptive immune memory responses elicited by RSV challenge, thus protecting mice against RSV challenge and allowing them to avoid VED.
These results give an important insight into how to inhibit vaccine-enhanced inflammation and immunopathology in mice. It has been widely accepted that on exposure to RSV, a Th2-biased cell response is responsible for VED. We first found that both strong Th2-like and strong Th17-like responses were associated with VED. IL-27, as a negative regulator of the development of Th2 and Th17 responses, could provide us with a potential way for preventing VED. More extensive studies will be required to elucidate the underlying mechanisms. Overall, our study provides hope for the development of a safe and effective RSV vaccine for use in humans.
ACKNOWLEDGMENTS
The present work was supported by grants from the Chinese National Natural Science Foundation (30972801), the Specialized Research Fund for the Doctoral Program of Higher Education, State Education Ministry (20091323120005), and the 100 Innovation Talents Project of Hebei Higher Education (CPRC030).
Footnotes
Published ahead of print 1 February 2012
REFERENCES
- 1. Acosta-Rodriguez EV, et al. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- 2. Artis D, et al. 2004. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J. Immunol. 173:5626–5634 [DOI] [PubMed] [Google Scholar]
- 3. Bermejo-Martin JF, et al. 2007. Persistence of proinflammatory response after severe respiratory syncytial virus disease in children. J. Allergy Clin. Immunol. 119:1547–1550 [DOI] [PubMed] [Google Scholar]
- 4. Calarota SA, et al. 2008. IL-15 as memory T-cell adjuvant for topical HIV-1 DermaVir vaccine. Vaccine 26:5188–5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castilow EM, Olson MR, Meyerholz DK, Varga SM. 2008. Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. J. Virol. 82:2196–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiyo M, et al. 2005. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int. J. Cancer 115:437–442 [DOI] [PubMed] [Google Scholar]
- 7. Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. 1989. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 245:308–310 [DOI] [PubMed] [Google Scholar]
- 8. Collins PL, Melero JA. 2011. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 162:80–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diveu C, et al. 2009. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 182:5748–5756 [DOI] [PubMed] [Google Scholar]
- 10. Ebbert JO, Limper AH. 2005. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration 72:263–269 [DOI] [PubMed] [Google Scholar]
- 11. Fan C, Mei X. 2005. Co-immunization of BALB/c mice with recombinant immunogens containing G protein fragment and chimeric CTL epitope of respiratory syncytial virus induces enhanced cellular immunity and high level of antibody response. Vaccine 23:4453–4461 [DOI] [PubMed] [Google Scholar]
- 12. Harrington LE, et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132 [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto K, et al. 2005. Respiratory syncytial virus infection in the absence of STAT1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J. Allergy Clin. Immunol. 116:550–557 [DOI] [PubMed] [Google Scholar]
- 14. Hashimoto K, et al. 2004. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and Gob-5. Am. J. Respir. Crit. Care Med. 170:306–312 [DOI] [PubMed] [Google Scholar]
- 15. Henrickson KJ, Hoover S, Kehl KS, Hua W. 2004. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr. Infect. Dis. J. 23:S11–S18 [DOI] [PubMed] [Google Scholar]
- 16. Hsu SC, Chargelegue D, Steward MW. 1998. Reduction of respiratory syncytial virus titer in the lungs of mice after intranasal immunization with a chimeric peptide consisting of a single CTL epitope linked to a fusion peptide. Virology 240:376–381 [DOI] [PubMed] [Google Scholar]
- 17. Hussell T, Baldwin CJ, O'Garra A, Openshaw PJ. 1997. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 27:3341–3349 [DOI] [PubMed] [Google Scholar]
- 18. Johnson TR, Parker RA, Johnson JE, Graham BS. 2003. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 170:2037–2045 [DOI] [PubMed] [Google Scholar]
- 19. Juntti H, et al. 2009. Cytokine responses in cord blood predict the severity of later respiratory syncytial virus infection. J. Allergy Clin. Immunol. 124:52–58 [DOI] [PubMed] [Google Scholar]
- 20. Kastelein RA, Hunter CA, Cua DJ. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25:221–242 [DOI] [PubMed] [Google Scholar]
- 21. Kim HW, et al. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422–434 [DOI] [PubMed] [Google Scholar]
- 22. Langrish CL, et al. 2004. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202:96–105 [DOI] [PubMed] [Google Scholar]
- 23. Lukacs NW, Smit JJ, Nunez G, Lindell DM. 2010. Respiratory virus-induced TLR7 activation controls IL-17 associated increase in mucus via IL-23 regulation. J. Immunol. 185:2231–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lukacs NW, et al. 2001. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J. Immunol. 167:1060–1065 [DOI] [PubMed] [Google Scholar]
- 25. Manjunath N, et al. 2001. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mok H, et al. 2007. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J. Virol. 81:13710–13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukherjee S, et al. 2011. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am. J. Pathol. 179:248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olson MR, Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424 [DOI] [PubMed] [Google Scholar]
- 29. Openshaw PJ. 1995. Immunopathological mechanisms in respiratory syncytial virus disease. Springer Semin. Immunopathol. 17:187–201 [DOI] [PubMed] [Google Scholar]
- 30. Oppmann B, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725 [DOI] [PubMed] [Google Scholar]
- 31. Power UF, et al. 2001. Differential histopathology and chemokine gene expression in lung tissues following respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV- or BBG2Na-immunized mice. J. Virol. 75:12421–12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Power UF, et al. 1997. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology 230:155–166 [DOI] [PubMed] [Google Scholar]
- 33. Prince GA, Curtis SJ, Yim KC, Porter DD. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine J. Gen. Virol. 82:2881–2888 [DOI] [PubMed] [Google Scholar]
- 34. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 35. Shimozato O, et al. 2006. The secreted form of the p40 subunit of interleukin (IL)-12 inhibits IL-23 functions and abrogates IL-23-mediated antitumour effects. Immunology 117:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sigurs N, et al. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 171:137–141 [DOI] [PubMed] [Google Scholar]
- 37. Stensballe LG, et al. 2009. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J. Allergy Clin. Immunol. 123:131–137 [DOI] [PubMed] [Google Scholar]
- 38. Stockinger B, Veldhoen M. 2007. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 19:281–286 [DOI] [PubMed] [Google Scholar]
- 39. Szabo SJ, et al. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655–669 [DOI] [PubMed] [Google Scholar]
- 40. Villarino A, et al. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19:645–655 [DOI] [PubMed] [Google Scholar]
- 41. Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 70:2852–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wua Q, et al. 2007. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshida H, et al. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15:569–578 [DOI] [PubMed] [Google Scholar]
- 44. Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. 2007. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 179:4415–4423 [DOI] [PubMed] [Google Scholar]
- 45. Yoshimura T, et al. 2006. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J. Immunol. 177:5377–5385 [DOI] [PubMed] [Google Scholar]
- 46. Zeng R, Gong W, Fan C, Mei X. 2006. Induction of balanced immunity in BALB/c mice by vaccination with a recombinant fusion protein containing a respiratory syncytial virus G protein fragment and a CTL epitope. Vaccine 24:941–947 [DOI] [PubMed] [Google Scholar]
- 47. Zeng R, Zhang Z, Mei X, Gong W, Wei L. 2008. Protective effect of a RSV subunit vaccine candidate G1F/M2 was enhanced by a HSP70-like protein in mice. Biochem. Biophys. Res. Commun. 377:495–499 [DOI] [PubMed] [Google Scholar]